The Influence of Weight Loss in Postural Control in Women Undergoing Sleeve Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Medical Examination and Postural Control Evaluation
2.3. Statistical Analysis
3. Results
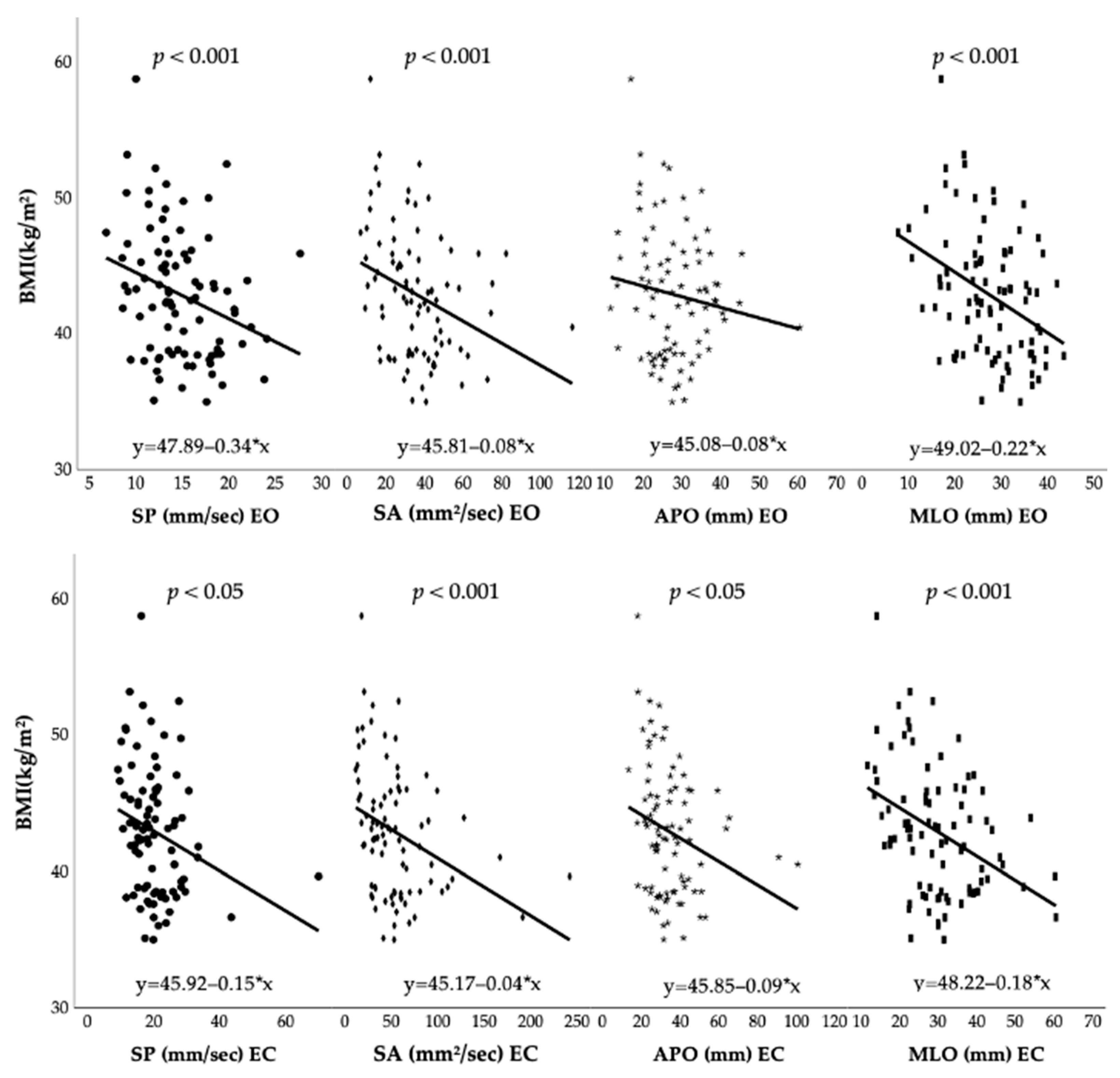
3.1. Pre-Surgery Anthropometric Characteristics and Postural Control Outcomes
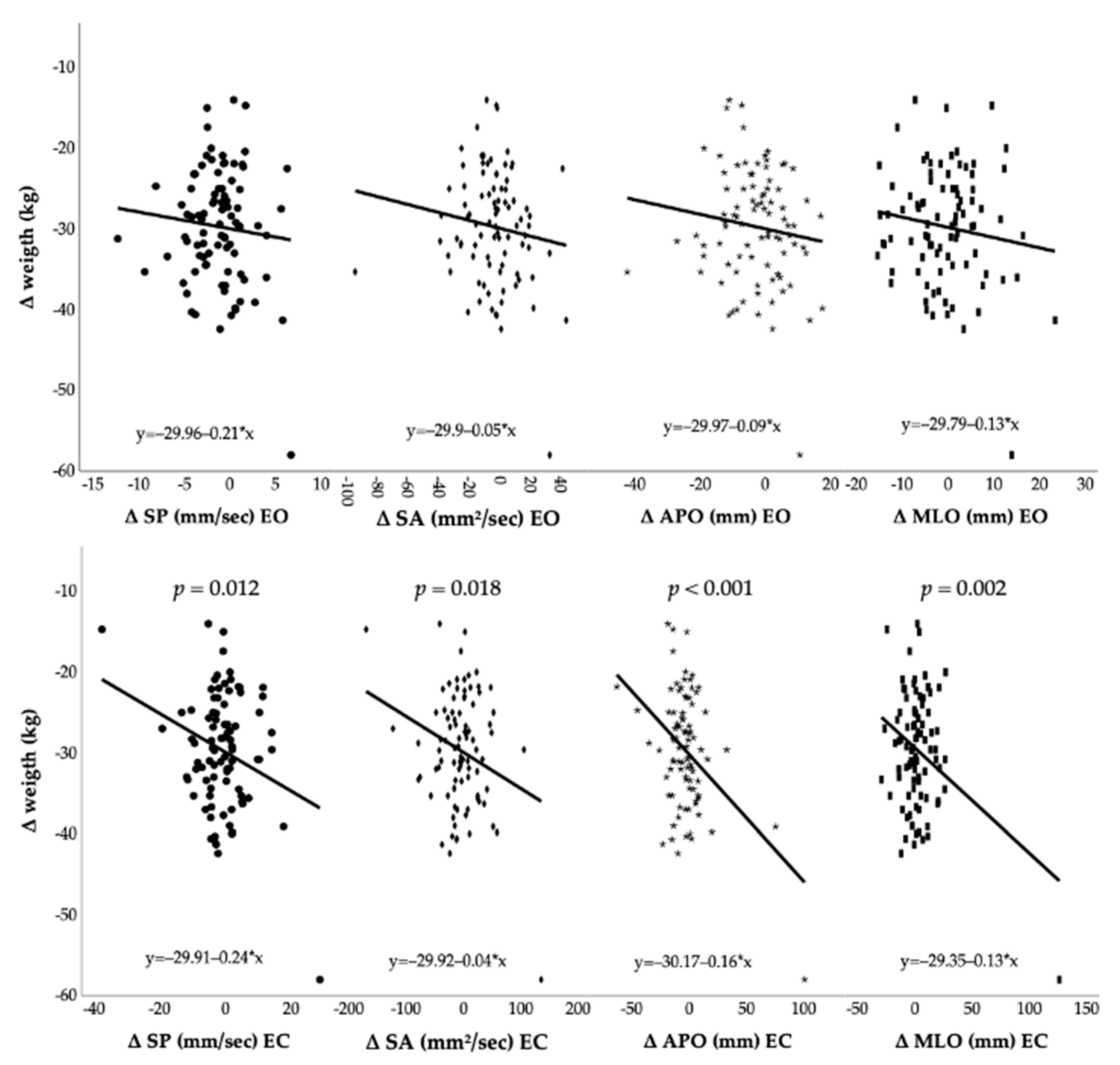
3.2. Post-Surgery Modification and Postural Control Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2014, 33, 673–689. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2021, 23, 120–133. [Google Scholar] [CrossRef]
- Fitch, A.K.; Bays, H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes. Pillars 2022, 1, 100004. [Google Scholar] [CrossRef]
- World Health Organization. WHO European Regional Obesity Report 2022. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf (accessed on 12 June 2021).
- Schutz, D.D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Reinmann, A.; Gafner, S.C.; Hilfiker, R.; Bruyneel, A.-V.; Pataky, Z.; Allet, L. Bariatric Surgery: Consequences on Functional Capacities in Patients With Obesity. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bobowik, P.; Wiszomirska, I. The impact of obesity and age on the risk of falls in elderly women. Acta Bioeng. Biomech. 2021, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pataky, Z.; Armand, S.; Müller-Pinget, S.; Golay, A.; Allet, L. Effects of obesity on functional capacity. Obesity 2013, 22, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Lord, S.R.; Harvey, L.A.; Close, J.C. Associations between obesity and overweight and fall risk, health status and quality of life in older people. Aust. N. Z. J. Public Health 2014, 38, 13–18. [Google Scholar] [CrossRef]
- Himes, C.L.; Reynolds, S.L. Effect of Obesity on Falls, Injury, and Disability. J. Am. Geriatr. Soc. 2012, 60, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, T.E.; Frames, C.W.; Soangra, R.; Lieberman, A. Effects of Obesity and Fall Risk on Gait and Posture of Community- Dwelling Older Adults. Int. J. Progn. Health Manag. 2019, 10, 019. [Google Scholar] [CrossRef] [PubMed]
- Rezaeipour, M. Evaluation of postural stability in overweight and obese middle-aged men. Turk. J. Med. Sci. 2018, 48, 1053–1057. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Silveira, E.A.; Mendonça, C.R.; Delpino, F.M.; Souza, G.V.E.; Rosa, L.P.D.S.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R.; Cugusi, L.; Bergamin, M.; Bini, V.; Fanelli, C.G.; Bullo, V.; Gobbo, S.; Di Blasio, A. Impact of BMI, Physical Activity, and Sitting Time Levels on Health-Related Outcomes in a Group of Overweight and Obese Adults with and without Type 2 Diabetes. J. Funct. Morphol. Kinesiol. 2022, 7, 12. [Google Scholar] [CrossRef]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef]
- Coen, P.M.; Goodpaster, B.H. A role for exercise after bariatric surgery? Diabetes Obes. Metab. 2016, 18, 16–23. [Google Scholar] [CrossRef]
- Alba, D.L.; Wu, L.; Cawthon, P.M.; Mulligan, K.; Lang, T.; Patel, S.; King, N.J.; Carter, J.T.; Rogers, S.J.; Posselt, A.M.; et al. Changes in Lean Mass, Absolute and Relative Muscle Strength, and Physical Performance After Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 711–720. [Google Scholar] [CrossRef]
- Jacobi, D.; Ciangura, C.; Couet, C.; Oppert, J.-M. Physical activity and weight loss following bariatric surgery. Obes. Rev. 2010, 12, 366–377. [Google Scholar] [CrossRef]
- Hue, O.; Berrigan, F.; Simoneau, M.; Marcotte, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Muscle Force and Force Control After Weight Loss in Obese and Morbidly Obese Men. Obes. Surg. 2008, 18, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Handrigan, G.; Hue, O.; Simoneau, M.; Corbeil, P.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Weight loss and muscular strength affect static balance control. Int. J. Obes. 2010, 34, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.; Cuthbertson, D.J.; Wilding, J.P.H. Impact of bariatric surgery on physical functioning in obese adults. Obes. Rev. 2015, 16, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Samaan, J.S.; Srinivasan, N.; Mirocha, J.; Premkumar, A.; Toubat, O.; Qian, E.; Subramanyam, C.; Malik, Y.; Lee, N.; Sandhu, K.; et al. Association of Postoperative Dieting, Exercise, Dietitian, and Surgeon Follow up With Bariatric Surgery Outcomes. Am. Surg. 2022, 88, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Lujan, J.; Tuero, C.; Landecho, M.F.; Moncada, R.; Cienfuegos, J.A.; Rotellar, F.; Silva, C.; Lapuente, F.; Martínez, P.; Frühbeck, G.; et al. Impact of Routine and Long-Term Follow-Up on Weight Loss after Bariatric Surgery. Obes. Surg. 2020, 30, 4293–4299. [Google Scholar] [CrossRef]
- Vieira, F.T.; de Oliveira, G.S.; Gonçalves, V.S.S.; Neri, S.G.R.; de Carvalho, K.M.B.; Dutra, E.S. Effect of physical exercise on muscle strength in adults following bariatric surgery: A systematic review and meta-analysis of different muscle strength assessment tests. PLoS ONE 2022, 17, e0269699. [Google Scholar] [CrossRef]
- Cibulková, N.; Daďová, K.; Mašková, K.; Busch, A.; Kobesová, A.; Vařeková, J.; Hašpicová, M.; Matoulek, M. Bariatric surgery and exercise: A pilot study on postural stability in obese individuals. PLoS ONE 2022, 17, e0262651. [Google Scholar] [CrossRef]
- Benetti, F.A.; Bacha, I.L.; Junior, A.B.G.; Greve, J.M.D. Analyses of balance and flexibility of obese patients undergoing bariatric surgery. Clinics 2016, 71, 78–81. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, S.; Li, R.; Dan, W.; Yang, L. Does weight loss affect the center of pressure of children with obesity: A follow-up study. J. Leather Sci. Eng. 2022, 4, 9. [Google Scholar] [CrossRef]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body weight is a strong predictor of postural stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef]
- Wearing, S.C.; Hennig, E.; Byrne, N.; Steele, J.; Hills, A.P. The biomechanics of restricted movement in adult obesity. Obes. Rev. 2006, 7, 13–24. [Google Scholar] [CrossRef]
- Teasdale, N.; Hue, O.; Marcotte, J.; Berrigan, F.; Simoneau, M.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A. Reducing weight increases postural stability in obese and morbid obese men. Int. J. Obes. 2006, 31, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar] [CrossRef]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Kováčiková, Z.; Svoboda, Z.; Neumannová, K.; Bizovská, L.; Cuberek, R.; Janura, M. Assessment of postural stability in overweight and obese middle-aged women. Acta Gymnica 2014, 44, 149–153. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Cieślińska-Świder, J.M.; Plewa, M.C.; Zahorska-Markiewicz, B.; Markiewicz, A. Effects of excessive body weight on postural control. J. Biomech. 2009, 42, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Cieślińska-Świder, J.M.; Błaszczyk, J.W. Posturographic characteristics of the standing posture and the effects of the treatment of obesity on obese young women. PLoS ONE 2019, 14, e0220962. [Google Scholar] [CrossRef] [PubMed]
- Menegoni, F.; Galli, M.; Tacchini, E.; Vismara, L.; Cavigioli, M.; Capodaglio, P. Gender-specific Effect of Obesity on Balance. Obesity 2009, 17, 1951–1956. [Google Scholar] [CrossRef]
- Greve, J.; Alonso, A.; Bordini, A.C.P.; Camanho, G.L. Correlation between body mass index and postural balance. Clinics 2007, 62, 717–720. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Haider, A.; Yang, A.; Cottell, K.E.; Tuppo, C.M.; Tong, K.-Y.; Pryor, A.D.; Rubin, C.T.; Chan, M.E. Postural Stability in Obese Preoperative Bariatric Patients Using Static and Dynamic Evaluation. Obes. Facts 2020, 13, 499–513. [Google Scholar] [CrossRef]
- de Souza, S.A.F.; Faintuch, J.; Valezi, A.C.; Sant’Anna, A.F.; Gama-Rodrigues, J.J.; Fonseca, I.C.D.B.; de Melo, R.D. Postural Changes in Morbidly Obese Patients. Obes. Surg. 2005, 15, 1013–1016. [Google Scholar] [CrossRef]
- Cieślińska-Świder, J.; Furmanek, M.P.; Błaszczyk, J.W. The influence of adipose tissue location on postural control. J. Biomech. 2017, 60, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, P.; Simoneau, M.; Rancourt, D.; Tremblay, A.; Teasdale, N. Increased risk for falling associated with obesity: Mathematical modeling of postural control. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Noria, S.; Grantcharov, T. Biological effects of bariatric surgery on obesity-related comorbidities. Can. J. Surg. 2013, 56, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Andreato, L.V.; de Oliveira, D.V.; Follmer, B.; Bertolini, S.M.M.G. The influence of age and overweight or obesity on foot sensitivity and postural control: A systematic review. Australas. J. Ageing 2020, 39, e251–e258. [Google Scholar] [CrossRef]
- Wu, X.; Madigan, M.L. Impaired plantar sensitivity among the obese is associated with increased postural sway. Neurosci. Lett. 2014, 583, 49–54. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Agosti, F.; Proietti, M.; Riva, D.; Resnik, M.; Lafortuna, C.L.; Sartorio, A. Postural instability of extremely obese individuals improves after a body weight reduction program entailing specific balance training. J. Endocrinol. Investig. 2005, 28, 2–7. [Google Scholar] [CrossRef]
- Zanotto, T.; Bergamin, M.; Roman, F.; Sieverdes, J.C.; Gobbo, S.; Zaccaria, M.; Ermolao, A. Effect of exercise on dual-task and balance on elderly in multiple disease conditions. Curr. Aging Sci. 2014, 7, 115–136. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD | Range (Max-Min) |
|---|---|---|
| Sex (n) | Women (88) | |
| Age (years) | 44.4 ± 11.2 | 18–68 |
| Height (m) | 1.6 ± 0.1 | 1.4–1.75 |
| Weight (kg) | 111.4 ± 13.5 | 82.5–140 |
| BMI (kg/m2) | 42.9 ± 4.7 | 35–58.77 |
| MMSE (score) | 29.2 ± 1 | 26–30 |
| Days from surgery to post-test | 189.36 ± 14.6 | 126–248 |
| Comorbidities (type) | Pre-diabetes (13), DMT2 (13), hypothyroidism (18), dyslipidemia (23), IPTS (32), OSAS (5), musculoskeletal disorders (27), other (34) | |
| Comorbidities (num) | No com. (18), 1 com. (20), 2 com. (18), 3 com. (16), 4 com. (10), 5 com. (5), >6 com. (1) | |
| Drugs (num) | No drug (26), 1 drug (23), 2 drugs (13), 3 drugs (7), 4 drugs (5), 5 drugs (6), >6 drugs (8) | |
| Obesity class (%) | II obesity class: 28 (33%) III obesity class: 59 (67%) | |
| Pre (m ± sd) | Post (m ± sd) | Δ (abs) C.I. (95%) | Δ (%) | ES | |
|---|---|---|---|---|---|
| Weight (kg) | 111.4 ± 13.5 | 81.7 ± 10.7 ** | −29.7 [−31.2; −28.2] | −26.7% | 2.2 |
| BMI (kg/m2) | 42.9 ± 4.7 | 31.5 ± 4.1 ** | −11.4 [−11.9; −10.9] | −26.6% | 2.4 |
| Pre-diabetes (num) | 13 | 7 | −6 | ||
| DMT2 (num) | 13 | 8 | −5 | ||
| Hypothyroidism (num) | 18 | 18 | 0 | ||
| Dyslipidemia (num) | 23 | 17 | −6 | ||
| IPTS (num) | 32 | 19 | −13 | ||
| OSAS (num) | 5 | 3 | −2 | ||
| MSDs (num) | 27 | 21 | −6 | ||
| Other (num) | 34 | 28 | −6 |
| Pre (M ± SD) | Post (M ± SD) | Δ (abs) C.I. (95%) | Δ (%) | ES | |
|---|---|---|---|---|---|
| SP (mm/s) EO | 14.8 ± 4 | 13.5 ± 3.4 ** | −1.2 [−1.9; −0.6] | −8.2% | 0.3 |
| SA (mm2/s) EO | 35.8 ± 18.4 | 31.9 ± 13.9 | −4 [−8; 0.1] | −11% | 0.2 |
| APO (mm) EO | 27.9 ± 8.2 | 25 ± 8 * | −2.9 [−5; −0.8] | −10.4% | 0.4 |
| MLO (mm) EO | 27.4 ± 7.9 | 26.8 ± 6.2 | −0.6 [−2.2; 1] | −2.2% | 0.1 |
| SP (mm/s) EC | 20.5 ± 8.1 | 19.7 ± 7.2 | −0.8 [−2.5; 0.9] | −4% | 0.1 |
| SA (mm2/s) EC | 54 ± 37.9 | 49.2 ± 31 | −4.8 [−13.4; 3.9] | −8.8% | 0.1 |
| APO (mm) EC | 34.6 ± 13.9 | 31.7 ± 15.5 | −2.9 [−6.9; 1.1] | −8.4% | 0.2 |
| MLO (mm) EC | 30 ± 10.3 | 32.7 ± 15.3 | +2.8 [−1; 6.5] | 9.2% | −0.3 |
| Postural Parameters | Model | R | R2 | Adjusted R2 | F | p Value |
|---|---|---|---|---|---|---|
| SP (mm/s) EO | (1) BMI | 0.289 | 0.083 | 0.073 | 7.814 | 0.006 |
| (2) BMI, age | 0.324 | 0.105 | 0.084 | 4.970 | 0.009 | |
| (3) BMI, age, height | 0.330 | 0.109 | 0.077 | 3.413 | 0.021 | |
| SA (mm2/s) EO | (1) BMI | 0.317 | 0.101 | 0.09 | 9.615 | 0.003 |
| (2) BMI, height | 0.333 | 0.111 | 0.09 | 5.305 | 0.007 | |
| (3) BMI, height, age | 0.351 | 0.123 | 0.092 | 3.945 | 0.011 | |
| APO (mm) EO | (1) Height | 0.286 | 0.082 | 0.071 | 7.651 | 0.007 |
| (2) Height, BMI | 0.298 | 0.089 | 0.067 | 4.127 | 0.019 | |
| (3) Height, BMI, age | 0.315 | 0.099 | 0.067 | 3.093 | 0.031 | |
| MLO (mm) EO | (1) BMI | 0.376 | 0.141 | 0.131 | 14.153 | <0.001 |
| (2) BMI, age | 0.384 | 0.147 | 0.127 | 7.350 | 0.001 | |
| (3) BMI, age, height | 0.391 | 0.153 | 0.122 | 5.046 | 0.003 | |
| SP (mm/s) EC | (1) BMI | 0.253 | 0.064 | 0.053 | 5.905 | 0.017 |
| (2) BMI, age | 0.320 | 0.102 | 0.081 | 4.844 | 0.010 | |
| (3) BMI, age, height | 0.323 | 0.104 | 0.072 | 3.251 | 0.026 | |
| SA (mm2/s) EC | (1) BMI | 0.338 | 0.114 | 0.104 | 11.074 | 0.001 |
| (2) BMI, age | 0.367 | 0.135 | 0.114 | 6.613 | 0.002 | |
| (3) BMI, age, height | 0.369 | 0.136 | 0.105 | 4.406 | 0.006 | |
| APO (mm) EC | (1) BMI | 0.252 | 0.063 | 0.053 | 5.824 | 0.018 |
| (2) BMI, height | 0.274 | 0.075 | 0.053 | 3.442 | 0.037 | |
| (3) BMI, height, age | 0.306 | 0.094 | 0.061 | 2.900 | 0.040 | |
| MLO (mm) EC | (1) BMI | 0.387 | 0.150 | 0.140 | 15.145 | <0.001 |
| (2) BMI, height | 0.390 | 0.152 | 0.132 | 7.615 | <0.001 | |
| (3) BMI, height, age | 0.399 | 0.159 | 0.129 | 5.294 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullo, V.; Gobbo, S.; Cugusi, L.; Di Blasio, A.; Bortoletto, A.; Pippi, R.; Cruz-Diaz, D.; Gasperetti, A.; Vettor, R.; Ermolao, A.; et al. The Influence of Weight Loss in Postural Control in Women Undergoing Sleeve Gastrectomy. J. Funct. Morphol. Kinesiol. 2022, 7, 117. https://doi.org/10.3390/jfmk7040117
Bullo V, Gobbo S, Cugusi L, Di Blasio A, Bortoletto A, Pippi R, Cruz-Diaz D, Gasperetti A, Vettor R, Ermolao A, et al. The Influence of Weight Loss in Postural Control in Women Undergoing Sleeve Gastrectomy. Journal of Functional Morphology and Kinesiology. 2022; 7(4):117. https://doi.org/10.3390/jfmk7040117
Chicago/Turabian StyleBullo, Valentina, Stefano Gobbo, Lucia Cugusi, Andrea Di Blasio, Alessandro Bortoletto, Roberto Pippi, David Cruz-Diaz, Andrea Gasperetti, Roberto Vettor, Andrea Ermolao, and et al. 2022. "The Influence of Weight Loss in Postural Control in Women Undergoing Sleeve Gastrectomy" Journal of Functional Morphology and Kinesiology 7, no. 4: 117. https://doi.org/10.3390/jfmk7040117
APA StyleBullo, V., Gobbo, S., Cugusi, L., Di Blasio, A., Bortoletto, A., Pippi, R., Cruz-Diaz, D., Gasperetti, A., Vettor, R., Ermolao, A., & Bergamin, M. (2022). The Influence of Weight Loss in Postural Control in Women Undergoing Sleeve Gastrectomy. Journal of Functional Morphology and Kinesiology, 7(4), 117. https://doi.org/10.3390/jfmk7040117







