Influence of Pennation Angle and Muscle Thickness on Mechanomyographic Amplitude–Torque Relationships and Sex-Related Differences in the Vastus Lateralis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ultrasound Imaging
2.3. Isometric Strength Testing
2.4. Mechanomyographic and Torque Signal Processing
2.5. Statistical Analysis
3. Results
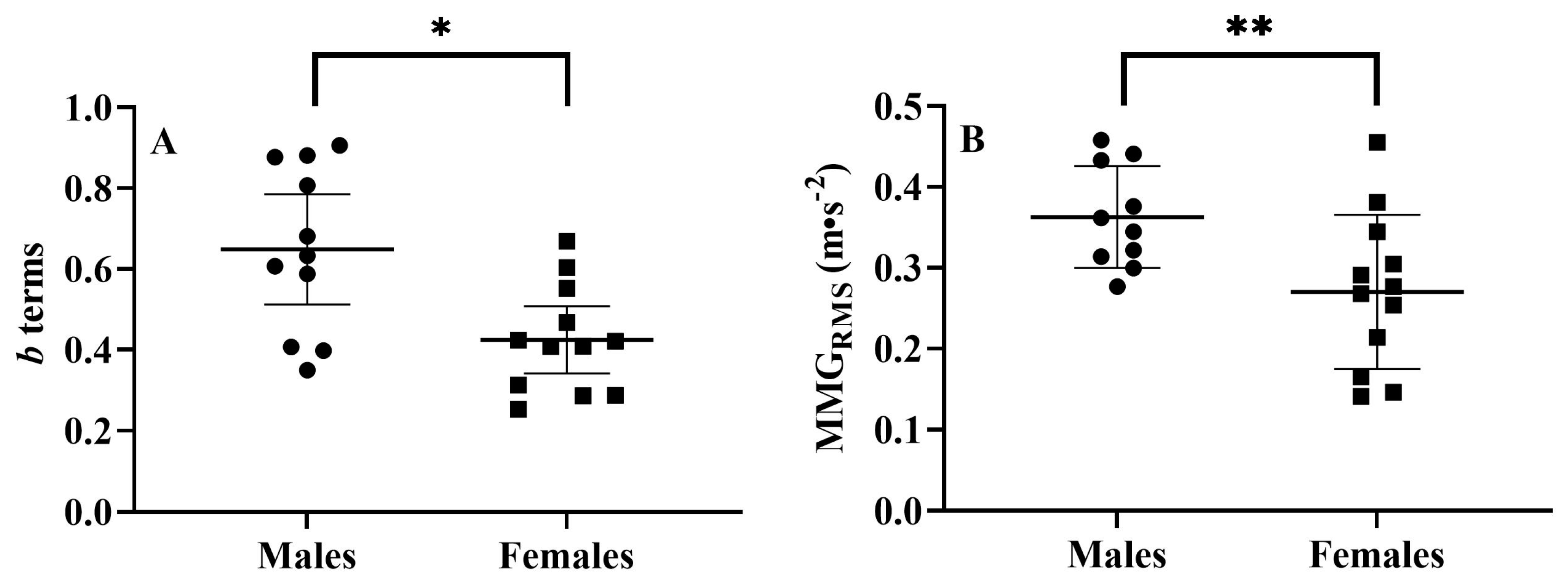
3.1. Sex-Related Comparisons
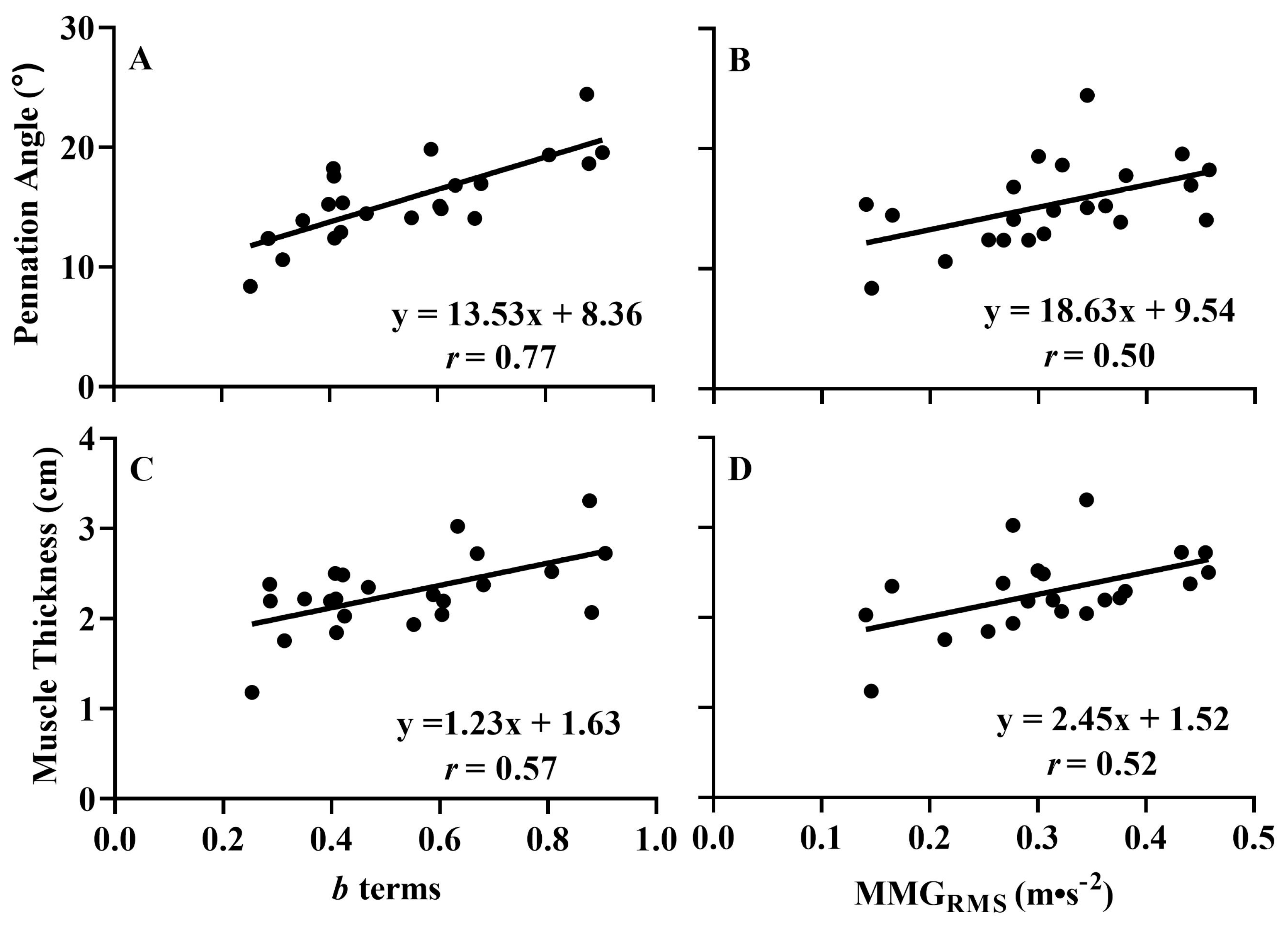
3.2. Correlations
3.3. Sequential Multiple Regression Models for Pennation Angle and Muscle Thickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodges, P.; Pengel, L.; Herbert, R.; Gandevia, S. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2003, 27, 682–692. [Google Scholar] [CrossRef]
- Miyatani, M.; Kanehisa, H.; Ito, M.; Kawakami, Y.; Fukunaga, T. The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur. J. Appl. Physiol. 2004, 91, 264–272. [Google Scholar]
- Aagaard, P.; Andersen, J.L.; Dyhre-Poulsen, P.; Leffers, A.M.; Wagner, A.; Magnusson, S.P.; Halkjær-Kristensen, J.; Simonsen, E.B. A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture. J. Physiol. 2001, 534, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.M.; Ward, K.; Sidhu, P.S.; Puthucheary, Z.; Steier, J.; Jolley, C.J.; Rafferty, G.; Polkey, M.I.; Moxham, J. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 2009, 64, 418–423. [Google Scholar] [CrossRef]
- Rekabizaheh, M.; Rezasoltani, A.; Lahouti, B.; Namavarian, N. Pennation angle and fascicle length of human skeletal muscles to predict the strength of an individual muscle using real-time ultrasonography: A review of literature. J. Clin. Physiother. Res. 2016, 1, 42–48. [Google Scholar]
- Jones, D.; Rutherford, O. Human muscle strength training: The effects of three different regimens and the nature of the resultant changes. J. Physiol. 1987, 391, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Fukumoto, K.; Fukuda, O. Prediction of the muscle strength by the muscle thickness and hardness using ultrasound muscle hardness meter. Springerplus 2013, 2, 457. [Google Scholar] [CrossRef]
- Freilich, R.; Kirsner, R.; Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord. 1995, 5, 415–422. [Google Scholar] [CrossRef]
- Cadore, E.L.; Izquierdo, M.; Pinto, S.S.; Alberton, C.L.; Pinto, R.S.; Baroni, B.M.; Vaz, M.A.; Lanferdini, F.J.; Radaelli, R.; González-Izal, M. Neuromuscular adaptations to concurrent training in the elderly: Effects of intrasession exercise sequence. Age 2013, 35, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Maganaris, C.N.; Baltzopoulos, V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 294–297. [Google Scholar] [CrossRef]
- Scott, S.H.; Winter, D.A. A comparison of three muscle pennation assumptions and their effect on isometric and isotonic force. J. Biomech. 1991, 24, 163–167. [Google Scholar] [CrossRef]
- Narici, M.V.; Binzoni, T.; Hiltbrand, E.; Fasel, J.; Terrier, F.; Cerretelli, P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J. Physiol. 1996, 496, 287–297. [Google Scholar] [CrossRef]
- Ito, M.; Kawakami, Y.; Ichinose, Y.; Fukashiro, S.; Fukunaga, T. Nonisometric behavior of fascicles during isometric contractions of a human muscle. J. Appl. Physiol. 1998, 85, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Manal, K.; Roberts, D.P.; Buchanan, T.S. Can pennation angles be predicted from EMGs for the primary ankle plantar and dorsiflexors during isometric contractions? J. Biomech. 2008, 41, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.; Karamanidis, K.; Stafilidis, S.; Morey-Klapsing, G.; DeMonte, G.; Brüggemann, G.-P. Effect of different ankle-and knee-joint positions on gastrocnemius medialis fascicle length and EMG activity during isometric plantar flexion. J. Biomech. 2006, 39, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Muñoz, M.; Cuesta-Vargas, A.I. Electromyography and sonomyography analysis of the tibialis anterior: A cross sectional study. J. Foot Ankle Res. 2014, 7, 11. [Google Scholar] [CrossRef]
- McMeeken, J.; Beith, I.; Newham, D.; Milligan, P.; Critchley, D. The relationship between EMG and change in thickness of transversus abdominis. Clin. Biomech. 2004, 19, 337–342. [Google Scholar] [CrossRef]
- Chauhan, B.; Hamzeh, M.A.; Cuesta-Vargas, A.I. Prediction of muscular architecture of the rectus femoris and vastus lateralis from EMG during isometric contractions in soccer players. Springerplus 2013, 2, 548. [Google Scholar] [CrossRef]
- Orizio, C. Muscle sound: Bases for the introduction of a mechanomyographic signal in muscle studies. Crit. Rev. Biomed. Eng. 1993, 21, 201–243. [Google Scholar]
- Coburn, J.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P.; Miller, J.M.; Beck, T.W.; Malek, M.H.; Johnson, G.O. Mechanomyographic time and frequency domain responses of the vastus medialis muscle during submaximal to maximal isometric and isokinetic muscle actions. Electromyogr. Clin. Neurophysiol. 2004, 44, 247–255. [Google Scholar] [PubMed]
- Coburn, J.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P.; Miller, J.M.; Beck, T.W.; Malek, M.H.; Johnson, G.O. Mechanomyographic and electromyographic responses of the vastus medialis muscle during isometric and concentric muscle actions. J. Strength. Cond. Res. 2005, 19, 412–420. [Google Scholar] [PubMed]
- Herda, T.J.; Housh, T.J.; Fry, A.C.; Weir, J.P.; Schilling, B.K.; Ryan, E.D.; Cramer, J.T. A noninvasive, log-transform method for fiber type discrimination using mechanomyography. J. Electromyogr. Kinesiol. 2010, 20, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W.; Housh, T.J.; Fry, A.C.; Cramer, J.T.; Weir, J.P.; Schilling, B.K.; Falvo, M.J.; Moore, C.A. The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyogr. Clin. Neurophysiol. 2007, 47, 221–232. [Google Scholar]
- Trevino, M.A.; Herda, T.J.; Fry, A.C.; Gallagher, P.M.; Vardiman, J.P.; Mosier, E.M.; Miller, J.D. The influence of myosin heavy chain isoform content on mechanical behavior of the vastus lateralis in vivo. J. Electromyogr. Kinesiol. 2016, 28, 143–151. [Google Scholar] [CrossRef]
- Sontag, S.A.; Sterczala, A.J.; Miller, J.D.; Deckert, J.A.; Olmos, A.A.; Parra, M.E.; Dimmick, H.L.; Gallagher, P.M.; Fry, A.C.; Herda, T.J. A Noninvasive Test for Estimating Myosin Heavy Chain of the Vastus Lateralis in Females with Mechanomyography. Med. Eng. Phys. 2022, 111, 103946. [Google Scholar] [CrossRef]
- Sontag, S.A.; Trevino, M.A.; Herda, T.J.; Sterczala, A.J.; Miller, J.D.; Parra, M.E.; Dimmick, H.L.; Deckert, J. Endurance training alters motor unit activation strategies for the vastus lateralis, yet sex-related differences and relationships with muscle size remain. Eur. J. Appl. Physiol. 2021, 121, 1367–1377. [Google Scholar] [CrossRef]
- Trevino, M.; Sterczala, A.; Miller, J.; Wray, M.; Dimmick, H.; Ciccone, A.; Weir, J.; Gallagher, P.; Fry, A.; Herda, T. Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol. 2019, 225, e13151. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Abe, T.; Kanehisa, H.; Fukunaga, T. Human skeletal muscle size and architecture: Variability and interdependence. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2006, 18, 845–848. [Google Scholar] [CrossRef]
- Ryan, E.D.; Beck, T.W.; Herda, T.J.; Hartman, M.J.; Stout, J.R.; Housh, T.J.; Cramer, J.T. Mechanomyographic amplitude and mean power frequency responses during isometric ramp vs. step muscle actions. J. Neurosci. Methods 2008, 168, 293–305. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef]
- Orizio, C.; Perini, R.; Veicsteinas, A. Muscular sound and force relationship during isometric contraction in man. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 528–533. [Google Scholar] [CrossRef]
- Zwarts, M.J.; Keidel, M. Relationship between electrical and vibratory output of muscle during voluntary contraction and fatigue. Muscle Nerve 1991, 14, 756–761. [Google Scholar] [CrossRef]
- Trevino, M.A.; Herda, T.J. The effects of chronic exercise training status on motor unit activation and deactivation control strategies. J. Sports Sci. 2015, 34, 199–208. [Google Scholar] [CrossRef]
- Olmos, A.A.; Herda, T.J.; Sontag, S.A.; Trevino, M.A. The influence of chronic training status on the mechanical behavior of the vastus lateralis during repetitive trapezoidal contractions. J. Musculoskelet. Neuronal Interact. 2022, 22, 161. [Google Scholar] [PubMed]
- Trevino, M.A.; Dimmick, H.L.; Parra, M.E.; Sterczala, A.J.; Miller, J.D.; Deckert, J.A.; Gallagher, P.M.; Fry, A.C.; Weir, J.P.; Herda, T.J. Effects of continuous cycling training on motor unit firing rates, input excitation, and myosin heavy chain of the vastus lateralis in sedentary females. Exp. Brain Res. 2022, 240, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Dimmick, H.L.; Miller, J.D.; Sterczala, A.J.; Trevino, M.A.; Herda, T.J. Vastus lateralis muscle tissue composition and motor unit properties in chronically endurance-trained vs. sedentary women. Eur. J. Appl. Physiol. 2018, 118, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Herda, T.J.; Vardiman, J.P.; Gallagher, P.M.; Fry, A.C. Relationships between skinfold thickness and electromyographic and mechanomyographic amplitude recorded during voluntary and non-voluntary muscle actions. J. Electromyogr. Kinesiol. 2014, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jaskolska, A.; Brzenczek, W.; Kisiel-Sajewicz, K.; Kawczynski, A.; Marusiak, J.; Jaskolski, A. The effect of skinfold on frequency of human muscle mechanomyogram. J. Electromyogr. Kinesiol. 2004, 14, 217–225. [Google Scholar] [CrossRef]
- Jeon, S.; Sontag, S.A.; Herda, T.J.; Trevino, M.A. Chronic training status affects muscle excitation of the vastus lateralis during repeated contractions. Sport. Med. Health Sci. 2023, 5, 42–49. [Google Scholar] [CrossRef]
- Contessa, P.; De Luca, C.J.; Kline, J.C. The compensatory interaction between motor unit firing behavior and muscle force during fatigue. J. Neurophysiol. 2016, 116, 1579–1585. [Google Scholar] [CrossRef]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S.; Cohen, J.; Cohen, J.; Cohen, P.; West, S.G.A.; Leona, S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Routledge: Oxford, UK, 2003. [Google Scholar]
- Kubo, K.; Kanehisa, H.; Azuma, K.; Ishizu, M.; Kuno, S.-Y.; Okada, M.; Fukunaga, T. Muscle architectural characteristics in young and elderly men and women. Int. J. Sport. Med. 2003, 24, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Abe, T.; Fukunaga, T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J. Appl. Physiol. 1993, 74, 2740–2744. [Google Scholar] [CrossRef]
- Miller, A.E.J.; MacDougall, J.; Tarnopolsky, M.; Sale, D. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.; Randall Fox, E.; Norgren, P.; Tydén, A. The relationship between the mean muscle fibre area and the muscle cross-sectional area of the thigh in subjects with large differences in thigh girth. Acta Physiol. Scand. 1981, 113, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.; Randall-Fox, E.; Hutchison, W.; Tydén, A.; Åstrand, P.O. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol. 1983, 117, 219–226. [Google Scholar] [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef]
- Trappe, S.; Gallagher, P.; Harber, M.; Carrithers, J.; Fluckey, J.; Trappe, T. Single muscle fibre contractile properties in young and old men and women. J. Physiol. 2003, 552, 47–58. [Google Scholar] [CrossRef]
- De Luca, C.J.; Contessa, P. Biomechanical benefits of the onion-skin motor unit control scheme. J. Biomech. 2015, 48, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Longo, S.; Mallinson, J.; Quinlan, J.I.; Taylor, T.; Greenhaff, P.L.; Narici, M.V. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand. J. Med. Sci. Sport. 2018, 28, 846–853. [Google Scholar] [CrossRef]
- Barry, D.T. Acoustic signals from frog skeletal muscle. Biophys. J. 1987, 51, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Handsfield, G.; Knaus, K.; Fiorentino, N.; Meyer, C.; Hart, J.; Blemker, S. Adding muscle where you need it: Non-uniform hypertrophy patterns in elite sprinters. Scand. J. Med. Sci. Sport. 2017, 27, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.; Faulkner, J.; Hyatt, G. Estimation of number of fibers in guinea pig skeletal muscles. J. Appl. Physiol. 1974, 37, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Fridén, J. Clinical significance of skeletal muscle architecture. Clin. Orthop. Relat. Res. 2001, 383, 140–151. [Google Scholar] [CrossRef]
- Lieber, R.L.; Fridén, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Lieber, R.L. Can we just forget about pennation angle? J. Biomech. 2022, 132, 110954. [Google Scholar] [CrossRef]
- Nonaka, H.; Mita, K.; Akataki, K.; Watakabe, M.; Itoh, Y. Sex differences in mechanomyographic responses to voluntary isometric contractions. Med. Sci. Sport. Exerc. 2006, 38, 1311–1316. [Google Scholar] [CrossRef]
- Cramer, J.T.; Housh, T.J.; Evetovich, T.K.; Johnson, G.O.; Ebersole, K.T.; Perry, S.R.; Bull, A.J. The relationships among peak torque, mean power output, mechanomyography, and electromyography in men and women during maximal, eccentric isokinetic muscle actions. Eur. J. Appl. Physiol. 2002, 86, 226–232. [Google Scholar] [CrossRef]
- Cramer, J.T.; Housh, T.J.; Johnson, G.O.; Ebersole, K.T.; Perry, S.R.; Bull, A.J. Mechanomyographic amplitude and mean power output during maximal, concentric, isokinetic muscle actions. Muscle Nerve 2000, 23, 1826–1831. [Google Scholar] [CrossRef]
- Cramer, J.T.; Housh, T.J.; WEIR, J.E.; Johnson, G.O.; Ebersole, K.T.; Perry, S.R.; Bull, A.J. Power output, mechanomyographic, and electromyographic responses to maximal, concentric, isokinetic muscle actions in men and women. J. Strength. Cond. Res. 2002, 16, 399–408. [Google Scholar]
- Cramer, J.T.; Housh, T.J.; Weir, J.P.; Johnson, G.O.; Berning, J.M.; Perry, S.R.; Bull, A.J. Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand. J. Med. Sci. Sport. 2004, 14, 116–127. [Google Scholar] [CrossRef]
- Evetovich, T.K.; Housh, T.J.; Johnson, G.O.; Smith, D.B.; Ebersole, K.T.; Perry, S.R. Gender comparisons of the mechanomyographic responses to maximal concentric and eccentric isokinetic muscle actions. Med. Sci. Sport. Exerc. 1998, 30, 1697–1702. [Google Scholar] [CrossRef]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Are there sex-specific neuromuscular or force responses to fatiguing isometric muscle actions anchored to a high perceptual intensity? J. Strength. Cond. Res. 2022, 36, 156–161. [Google Scholar] [CrossRef]
- Miller, J.D.; Lund, C.; Gingrich, M.D.; Schtul, K.L.; Wray, M.E.; Herda, T.J. The effect of rate of torque development on motor unit recruitment and firing rates during isometric voluntary trapezoidal contractions. Exp. Brain Res. 2019, 237, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Garnett, R.; O’donovan, M.; Stephens, J.; Taylor, A. Motor unit organization of human medial gastrocnemius. J. Physiol. 1979, 287, 33–43. [Google Scholar] [CrossRef]
- Fry, A.C.; Allemeier, C.A.; Staron, R.S. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Trevino, M.A.; Herda, T.J.; Fry, A.C.; Gallagher, P.M.; Vardiman, J.P.; Mosier, E.M.; Miller, J.D. Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. J. Neurophysiol. 2016, 116, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Bichler, E. Mechanomyograms recorded during evoked contractions of single motor units in the rat medial gastrocnemius muscle. Eur. J. Appl. Physiol. 2000, 83, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C.; Housh, T.J.; Cramer, J.B.; Weir, J.P.; Beck, T.W.; Schilling, B.K.; Miller, J.D.; Nicoll, J.X. Noninvasive Assessment of Skeletal Muscle Myosin Heavy Chain Expression in Trained and Untrained Men. J. Strength. Cond. Res. 2017, 31, 2355–2362. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevino, M.; Perez, S.; Sontag, S.; Olmos, A.; Jeon, S.; Richardson, L. Influence of Pennation Angle and Muscle Thickness on Mechanomyographic Amplitude–Torque Relationships and Sex-Related Differences in the Vastus Lateralis. J. Funct. Morphol. Kinesiol. 2023, 8, 53. https://doi.org/10.3390/jfmk8020053
Trevino M, Perez S, Sontag S, Olmos A, Jeon S, Richardson L. Influence of Pennation Angle and Muscle Thickness on Mechanomyographic Amplitude–Torque Relationships and Sex-Related Differences in the Vastus Lateralis. Journal of Functional Morphology and Kinesiology. 2023; 8(2):53. https://doi.org/10.3390/jfmk8020053
Chicago/Turabian StyleTrevino, Michael, Sergio Perez, Stephanie Sontag, Alex Olmos, Sunggun Jeon, and Lyric Richardson. 2023. "Influence of Pennation Angle and Muscle Thickness on Mechanomyographic Amplitude–Torque Relationships and Sex-Related Differences in the Vastus Lateralis" Journal of Functional Morphology and Kinesiology 8, no. 2: 53. https://doi.org/10.3390/jfmk8020053
APA StyleTrevino, M., Perez, S., Sontag, S., Olmos, A., Jeon, S., & Richardson, L. (2023). Influence of Pennation Angle and Muscle Thickness on Mechanomyographic Amplitude–Torque Relationships and Sex-Related Differences in the Vastus Lateralis. Journal of Functional Morphology and Kinesiology, 8(2), 53. https://doi.org/10.3390/jfmk8020053







