Use of Robot-Assisted Ankle Training in a Patient with an Incomplete Spinal Cord Injury: A Case Report
Abstract
1. Introduction
2. Case Report
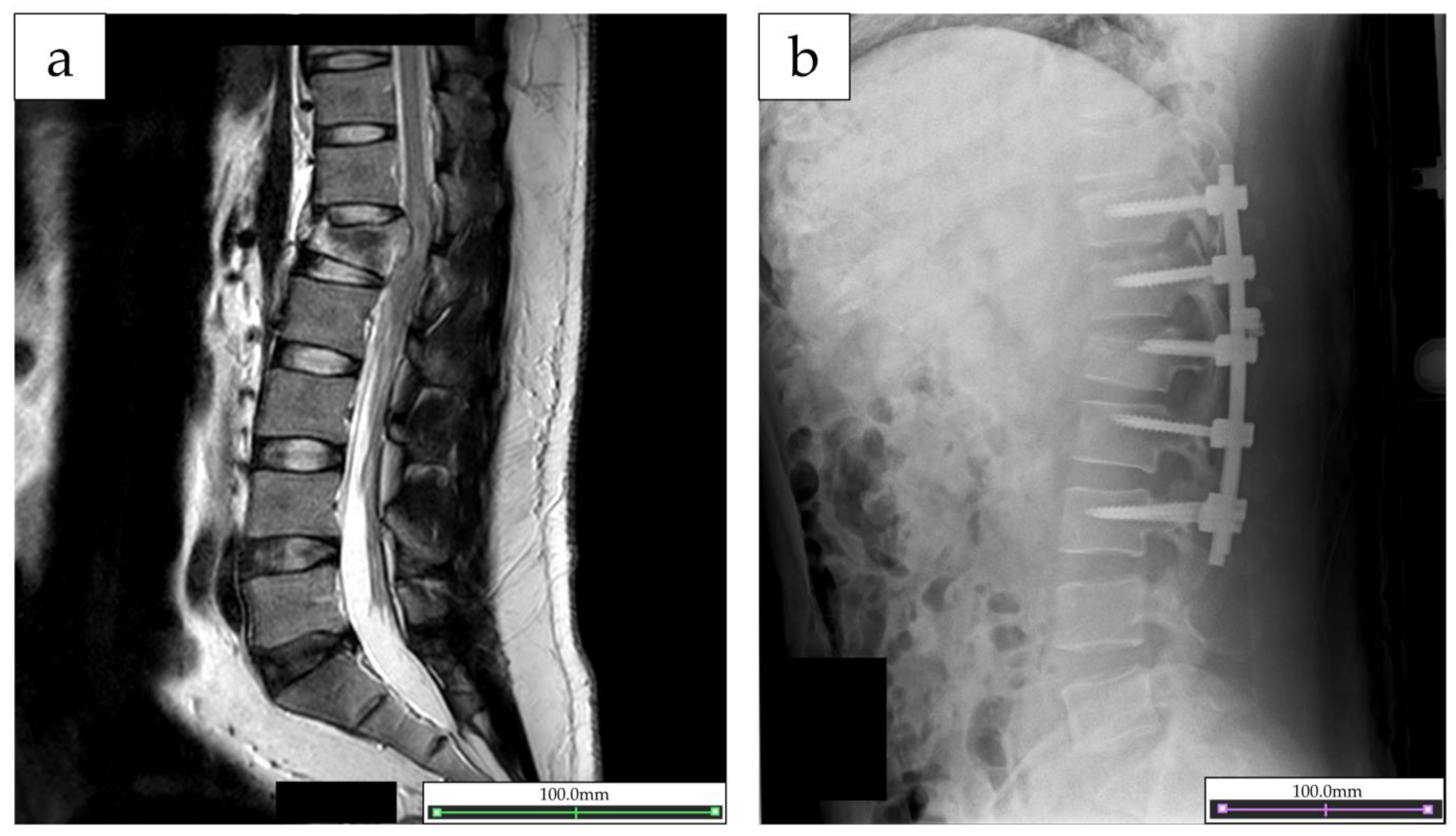
2.1. Participant
2.2. Intervention Used
2.3. Outcome Measurement
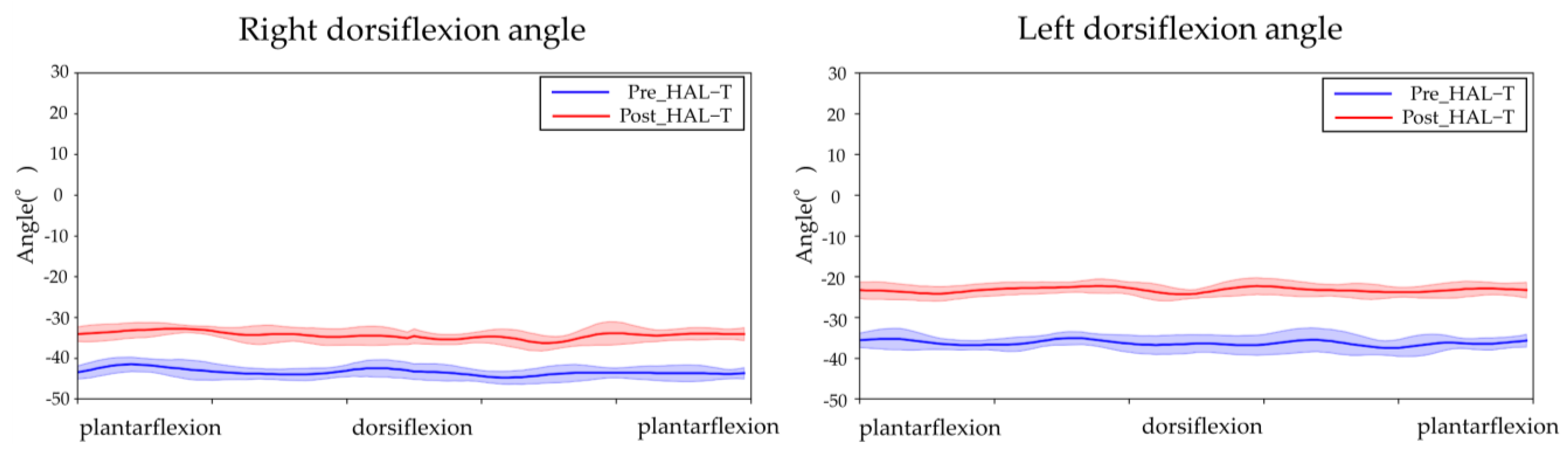
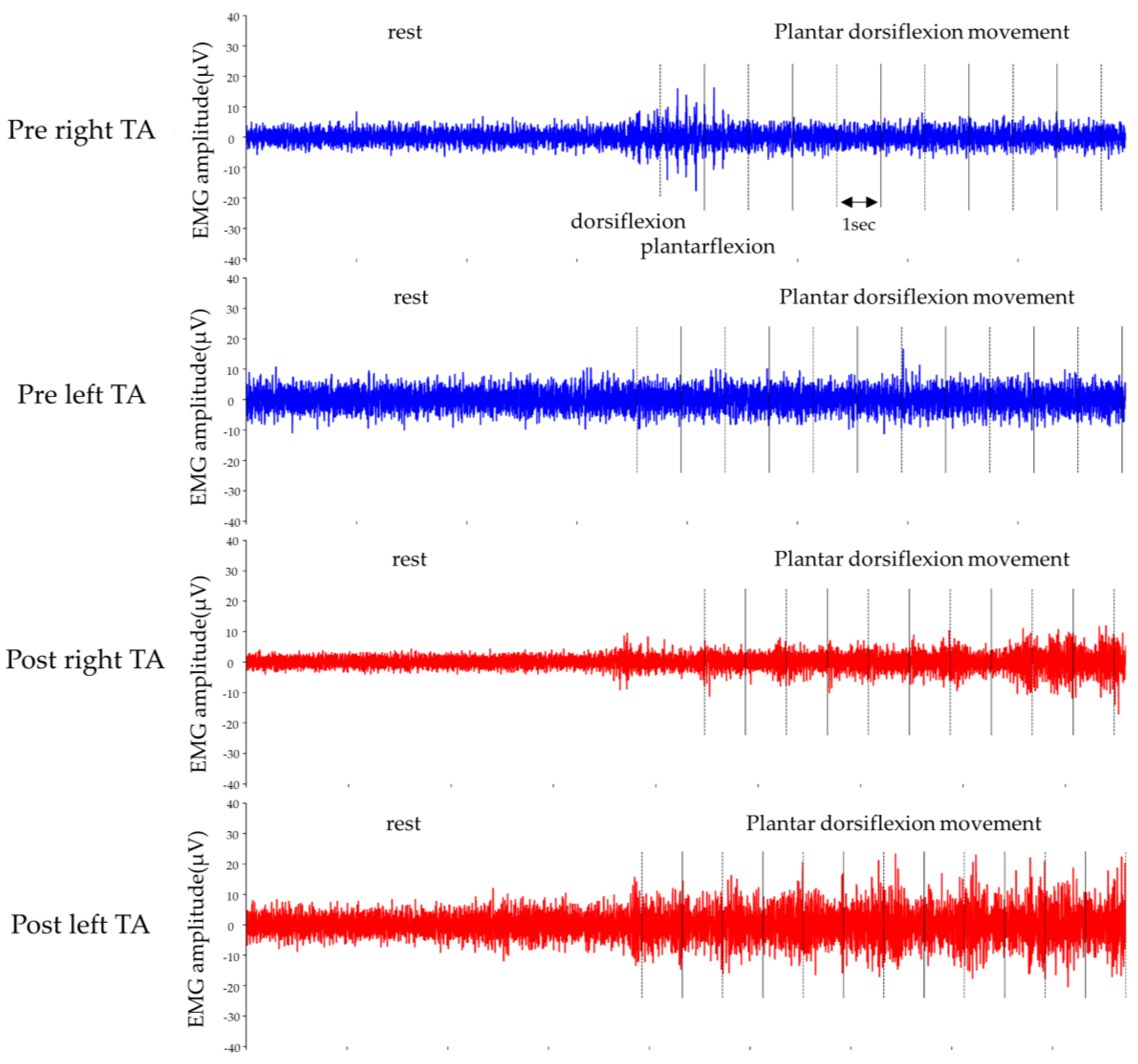
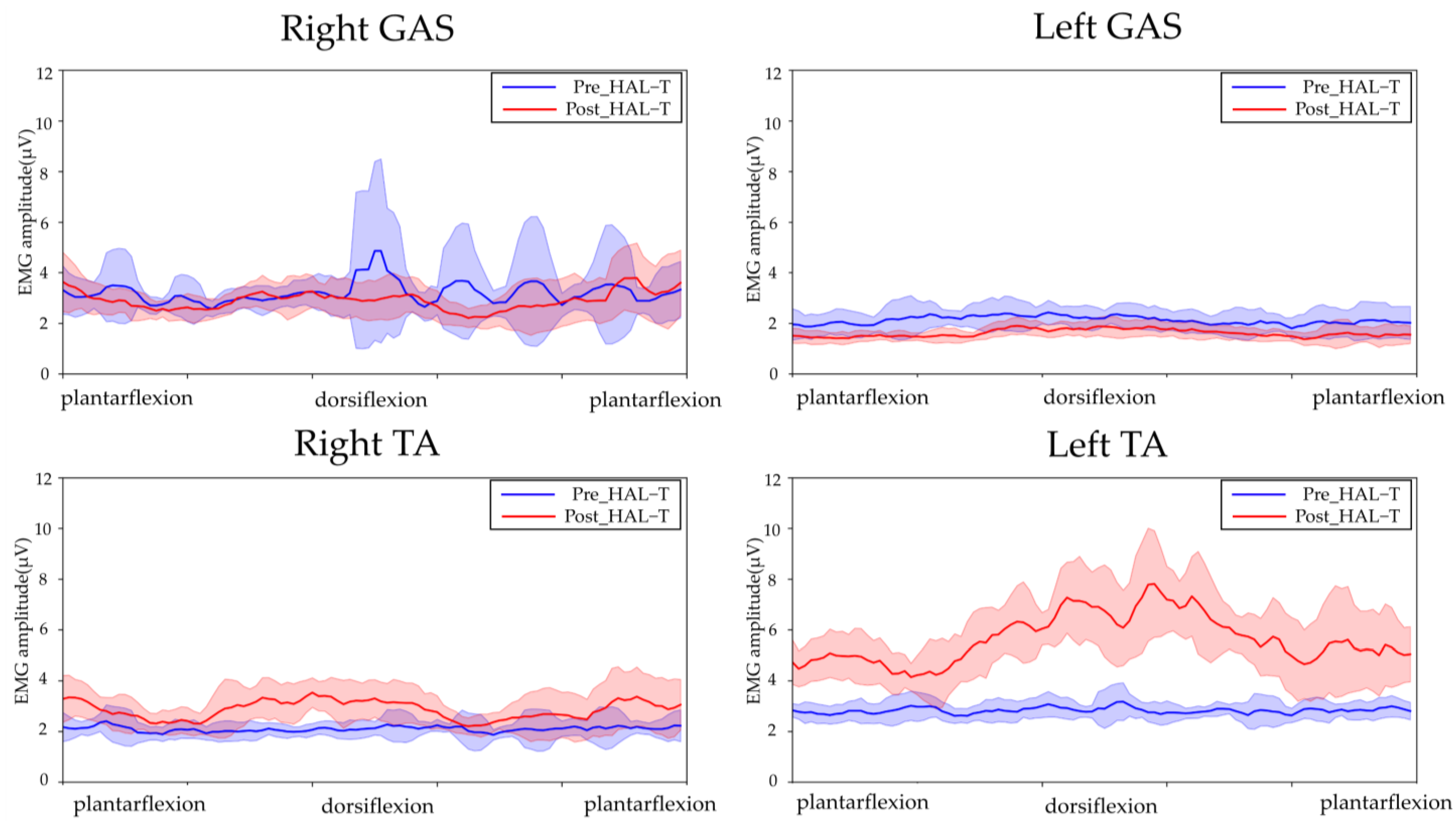
2.4. Outcome of the Intervention
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, J.; Chan, L.; Flynn, S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Suda, K.; Kudo, D.; Sakai, H.; Nakagawa, Y.; Mikami, Y.; Suzuki, S.; Tokioka, T.; Tokuhiro, A.; Takei, H.; et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord 2021, 59, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Holanda, L.J.; Silva, P.M.M.; Amorim, T.C.; Lacerda, M.O.; Simão, C.R.; Morya, E. Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 126. [Google Scholar] [CrossRef]
- Mekki, M.; Delgado, A.D.; Fry, A.; Putrino, D.; Huang, V. Robotic rehabilitation and spinal cord injury: A narrative review. Neurotherapeutics 2018, 15, 604–617. [Google Scholar] [CrossRef]
- Saita, K.; Morishita, T.; Hyakutake, K.; Fukuda, H.; Shiota, E.; Sankai, Y.; Inoue, T. Combined therapy using botulinum toxin A and single-joint hybrid assistive limb for upper-limb disability due to spastic hemiplegia. J. Neurol. Sci. 2017, 373, 182–187. [Google Scholar] [CrossRef]
- Goto, K.; Morishita, T.; Kamada, S.; Saita, K.; Fukuda, H.; Shiota, E.; Sankai, Y.; Inoue, T. Feasibility of rehabilitation using the single-joint hybrid assistive limb to facilitate early recovery following total knee arthroplasty: A pilot study. Assist. Technol. 2017, 29, 197–201. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Koseki, K.; Endo, Y.; Yamamoto, S.; Kanae, K.; Takeuchi, R.; Yozu, A.; Mutsuzaki, H. Adjusting assistance commensurates with patient effort during robot-assisted upper limb training for a patient with spasticity after cervical spinal cord injury: A case report. Medicina 2019, 55, 404. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kubota, S.; Sugaya, H.; Arai, N.; Hyodo, K.; Kanamori, A.; Yamazaki, M. Feasibility and efficacy of knee extension training using a single-joint hybrid assistive limb, versus conventional rehabilitation during the early postoperative period after total knee arthroplasty. J. Rural Med. 2021, 16, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mutsuzaki, H.; Yoshikawa, K.; Yamamoto, S.; Koseki, K.; Takeuchi, R.; Mataki, Y.; Iwasaki, N. Robot-assisted ankle rehabilitation using the hybrid assistive limb for children after equinus surgery: A report of two cases. Pediatr. Rep. 2022, 14, 41. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kadone, H.; Kubota, S.; Ikumi, A.; Abe, T.; Marushima, A.; Ueno, T.; Endo, A.; Kawamoto, H.; Saotome, K.; et al. Active elbow flexion is possible in C4 quadriplegia using hybrid assistive limb (HAL®) technology: A case study. J. Spinal Cord Med. 2017, 40, 456–462. [Google Scholar] [CrossRef] [PubMed]
- ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What’s new? Spinal Cord 2019, 57, 815–817. [Google Scholar] [CrossRef]
- Dietz, V.; Fouad, K. Restoration of sensorimotor functions after spinal cord injury. Brain 2014, 137, 654–667. [Google Scholar] [CrossRef]
- Govil, K.; Noohu, M.M. Effect of EMG biofeedback training of gluteus maximus muscle on gait parameters in incomplete spinal cord injury. NeuroRehabilitation 2013, 33, 147–152. [Google Scholar] [CrossRef]
- Roosink, M.; Mercier, C. Virtual feedback for motor and pain rehabilitation after spinal cord injury. Spinal Cord 2014, 52, 860–866. [Google Scholar] [CrossRef]
- Cheung, E.Y.Y.; Yu, K.K.K.; Kwan, R.L.C.; Ng, C.K.M.; Chau, R.M.W.; Cheing, G.L.Y. Effect of EMG-biofeedback robotic-assisted body weight supported treadmill training on walking ability and cardiopulmonary function on people with subacute spinal cord injuries—A randomized controlled trial. BMC Neurol. 2019, 19, 140. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, F.; Li, J.; Yang, M.; Li, J.; Yang, D.; Du, L. Effect of electromyographic biofeedback training on motor function of quadriceps femoris in patients with incomplete spinal cord injury: A randomized controlled trial. NeuroRehabilitation 2021, 48, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V. Body weight supported gait training: From laboratory to clinical setting. Brain Res. Bull. 2008, 76, 459–463. [Google Scholar] [CrossRef]
- Benito-Penalva, J.; Edwards, D.J.; Opisso, E.; Cortes, M.; Lopez-Blazquez, R.; Murillo, N.; Costa, U.; Tormos, J.M.; Vidal-Samso, J.; Valls-Solé, J.; et al. Gait training in human spinal cord injury using electromechanical systems: Effect of device type and patient characteristics. Arch. Phys. Med. Rehabil. 2012, 93, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Field-Fote, E.C.; Roach, K.E. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Phys. Ther. 2011, 91, 48–60. [Google Scholar] [CrossRef]
- Burns, A.S.; Ditunno, J.F. Establishing prognosis and maximizing functional outcomes after spinal cord injury: A review of current and future directions in rehabilitation management. Spine 2001, 26, S137–S145. [Google Scholar] [CrossRef]
- Lee, J.W.; Chan, K.; Unger, J.; Yoo, J.; Musselman, K.E.; Masani, K. Interjoint coordination between the ankle and hip joints during quiet standing in individuals with motor incomplete spinal cord injury. J. Neurophysiol. 2021, 125, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Billeri, L.; Ciappina, F.; Balletta, T.; Porcari, B.; Cannavo, A.; Pignolo, L.; Manuli, A.; Naro, A. Toward improving functional recovery in spinal cord injury using robotics: A pilot study focusing on ankle rehabilitation. Expert Rev. Med. Devices 2022, 19, 83–95. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koseki, K.; Takahashi, K.; Yamamoto, S.; Yoshikawa, K.; Abe, A.; Mutsuzaki, H. Use of Robot-Assisted Ankle Training in a Patient with an Incomplete Spinal Cord Injury: A Case Report. J. Funct. Morphol. Kinesiol. 2023, 8, 31. https://doi.org/10.3390/jfmk8010031
Koseki K, Takahashi K, Yamamoto S, Yoshikawa K, Abe A, Mutsuzaki H. Use of Robot-Assisted Ankle Training in a Patient with an Incomplete Spinal Cord Injury: A Case Report. Journal of Functional Morphology and Kinesiology. 2023; 8(1):31. https://doi.org/10.3390/jfmk8010031
Chicago/Turabian StyleKoseki, Kazunori, Kazushi Takahashi, Satoshi Yamamoto, Kenichi Yoshikawa, Atsushi Abe, and Hirotaka Mutsuzaki. 2023. "Use of Robot-Assisted Ankle Training in a Patient with an Incomplete Spinal Cord Injury: A Case Report" Journal of Functional Morphology and Kinesiology 8, no. 1: 31. https://doi.org/10.3390/jfmk8010031
APA StyleKoseki, K., Takahashi, K., Yamamoto, S., Yoshikawa, K., Abe, A., & Mutsuzaki, H. (2023). Use of Robot-Assisted Ankle Training in a Patient with an Incomplete Spinal Cord Injury: A Case Report. Journal of Functional Morphology and Kinesiology, 8(1), 31. https://doi.org/10.3390/jfmk8010031





