Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Time Course of Proceduresv
2.3. OMNI-RES Scale Standardized Anchoring Instructions
2.4. Orientation Session
2.5. Testing Visit
2.6. Electromyographic, Mechanomyographic, and Torque Acquisition
2.7. Statistical Analysis
3. Results
3.1. Reliability
3.2. Time to Task Failure
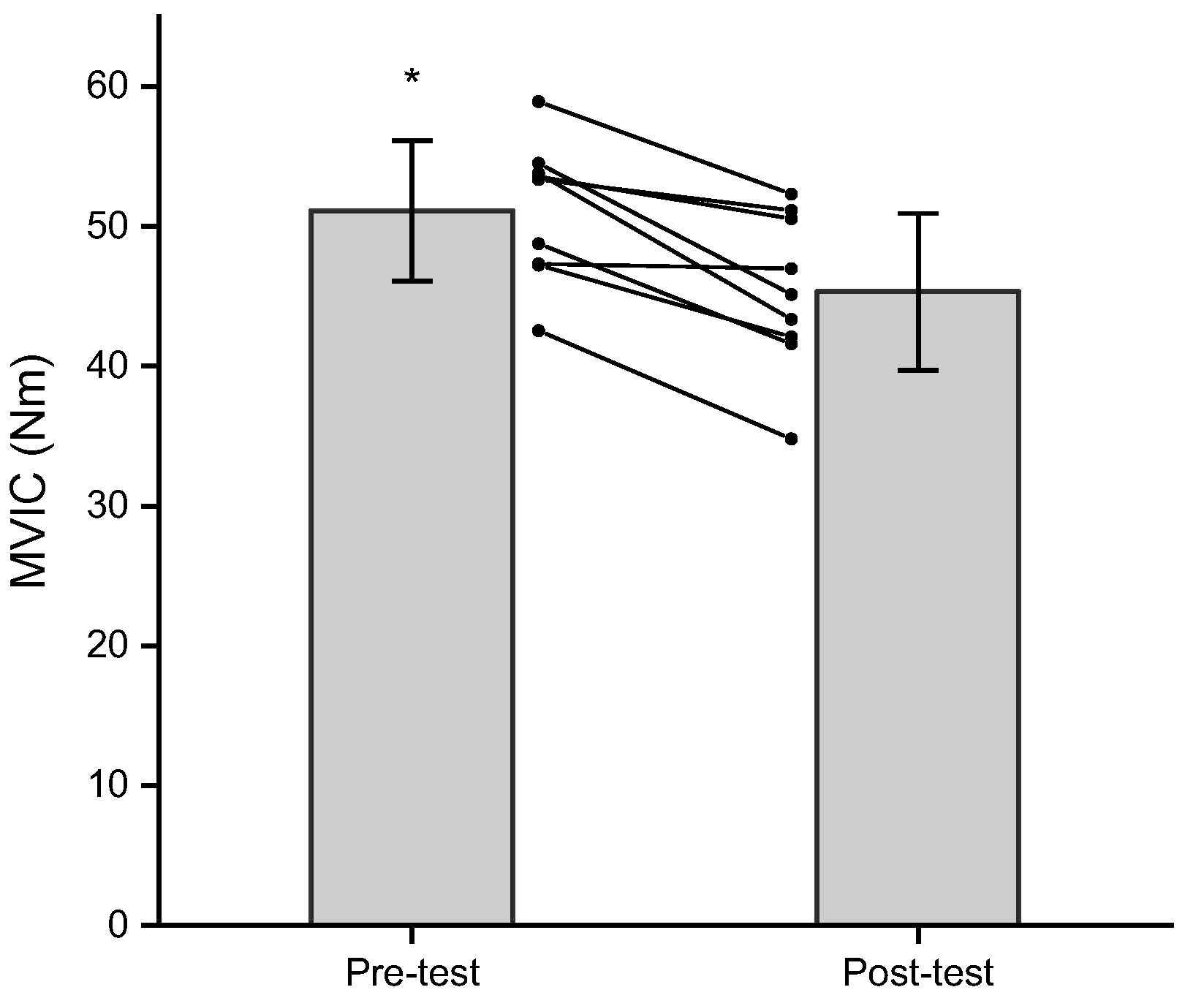
3.3. Maximal Voluntary Isometric Contraction
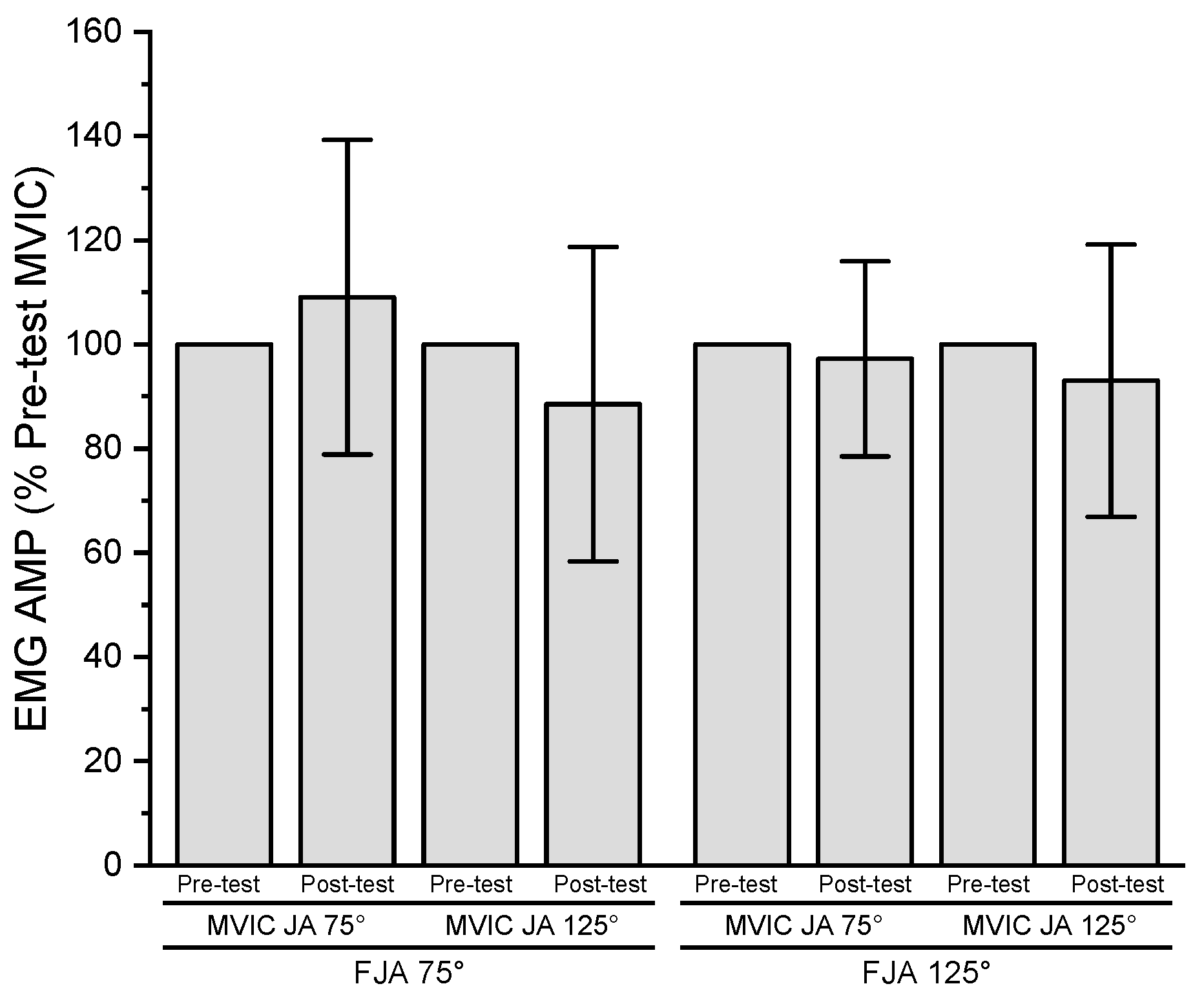
3.4. Electromyographic Amplitude
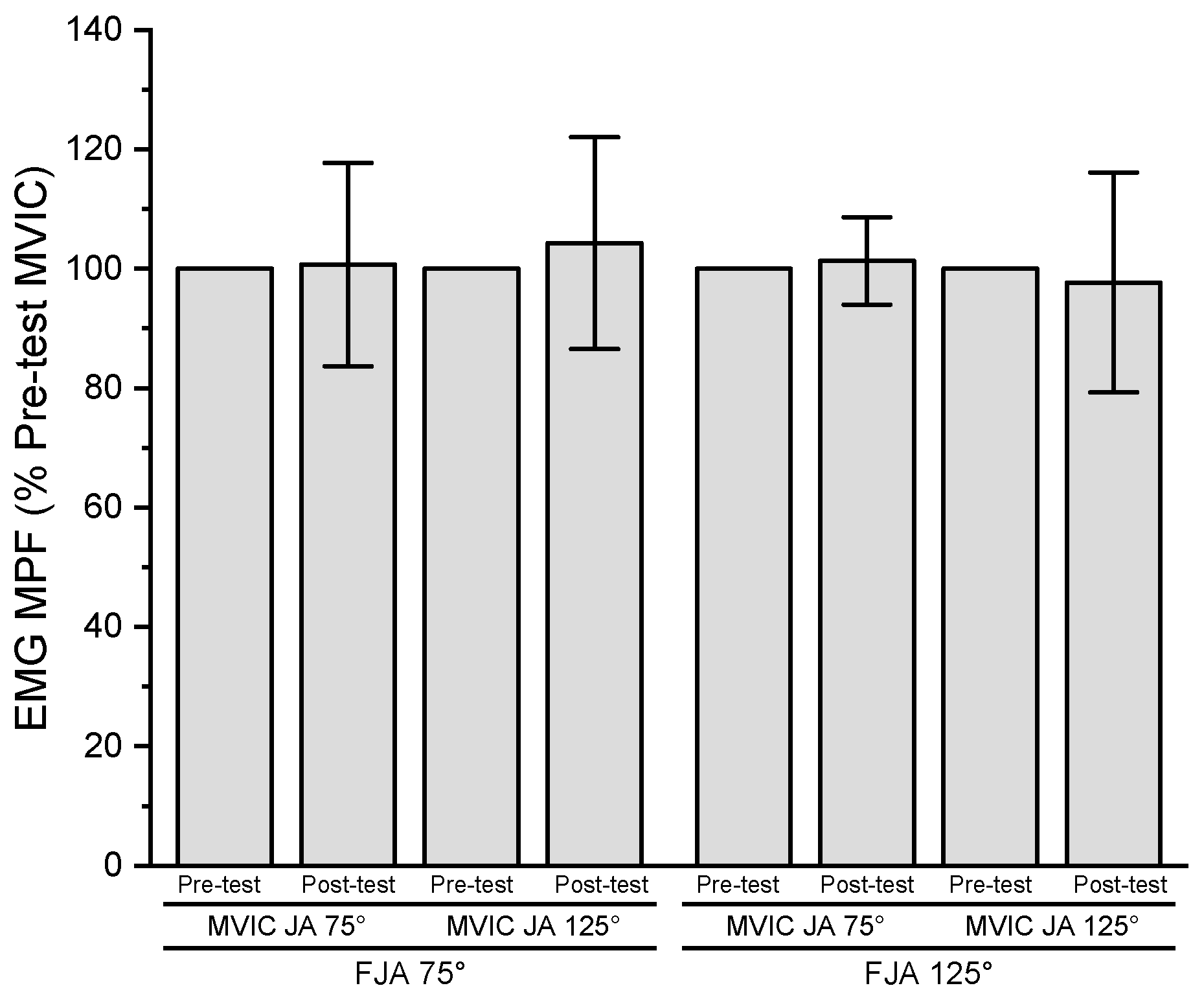
3.5. Electromyographic Mean Power Frequency
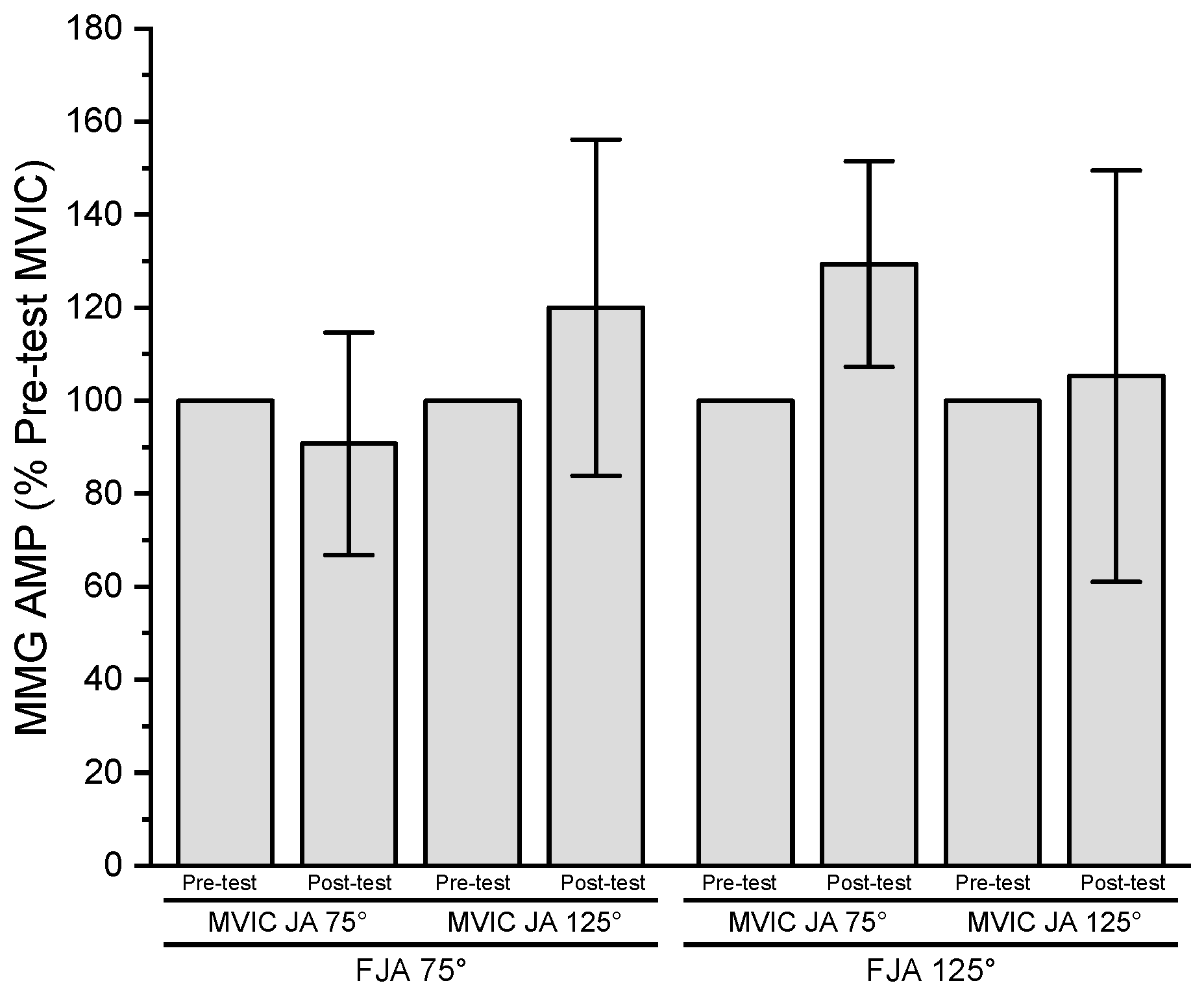
3.6. Mechanomyographic Amplitude
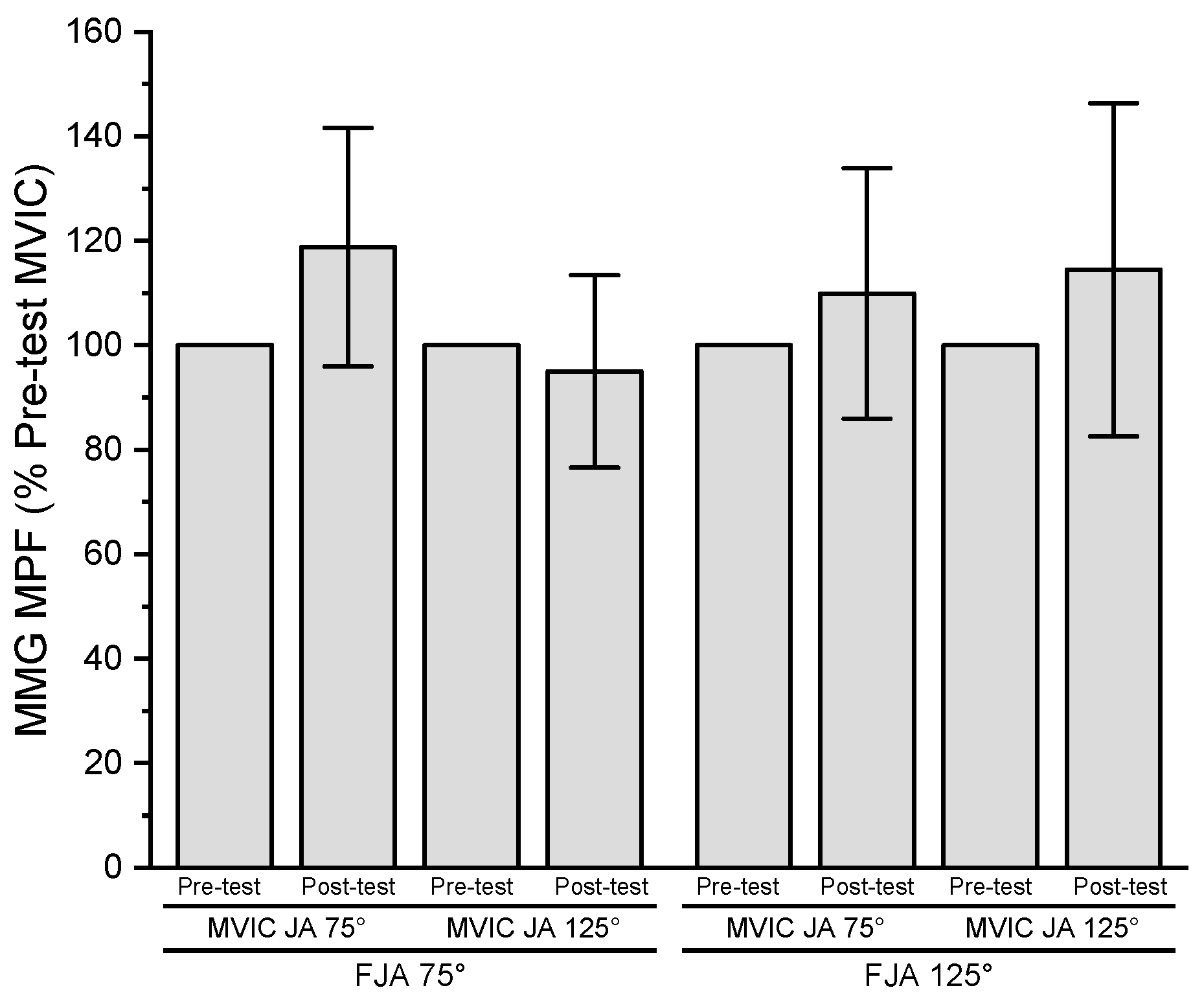
3.7. Mechanomyographic Mean Power Frequency
3.8. Neuromuscular Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enoka, R.M.; Stuart, D.G. Neurobiology of Muscle Fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Arnett, J.E.; Smith, R.W.; Neltner, T.J.; Anders, J.P.V.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. The RPE Clamp Model and Fatigability Following a Sustained, Isometric Task to Failure. J. Exerc. Physiol. Online 2022, 25, 13–26. [Google Scholar]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Are There Sex-Specific Neuromuscular or Force Responses to Fatiguing Isometric Muscle Actions Anchored to a High Perceptual Intensity? J. Strength Cond. Res. 2022, 36, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle Fatigue: What, Why and How It Influences Muscle Function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef]
- Cairns, S.P.; Knicker, A.J.; Thompson, M.W.; Sjøgaard, G. Evaluation of Models Used to Study Neuromuscular Fatigue. Exerc. Sport Sci. Rev. 2005, 33, 9–16. [Google Scholar]
- Robertson, R.J.; Noble, B.J. Perception of Physical Exertion: Methods, Mediators, and Applications. Exerc. Sport Sci. Rev. 1997, 25, 407–452. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Tucker, R. The Anticipatory Regulation of Performance: The Physiological Basis for Pacing Strategies and the Development of a Perception-Based Model for Exercise Performance. Br. J. Sports Med. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Arnett, J.E.; Smith, R.W.; Neltner, T.J.; Anders, J.P.V.; Ortega, D.G.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. The Effects of Joint Angle and Anchoring Scheme on Performance Fatigability and Neuromuscular Responses Following Isometric Forearm Flexion Tasks to Failure. NeuroSports 2023, 1, 7. [Google Scholar]
- Keller, J.L.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Schmidt, R.J.; Johnson, G.O. Anchor Scheme, Intensity, and Time-to-task failure Do Not Influence Performance Fatigability or Changes in Neuromuscular Responses Following Bilateral Leg Extensions. J. Exerc. Physiol. Online 2020, 23, 119–134. [Google Scholar]
- Smith, R.W.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Keller, J.L.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Perceptual Fatigability and Neuromuscular Responses during a Sustained, Isometric Forearm Flexion Muscle Action Anchored to a Constant Level of Perceived Exertion. NeuroSports 2021, 1, 2. [Google Scholar]
- Smith, R.W.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Ortega, D.G.; Schmidt, R.J.; Johnson, G.O. Torque and Neuromuscular Responses Are Not Joint Angle Dependent During a Sustained, Isometric Task Anchored to a High Perceptual Intensity. Am. J. Sports Sci. Med. 2022, 10, 29–39. [Google Scholar] [CrossRef]
- Basmajian, J.V.; De Luca, C.J. Muscles Alive: Their Functions Revealed by Electromyography; Williams & Wilkins: Baltimore, MD, USA, 1985; ISBN 0-683-00414-X. [Google Scholar]
- Arendt-Nielsen, L.; Mills, K.R. The Relationship between Mean Power Frequency of the EMG Spectrum and Muscle Fibre Conduction Velocity. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Brewer, R.E.; Bush, J.A.; McCoy, R.W.; Volek, J.S.; Kraemer, W.J. Neuromuscular Disturbance Outlasts Other Symptoms of Exercise-Induced Muscle Damage. J. Neurol. Sci. 2000, 174, 92–99. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The Extraction of Neural Strategies from the Surface EMG: An Update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef]
- Miller, R.G.; Giannini, D.; Milner-Brown, H.S.; Layzer, R.B.; Koretsky, A.P.; Hooper, D.; Weiner, M.W. Effects of Fatiguing Exercise on High-Energy Phosphates, Force, and EMG: Evidence for Three Phases of Recovery. Muscle Nerve 1987, 10, 810–821. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Weir, J.P.; Cramer, J.T.; Coburn, J.W.; Malek, M.H. Mechanomyographic Amplitude and Mean Power Frequency versus Torque Relationships during Isokinetic and Isometric Muscle Actions of the Biceps Brachii. J. Electromyogr. Kinesiol. 2004, 14, 555–564. [Google Scholar] [CrossRef]
- Orizio, C.; Gobbo, M.; Diemont, B.; Esposito, F.; Veicsteinas, A. The Surface Mechanomyogram as a Tool to Describe the Influence of Fatigue on Biceps Brachii Motor Unit Activation Strategy. Historical Basis and Novel Evidence. Eur. J. Appl. Physiol. 2003, 90, 326–336. [Google Scholar] [CrossRef]
- Kulig, K.; Andrews, J.G.; Hay, J.G. Human Strength Curves. Exerc. Sport Sci. Rev. 1984, 12, 417–466. [Google Scholar] [CrossRef] [PubMed]
- Huijing, P.A. Mechanical Muscle Models. In Strength and Power in Sport, 1st ed.; Komi, P.V., Ed.; Blackwell Scientific Publications: Oxford, UK, 1992; pp. 130–150. [Google Scholar]
- Petrofsky, J.S.; Phillips, C.A. The Effect of Elbow Angle on the Isometric Strength and Endurance of the Elbow Flexors in Men and Women. J. Hum. Ergol. 1980, 9, 125–131. [Google Scholar]
- Weir, J.P.; Ayers, K.M.; Lacefield, J.F.; Walsh, K.L. Mechanomyographic and Electromyographic Responses during Fatigue in Humans: Influence of Muscle Length. Eur. J. Appl. Physiol. 2000, 81, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K. The Relevance of Sex Differences in Performance Fatigability. Med. Sci. Sports Exerc. 2016, 48, 2247–2256. [Google Scholar] [CrossRef]
- Hunter, S.K. Sex Differences in Human Fatigability: Mechanisms and Insight to Physiological Responses. Acta Physiol. 2014, 210, 768–789. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Walker, E.H.E.; Perreault, E.J. Arm Dominance Affects Feedforward Strategy More than Feedback Sensitivity during a Postural Task. Exp. Brain Res. 2015, 233, 2001–2011. [Google Scholar] [CrossRef]
- Gearhart, R.F., Jr.; Goss, F.L.; Lagally, K.M.; Jakicic, J.M.; Gallagher, J.; Robertson, R.J. Standardized Scaling Procedures for Rating Perceived Exertion during Resistance Exercise. J. Strength Cond. Res. 2001, 15, 320–325. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Kwatny, E.; Thomas, D.H.; Kwatny, H.G. An Application of Signal Processing Techniques to the Study of Myoelectric Signals. IEEE Trans. Biomed. Eng. 1970, 17, 303–313. [Google Scholar] [CrossRef]
- Jones, A.A.; Power, G.A.; Herzog, W. History Dependence of the Electromyogram: Implications for Isometric Steady-State EMG Parameters Following a Lengthening or Shortening Contraction. J. Electromyogr. Kinesiol. 2016, 27, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Hill, E.C.; Housh, T.J.; Keller, J.L.; Smith, C.M.; Anders, J.V.; Schmidt, R.J.; Johnson, G.O.; Cramer, J.T. Low-Load Blood Flow Restriction Elicits Greater Concentric Strength than Non-Blood Flow Restriction Resistance Training but Similar Isometric Strength and Muscle Size. Eur. J. Appl. Physiol. 2020, 120, 425–441. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- LeVeau, B.; Andersson, G. Output Forms: Data Analysis and Applications. Interpretation of the Electromyographic Signal. Sel. Top. Surf. Electromyogr. Occup. Setting Expert Perspect. 1992, 91, 100. [Google Scholar]
- Halaki, M.; Ginn, K. Normalization of EMG Signals: To Normalize or Not to Normalize and What to Normalize to. In Computational Intelligence in Electromyography Analysis-A Perspective on Current Applications and Future Challenges; IntechOpen: London, UK, 2012; Volume 10, p. 49957. [Google Scholar]
- Jørgensen, K.; Bankov, S. Maximum Strength of Elbow Flexors with Pronated and Supinated Forearm. Med. Sport Sci. 1969, 6, 174–180. [Google Scholar]
- Knapik, J.J.; Wright, J.E.; Mawdsley, R.H.; Braun, J. Isometric, Isotonic, and Isokinetic Torque Variations in Four Muscle Groups through a Range of Joint Motion. Phys. Ther. 1983, 63, 938–947. [Google Scholar] [CrossRef]
- Singh, M.; Karpovich, P.V. Isotonic and Isometric Forces of Forearm Flexors and Extensors. J. Appl. Physiol. 1966, 21, 1435–1437. [Google Scholar] [CrossRef]
- Williams, M.; Stutzman, L. Strength Variation through the Range of Joint Motion. Phys. Ther. 1959, 39, 145–152. [Google Scholar] [CrossRef]
- Fitch, S.; McComas, A. Influence of Human Muscle Length on Fatigue. J. Physiol. 1985, 362, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Goodall, S.; Howatson, G. Performance Fatigability Is Not Regulated to a Peripheral Critical Threshold. Exerc. Sport Sci. Rev. 2018, 46, 240–246. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The Extraction of Neural Strategies from the Surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef]
- Hureau, T.J.; Romer, L.M.; Amann, M. The ‘Sensory Tolerance Limit’: A Hypothetical Construct Determining Exercise Performance? Eur. J. Sport Sci. 2018, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G.; Lannergren, J. Muscle Fatigue: Lactic Acid or Inorganic Phosphate the Major Cause? Physiology 2002, 17, 17–21. [Google Scholar] [CrossRef]
- Hureau, T.J.; Broxterman, R.M.; Weavil, J.C.; Lewis, M.T.; Layec, G.; Amann, M. On the Role of Skeletal Muscle Acidosis and Inorganic Phosphates as Determinants of Central and Peripheral Fatigue: A 31P-MRS Study. J. Physiol. 2022, 600, 3069–3081. [Google Scholar] [CrossRef]
- Hill, E.C.; Housh, T.J.; Smith, C.M.; Cochrane, K.C.; Jenkins, N.D.M.; Cramer, J.T.; Schmidt, R.J.; Johnson, G.O. Effect of Sex on Torque, Recovery, EMG, and MMG Responses to Fatigue. J. Musculoskelet. Neuronal Interact. 2016, 16, 310–317. [Google Scholar]
- Smith, C.M.; Housh, T.J.; Hill, E.C.; Cochrane, K.C.; Jenkins, N.D.M.; Schmidt, R.J.; Johnson, G.O. Effects of Fatiguing Constant versus Alternating Intensity Intermittent Isometric Muscle Actions on Maximal Torque and Neuromuscular Responses. J. Musculoskelet. Neuronal Interact. 2016, 16, 318–326. [Google Scholar] [PubMed]
- Bigland-Ritchie, B.; Rice, C.L.; Garland, S.J.; Walsh, M.L. Task-Dependent Factors in Fatigue of Human Voluntary Contractions. In Fatigue: Neural and Muscular Mechanisms, 1st ed.; Gandevia, S.C., Enoka, R.M., McComas, A.J., Stuart, D.G., Thomas, C.K., Eds.; Springer: New York, NY, USA, 1995; pp. 361–380. [Google Scholar]


| Orientation Session | Testing Visits 1 and 2 |
|---|---|
|
|
| Fatiguing Joint Angle 75° | Fatiguing Joint Angle 125° | |||
|---|---|---|---|---|
| Pre-Test | Post-Test | Pre-Test | Post-Test | |
| MVIC (Nm) | ||||
| Joint Angle | ||||
| 75° | 50.0 ± 8.0 | 44.4 ± 7.6 | 47.5 ± 6.1 | 41.9 ± 8.0 |
| 125° | 54.9 ± 6.7 | 48.6 ± 7.2 | 52.0 ± 5.9 | 46.5 ± 5.8 |
| EMG AMP (µVrms) | ||||
| Joint Angle | ||||
| 75° | 987.0 ± 334.8 | 1035.9 ± 365.0 | 1195.6 ± 501.4 | 1123.9 ± 439.9 |
| 125° | 1198.5 ± 551.0 | 954.4 ± 317.7 | 976.7 ± 495.0 | 885.1 ± 507.6 |
| EMG MPF (Hz) | ||||
| Joint Angle | ||||
| 75° | 71.4 ± 13.7 | 71.4 ± 15.7 | 73.6 ± 11.8 | 74.4 ± 12.1 |
| 125° | 67.0 ± 8.5 | 69.4 ± 11.5 | 74.0 ± 15.4 | 70.5 ± 11.0 |
| MMG AMP (m∙s−2) | ||||
| Joint Angle | ||||
| 75° | 0.48 ± 0.19 | 0.43 ± 0.16 | 0.37 ± 0.19 | 0.47 ± 0.23 |
| 125° | 0.49 ± 0.17 | 0.56 ± 0.14 | 0.47 ± 0.17 | 0.45 ± 0.17 |
| MMG MPF (Hz) | ||||
| Joint Angle | ||||
| 75° | 21.9 ± 6.0 | 26.1 ± 8.1 | 22.5 ± 5.6 | 25.0 ± 9.2 |
| 125° | 25.9 ± 6.1 | 24.8 ± 8.4 | 26.7 ± 8.8 | 28.3 ± 6.0 |
| NME | ||||
| Joint Angle | ||||
| 75° | 1.00 ± 0.00 | 0.87 ± 0.24 | 1.00 ± 0.00 | 0.93 ± 0.19 |
| 125° | 1.00 ± 0.00 | 1.08 ± 0.31 | 1.00 ± 0.00 | 1.05 ± 0.44 |
| MVIC (mean ± SD) | Visit 1 | Visit 2 | P | ICC | ICC95% |
| Forearm flexion at JA75 (Nm) | 49.1 ± 8.6 | 48.4 ± 5.6 | 0.771 | 0.559 | −0.164–0.882 |
| Forearm flexion at JA125 (Nm) | 54.2 ± 6.3 | 52.7 ± 6.6 | 0.574 | 0.388 | −0.365–0.822 |
| Neuromuscular Parameters (mean ± SD) | |||||
| EMG AMP at JA75 (µVrms) | 989.7 ± 342.5 | 1194.0 ± 497.5 | 0.222 | 0.393 | −0.237–0.814 |
| EMG AMP at JA125 (µVrms) | 1174.7 ± 557.8 | 1000.5 ± 498.0 | 0.332 | 0.540 | −0.100–0.872 |
| EMG MPF at JA75 (Hz) | 71.7 ± 14.5 | 73.2 ± 10.9 | 0.551 | 0.829 | 0.425–0.959 |
| EMG MPF at JA125 (Hz) | 66.9 ± 9.9 | 74.1 ± 14.5 | 0.247 | 0.025 | −0.556–0.633 |
| MMG AMP at JA75 (m∙s−2) | 0.44 ± 0.20 | 0.41 ± 0.19 | 0.550 | 0.791 | 0.326–0.949 |
| MMG AMP at JA125 (m∙s−2) | 0.49 ± 0.16 | 0.47 ± 0.18 | 0.843 | 0.158 | −0.641–0.733 |
| MMG MPF at JA75 (Hz) | 21.1 ± 4.7 | 23.3 ± 6.4 | 0.089 | 0.774 | 0.276–0.944 |
| MMG MPF at JA125 (Hz) | 27.7 ± 7.8 | 24.8 ± 7.1 | 0.277 | 0.491 | −0.148–0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, D.G.; Housh, T.J.; Smith, R.W.; Arnett, J.E.; Neltner, T.J.; Anders, J.P.V.; Schmidt, R.J.; Johnson, G.O. Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men. J. Funct. Morphol. Kinesiol. 2023, 8, 114. https://doi.org/10.3390/jfmk8030114
Ortega DG, Housh TJ, Smith RW, Arnett JE, Neltner TJ, Anders JPV, Schmidt RJ, Johnson GO. Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men. Journal of Functional Morphology and Kinesiology. 2023; 8(3):114. https://doi.org/10.3390/jfmk8030114
Chicago/Turabian StyleOrtega, Dolores G., Terry J. Housh, Robert W. Smith, Jocelyn E. Arnett, Tyler J. Neltner, John Paul V. Anders, Richard J. Schmidt, and Glen O. Johnson. 2023. "Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men" Journal of Functional Morphology and Kinesiology 8, no. 3: 114. https://doi.org/10.3390/jfmk8030114
APA StyleOrtega, D. G., Housh, T. J., Smith, R. W., Arnett, J. E., Neltner, T. J., Anders, J. P. V., Schmidt, R. J., & Johnson, G. O. (2023). Fatiguing Joint Angle Does Not Influence Torque and Neuromuscular Responses Following Sustained, Isometric Forearm Flexion Tasks Anchored to Perceptual Intensity in Men. Journal of Functional Morphology and Kinesiology, 8(3), 114. https://doi.org/10.3390/jfmk8030114






