Acute Hormonal Responses to Multi-Joint Resistance Exercises with Blood Flow Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
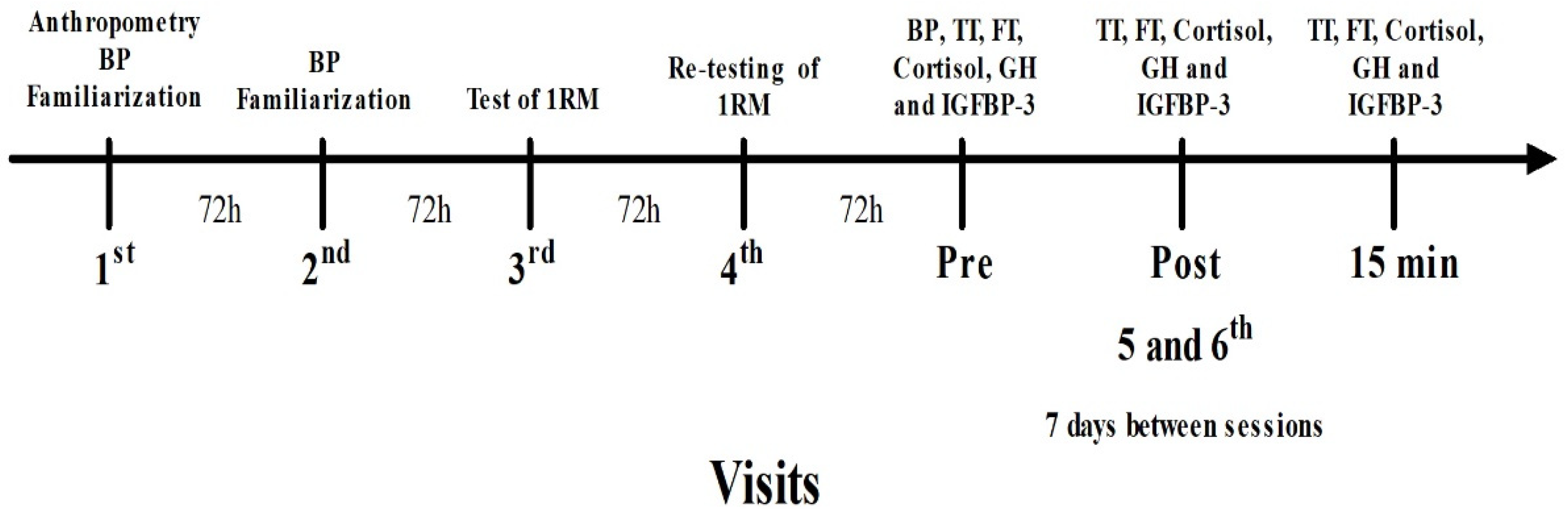
2.2. Study Design
2.3. Blood Pressure Measurements
2.4. One-Repetition Test
2.5. Blood Samples
2.6. Experimental Sessions
2.7. Statical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACSM. American College of Sports Medicine. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, L.C.; Nunes, P.R.; Souza, L.R.; Oliveira, A.A.; Furlanetto, R.; Marocolo, M.; Orsatti, F. Low-load resistance training promotes muscular adaptation regardless of vascular occlusion, load, or volume. Eur. J. Appl. Physiol. 2015, 115, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, G.C.; Ugrinowitsch, C.; Roschel, H.; Aoki, M.S.; Soares, A.G.; Aihara, A.Y.; Rocha, C.F.; Tricoli, V. Strength training with blood flow restriction diminishes myostatin gene expression. Med. Sci. Sports Exerc. 2012, 44, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Monazzami, A.; Nikousefat, Z.; Heyrani, A.; Yari, K. The acute and chronic effects of resistance training with blood flow restriction on hormonal responses in untrained young men: A comparison of frequency. Cell. Mol. Biol. 2020, 66, 1–8. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef]
- Vechin, F.C.; Libardi, C.A.; Conceição, M.S.; Damas, F.R.; Lixandrão, M.E.; Berton, R.P.B.; Tricoli, V.; Roschel, H.A.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J. Strength Cond. Res. 2015, 29, 1071–1076. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Wilson, J.M.; Bemben, M.G. Blood flow restriction: The metabolite/volume threshold theory. Med. Hypotheses 2011, 77, 748–752. [Google Scholar] [CrossRef]
- Pope, Z.K.; Willardson, J.M.; Schoenfeld, B.J. Exercise And Blood Flow Restriction. J. Strength Cond. Res. 2013, 27, 2914–2926. [Google Scholar] [CrossRef]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.-I.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 2005, 95, 65–73. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Murphy, B.G.; LaBarbera, K.E. Neuromuscular function following a bout of low-load blood flow restricted exercise. Med. Sci. Sports Exerc. 2013, 45, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Tsoukos, P.; Zafeiridis, A.; Spassis, A.; Tokmakidis, S.P. Hormonal responses after resistance exercise performed with maximum and submaximum movement velocities. Appl. Physiol. Nutr. Metab. 2014, 39, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Yasuda, T.; Midorikawa, T.; Sato, Y.; Kearns, C.F.; Inoue, K.; Koizumi, K.; Ishii, N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 2005, 1, 6–12. [Google Scholar] [CrossRef]
- Manini, T.M.; Yarro, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Madarame, H.; Sasaki, K.; Ishii, N. Endocrine responses to upper- and lower-limb resistance exercises with blood flow restriction. Acta Physiol. Hung. 2010, 97, 192–200. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, S.; Li, Y. Acute effects of low load resistance training with blood flow restriction on serum growth hormone, insulin-like growth factor-1, and testosterone in patients with mild to moderate unilateral knee osteoarthritis. Heliyon 2022, 8, e11051. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Gregg, L.D.; Kim, D.; Sherk, V.D.; Bemben, M.G.; Bemben, D.A. Hormone responses to an acute bout of low intensity blood flow restricted resistance exercise in college-aged females. J. Sports Sci. Med. 2014, 13, 91–96. [Google Scholar]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Parlavantzas, A.; Tokmakidis, S.P. Hormonal Responses after a Strength Endurance Resistance Exercise Protocol in Young and Elderly Males. Endoscopy 2006, 28, 401–406. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Loenneke, J.P.; Ugrinowitsch, C.; Aoki, M.S.; Soares, A.G.; Roschel, H.; Tricoli, V. Blood-Flow-Restriction- Training induced hormonal response is not associated with gains in muscle size and strength. J. Human Kinet. 2022, 83, 235–243. [Google Scholar] [CrossRef]
- West, D.W.D.; Phillips, S.M. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur. J. Appl. Physiol. 2011, 112, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fujita, S.; Ogasawara, R.; Sato, Y.; Abe, T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: A pilot study. Clin. Physiol. Funct. Imaging 2010, 30, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanami, S.; Volpi, E.; Rasmussen, B.B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Abe, T.; Drummond, M.J.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar]
- Hollander, D.B.; Reeves, G.V.; Clavier, J.D.; Francois, M.R.; Thomas, C.; Kraemer, R.R. Partial occlusion during resistance exercise alters effort sense and pain. J. Strength Cond. Res. 2010, 24, 235–243. [Google Scholar] [CrossRef]
- Moir, L.M. Muscular Strength. In Book NSCA’s Guide to Tests and Assessments; Miller, T., Ed.; Human Kinetics: Champaign, IL, USA, 2012; pp. 164–171. [Google Scholar]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood flow restriction exercise: Considerations of Methodology, application, and safety. Front. Physiol. 2019, 10, 533. [Google Scholar]
- Beck, T.W. The Importance of A Priori Sample Size Estimation in Strength and Conditioning Research. J. Strength Cond. Res. 2013, 27, 2323–2337. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Meth. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for The Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Gotshalk, L.A.; Loebel, C.C.; Nindl, B.C.; Putukian, M.; Sebastianelli, W.J.; Newton, R.U.; Häkkinen, K.; Kraemer, W.J. Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can. J. Appl. Physiol. 1997, 21, 244–255. [Google Scholar] [CrossRef]
- Marx, J.O.; Ratamess, N.A.; Nindl, B.C.; Gotshalk, L.A.; Volek, J.S.; Dohi, K.; Bush, J.A.; Gomez, A.L.; Mazzetti, S.A.; Fleck, S.J. Low-volume circuit versus high-volume periodized resistance training in women. Med. Sci. Sports Exerc. 2001, 33, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.V.; Kraemer, R.R.; Hollander, D.B.; Clavier, J.; Thomas, C.; Francois, M.; Castracane, V.D. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J. Appl. Physiol. 2006, 101, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshitomi, A.; Abe, T. Acute growth hormone response to low-intensity KAATSU resistance exercise: Comparison between arm and leg. Int. J. KAATSU Train. Res. 2005, 1, 45–50. [Google Scholar] [CrossRef]
- Birzniece, V. Exercise and the growth hormone–insulin-like growth factor axis. Curr. Opin. Endocr. Metab. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, G.J.; Wilson, J.M. A mechanistic approach to blood flow occlusion. Int. J. Sports Med. 2010, 31, 1–4. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S.M. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef]
- Jones, A.Y.; Dean, E. Body position change and its effect on hemodynamic and metabolic status. Heart Lung 2004, 33, 281–290. [Google Scholar] [CrossRef]
- Araujo, J.P.; Neto, G.R.; Loenneke, J.P.; Bemben, M.G.; Laurentino, G.; Batista, G.; Silva, J.C.; Freitas, E.; Cirilo-Sousa, M.S. The effects of water aerobics in combination with blood flow restriction on strength and functional capacity in post-menopausal women. Age 2015, 37, 110. [Google Scholar] [CrossRef]
- Gil, A.L.; Neto, G.R.; Sousa, M.S.C.; Dias, I.; Vianna, J.; Nunes, R.A.; Novaes, J.S. Effect of strength training with blood flow restriction on muscle power and submaximal strength in eumenorrheic women. Clin. Physiol. Funct. Imaging 2015, 35, 221–228. [Google Scholar] [CrossRef]
- Neto, G.R.; Sousa, M.S.C.; Costa, P.B.; Salles, B.F.; Novaes, G.S.; Novaes, J.S. Hypotensive effects of resistance exercises with blood flow restriction. J. Strength Cond. Res. 2015, 29, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Neto, G.R.; Sousa, M.S.C.; Silva, G.V.C.; Gil, A.L.S.; Salles, B.F.; Novaes, J.S. Acute resistance exercise with blood flow restriction effects on heart rate, double product, oxygen saturation and perceived exertion. Clin. Physiol. Funct. Imaging 2014, 34, 53–59. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean ± Standard Deviation (n = 10) |
|---|---|
| Age (years) | 22.50 ± 3.24 |
| Height (cm) | 177.30 ± 4.76 |
| Body mass (kg) | 72.20 ± 8.06 |
| Estimated body fat (%) | 8.23 ± 2.53 |
| Systolic blood pressure (mmHg) | 121.40 ± 4.55 |
| Bench press (kg)—1RM | 61.50 ± 18.27 |
| Half Squat (kg)—1RM | 108.00 ± 28.98 |
| Variables | Protocols | PRE | POST0 | POST15 |
|---|---|---|---|---|
| TT (nmol.L−1) | HI | 20.10 ± 13.92 | 15.30 ± 6.70 | 14.59 ± 5.29 |
| LI + BFR | 16.50 ± 7.29 | 14.40 ± 5.32 | 16.74 ± 13.71 | |
| FT (nmol.L−1) | HI | 11.75 ± 4.96 | 13.41 ± 3.95 | 11.17 ± 3.58 |
| LI + BFR | 11.31 ± 3.83 | 11.95 ± 4.34 | 11.52 ± 3.85 | |
| Cortisol (nmol.L−1) | HI | 359.50 ± 107.33 | 347.08 ± 141.51 | 354.81 ± 155.31 |
| LI + BFR | 399.51 ± 135.82 | 461.00 ± 141.06 | 429.58 ± 151.29 | |
| GH (μg.L−1) | HI | 0.11 ± 0.08 | 0.93 ± 1.28 | 2.39 ± 2.85 * |
| LI + BFR | 0.14 ± 0.13 | 2.05 ± 2.46 | 3.33 ± 3.118 * | |
| IGFBP-3 (mg.L−1) | HI | 4.64 ± 1.37 | 5.35 ± 1.79 ! | 5.02 ± 1.66 ** |
| LI + BFR | 4.59 ± 1.28 | 5.20 ± 1.56 ! | 4.60 ± 1.33 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaça-Alves, J.; Magalhães, P.S.; Rosa, C.V.; Reis, V.M.; Garrido, N.D.; Payan-Carreira, R.; Neto, G.R.; Costa, P.B. Acute Hormonal Responses to Multi-Joint Resistance Exercises with Blood Flow Restriction. J. Funct. Morphol. Kinesiol. 2023, 8, 3. https://doi.org/10.3390/jfmk8010003
Vilaça-Alves J, Magalhães PS, Rosa CV, Reis VM, Garrido ND, Payan-Carreira R, Neto GR, Costa PB. Acute Hormonal Responses to Multi-Joint Resistance Exercises with Blood Flow Restriction. Journal of Functional Morphology and Kinesiology. 2023; 8(1):3. https://doi.org/10.3390/jfmk8010003
Chicago/Turabian StyleVilaça-Alves, José, Patrício S. Magalhães, Claudio V. Rosa, Victor M. Reis, Nuno D. Garrido, Rita Payan-Carreira, Gabriel R. Neto, and Pablo B. Costa. 2023. "Acute Hormonal Responses to Multi-Joint Resistance Exercises with Blood Flow Restriction" Journal of Functional Morphology and Kinesiology 8, no. 1: 3. https://doi.org/10.3390/jfmk8010003
APA StyleVilaça-Alves, J., Magalhães, P. S., Rosa, C. V., Reis, V. M., Garrido, N. D., Payan-Carreira, R., Neto, G. R., & Costa, P. B. (2023). Acute Hormonal Responses to Multi-Joint Resistance Exercises with Blood Flow Restriction. Journal of Functional Morphology and Kinesiology, 8(1), 3. https://doi.org/10.3390/jfmk8010003












