Association between Normal Weight Obesity and Skeletal Muscle Mass Index in Female University Students with Past Exercise Habituation
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Estimation of PAL Using the Factorial Method
2.3. Assessment of Muscle Mass and NW-O
2.4. Statistical Analysis
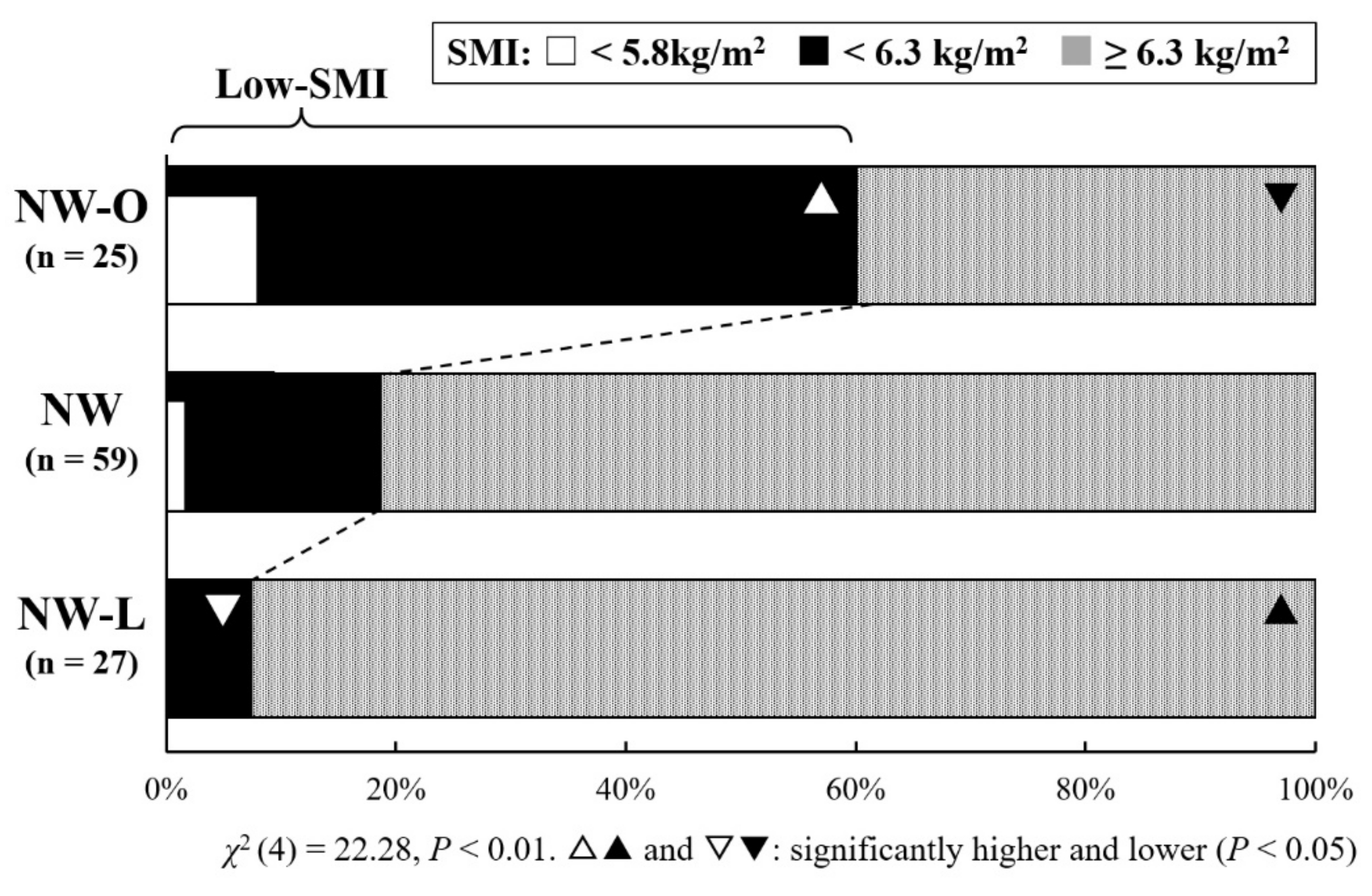
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, I. Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Hayashi, F.; Takimoto, H.; Yoshita, K.; Yoshiike, N. Perceived body size and desire for thinness of young Japanese women: A population-based survey. Br. J. Nutr. 2006, 96, 1154–1162. [Google Scholar] [CrossRef]
- Sugawara, A.; Saito, K.; Sato, M.; Kodama, S.; Sone, H. Thinness in Japanese young women. Epidemiology 2009, 20, 464–465. [Google Scholar] [CrossRef]
- Oshita, K.; Myotsuzono, R. An association between the physical activity level and skeletal muscle mass index in female university students with a past exercise habituation. Osteoporos. Sarcopenia 2021, 7, 146–152. [Google Scholar] [CrossRef]
- Oshita, K.; Myotsuzono, R.; Tashiro, T. Association between nutritional intake status and appendicular muscle mass in female university students with a high physical activity level. Jpn. J. Phys. Educ. Health Sport Sci. 2022, 67, 673–686. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Martinoli, R.; Vaia, F.; Di Renzo, L. Normal weight obese (NWO) women: An evaluation of a candidate new syndrome. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 513–523. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; González-Ruíz, K.; Rincón-Pabón, D.; Izquierdo, M.; García-Hermoso, A.; Agostinis-Sobrinho, C.; Sánchez-Capacho, N.; Roa-Cubaque, M.A.; Ramírez-Vélez, R. Normal-Weight Obesity Is Associated with Increased Cardiometabolic Risk in Young Adults. Nutrients 2020, 12, 1106. [Google Scholar] [CrossRef]
- Wijayatunga, N.N.; Dhurandhar, E.J. Normal weight obesity and unaddressed cardiometabolic health risk-a narrative review. Int. J. Obes. 2021, 45, 2141–2155. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Sakurai, S.; Kawata, Y.; Suzuki, Y.; Takaya, M.; Fujita, S.; Sakuraba, K.; Hirosawa, M.; Okada, T. Status of normal weight obesity among Japanese women under 40 years old. Juntendo Med. J. 2020, 66, 337–345. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. The Summary Report from the Scientific Committee of “Dietary Reference Intakes for Japanese” 2019; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2020.
- Oshita, K.; Nagamine, K.; Tashiro, T.; Hikita, A.; Yamaguchi, K. Association between skeletal muscle mass index (SMI), skipping meal(s), and lack of physical activity among female university students who desire to lose weight. Jpn. J. Physiol. Anthropol. 2019, 24, 27–34. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Mase, T.; Miyawaki, C.; Kouda, K.; Fujita, Y.; Okita, Y.; Ohara, K.; Mimasa, F.; Nakamura, H. Association between normal weight obesity and diet behaviors in female students. Jpn. J. Public Health 2012, 59, 371–380. [Google Scholar] [CrossRef]
- Ráthonyi, G.; Takács, V.; Szilágyi, R.; Bácsné Bába, É.; Müller, A.; Bács, Z.; Harangi-Rákos, M.; Balogh, L.; Ráthonyi-Odor, K. Your Physical Activity Is in Your Hand-Objective Activity Tracking Among University Students in Hungary, One of the Most Obese Countries in Europe. Front. Public Health 2021, 9, 661471. [Google Scholar] [CrossRef]
- Arias-Palencia, N.M.; Solera-Martínez, M.; Gracia-Marco, L.; Silva, P.; Martínez-Vizcaíno, V.; Cañete-García-Prieto, J.; Sánchez-López, M. Levels and Patterns of Objectively Assessed Physical Activity and Compliance with Different Public Health Guidelines in University Students. PLoS ONE 2015, 10, e0141977. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Zhang, M.; Schumann, M.; Huang, T.; Törmäkangas, T.; Cheng, S. Normal weight obesity and physical fitness in Chinese university students: An overlooked association. BMC Public Health 2018, 18, 1334. [Google Scholar] [CrossRef]
- Frisch, R.E. Fatness and fertility. Sci. Am. 1988, 258, 88–95. [Google Scholar] [CrossRef]
- Frisch, R.E. The right weight: Body fat, menarche and fertility. Proc. Nutr. Soc. 1994, 53, 113–129. [Google Scholar] [CrossRef]
| Height (cm) | 159.5 | ± | 4.8 | |
| Weight (kg) | 55.3 | ± | 5.3 | |
| BMI (kg/m2) | 21.7 | ± | 1.8 | |
| %BF (%) | 24.9 | ± | 5.0 | |
| SMI (kg/m2) | 7.12 | ± | 0.93 | |
| Muscle mass | Upper limb (kg) | 3.4 | ± | 0.4 |
| Lower limb (kg) | 14.7 | ± | 2.2 | |
| Appendicular (kg) | 18.1 | ± | 2.6 | |
| PAL | 1.8 | ± | 0.2 | |
| Body Weight—Boby Composition | Effect Size (Cohen’s d) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NW-L | NW | NW-O | NW-L vs. NW | NW-L vs. NW-O | NW vs. NW-O | ||||||||||
| BMI = 20.4 ± 1.2 | BMI = 21.8 ± 1.3 | BMI = 22.8 ± 1.2 | |||||||||||||
| %BF = 18.5 ± 1.8 | %BF = 24.8 ± 2.4 | %BF = 30.7 ± 1.3 | |||||||||||||
| Height (cm) | 160.6 | ± | 4.5 | 158.9 | ± | 5.1 | 159.4 | ± | 4.9 | 0.4 | 0.3 | 0.1 | |||
| Weight (kg) ** | 52.7 | ± | 3.7 | 55.1 | ± | 4.8 | 58.1 | ± | 4.1 | †, ‡ | 0.5 | 1.4 | 0.6 | ||
| SMI (kg/m2) ** | 7.6 | ± | 0.8 | 7.2 | ± | 0.9 | 6.5 | ± | 0.7 | †, ‡ | 0.5 | 1.5 | 0.9 | ||
| Muscle mass | Upper limb (kg) | 3.5 | ± | 0.4 | 3.4 | ± | 0.4 | 3.3 | ± | 0.4 | 0.4 | 0.6 | 0.2 | ||
| Lower limb (kg) ** | 16.1 | ± | 1.8 | 14.9 | ± | 2.2 | † | 13.1 | ± | 1.5 | †, ‡ | 0.6 | 1.8 | 0.9 | |
| Appendicular (kg) ** | 19.7 | ± | 2.2 | 18.3 | ± | 2.6 | † | 16.5 | ± | 1.8 | †, ‡ | 0.6 | 1.6 | 0.8 | |
| PAL * | 1.9 | ± | 0.2 | 1.8 | ± | 0.2 | 1.7 | ± | 0.2 | † | 0.6 | 1.0 | 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshita, K.; Myotsuzono, R.; Tashiro, T. Association between Normal Weight Obesity and Skeletal Muscle Mass Index in Female University Students with Past Exercise Habituation. J. Funct. Morphol. Kinesiol. 2022, 7, 92. https://doi.org/10.3390/jfmk7040092
Oshita K, Myotsuzono R, Tashiro T. Association between Normal Weight Obesity and Skeletal Muscle Mass Index in Female University Students with Past Exercise Habituation. Journal of Functional Morphology and Kinesiology. 2022; 7(4):92. https://doi.org/10.3390/jfmk7040092
Chicago/Turabian StyleOshita, Kazushige, Ryota Myotsuzono, and Tomoki Tashiro. 2022. "Association between Normal Weight Obesity and Skeletal Muscle Mass Index in Female University Students with Past Exercise Habituation" Journal of Functional Morphology and Kinesiology 7, no. 4: 92. https://doi.org/10.3390/jfmk7040092
APA StyleOshita, K., Myotsuzono, R., & Tashiro, T. (2022). Association between Normal Weight Obesity and Skeletal Muscle Mass Index in Female University Students with Past Exercise Habituation. Journal of Functional Morphology and Kinesiology, 7(4), 92. https://doi.org/10.3390/jfmk7040092






