Age-Related Changes in Skeletal Muscle Oxygen Utilization
Abstract
1. Introduction
2. Common Methods of Assessment
2.1. Historical Perspective
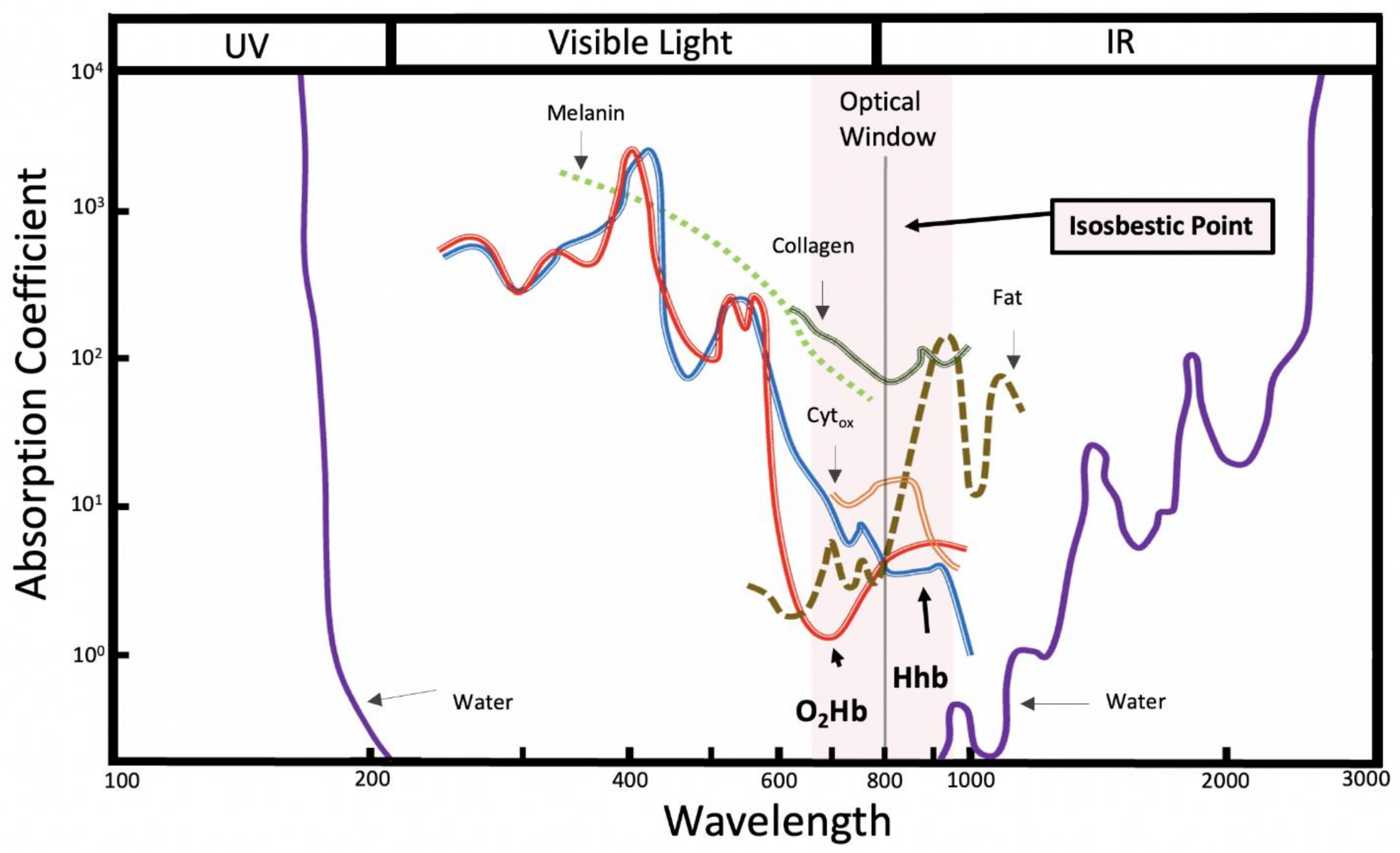
2.2. NIRS Validation, Advantages, and Limitations
| NIRS Device and Description | Advantages | Limitations | |
|---|---|---|---|
NIRcw—Continuous Wave
First used in evaluation of exercise: ~1992 The oldest and most widely used commercial NIRS equipment is the continuous wave (CW) sensor. These devices use a photomultiplier, photodiode, or avalanche photodiode detector to measure light attenuation. | 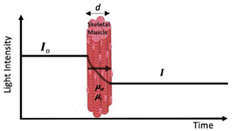 | Economical cost Lightweight and portable Sampling rate (i.e., number of readings taken per second) Simplicity and ease of use (i.e., more applicable for monitoring) | Difficult to separate absorption and scattering Limited to monitoring oxygenation trends; however, it is possible to quantify changes in concentrations of chromophores Penetration depth |
| NIRTD—Time Domain (Time-of-Flight or Time-Resolved) First used for in vivo: ~1987 First used in evaluation of exercise: ~2004 Ultrashort pulses typically generated using a semiconductor or solid-state laser. Synchro scan streak camera or a time-correlated single-photon counting method is used to measure photons according to their arrival time. | 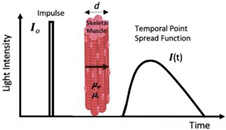 | Most accurate spectrometer in separating absorption and scattering. Penetration depth Superior spatial resolution | Cooling required Cost Excessive weight and size. Lack of stabilization. Sampling rate (i.e., number of readings taken per second) |
| NIRFD—Frequency Domain (Frequency-Resolved or Intensity/Phase Modulated Systems) First used for in vivo: ~1995 First used in evaluation of exercise: ~1995 Photon-counting detector or a gain-modulated area detector is employed to assess the attenuation, phase shift (Φ), and modulation (M) depth of the outgoing light. | 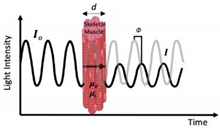 | Relative accuracy in uncoupling of absorption and scattering effects Sampling rate (i.e., number of readings) | Cost Complexity of use Excessive size of device Lack of scalability Penetration depth Radio frequency-modulated light cannot exceed 200 MHz Note: The above limitations have slowed NIRSFD translation to clinical applications. |
3. Aging and Muscle Oxygen Utilization
3.1. Submaximal Exercise
3.2. Maximal Exercise
3.3. Recovery Time
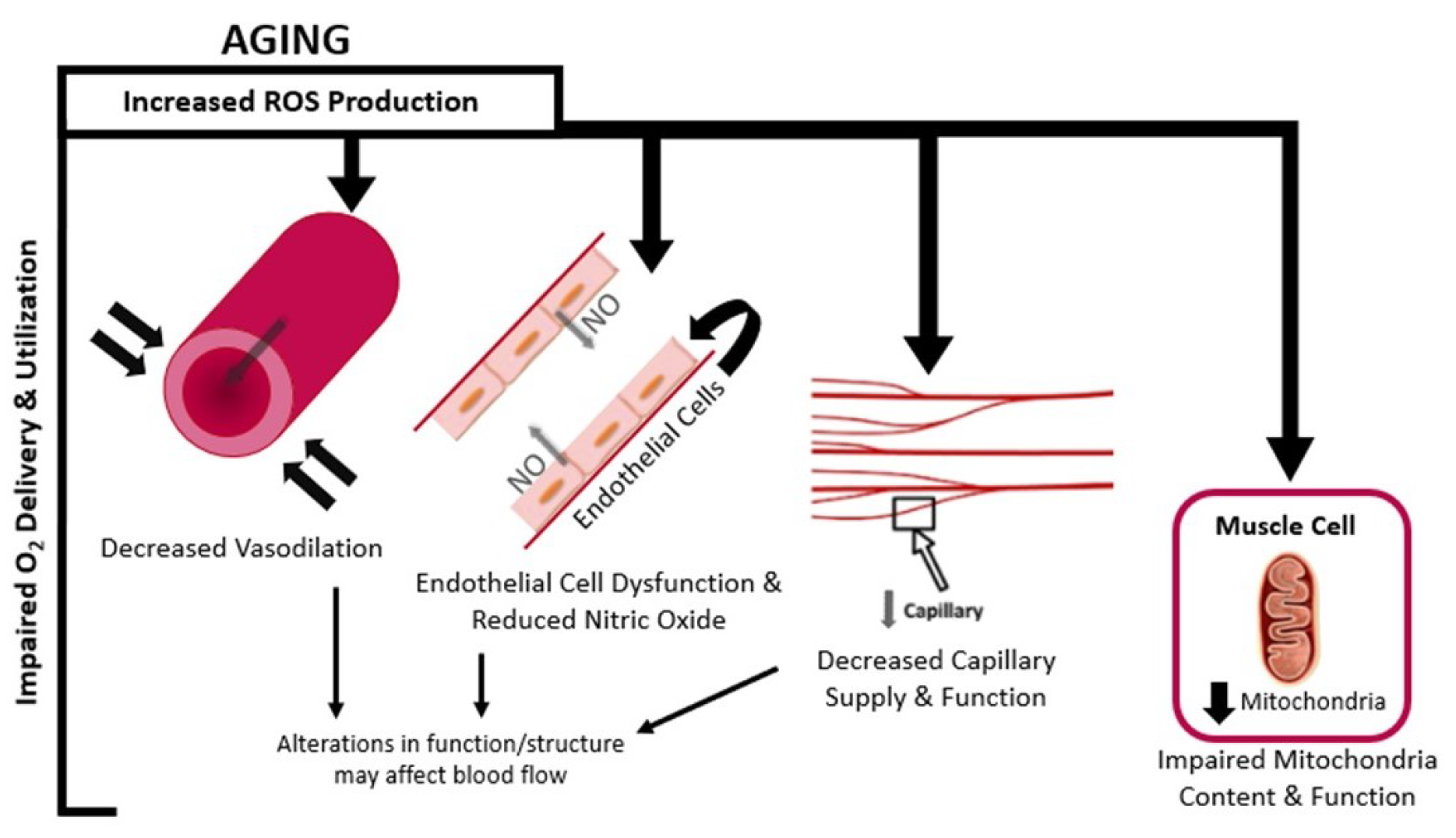
4. Age-Related Changes in the Oxygen Delivery Cascade
4.1. Blood Flow
4.2. Capillary Supply
4.3. Endothelial Cells
4.4. Nitric Oxide
4.5. Mitochondrial Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sidney, S.; Go, A.S.; Jaffe, M.G.; Solomon, M.D.; Ambrosy, A.P.; Rana, J.S. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019, 4, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Heart Disease & Stroke Statistical Update Fact Sheet Older Americans & Cardiovascular Diseases. 2021. Available online: https://professional.heart.org/-/media/PHD-Files-2/Science-News/2/2021-Heart-and-Stroke-Stat-Update/2021_Stat_Update_factsheet_Older_and_CVD.pdf (accessed on 2 January 2022).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Disease: A Costly Burden for America Projections through 2035. Available online: https://www.heart.org/-/media/Files/Get-Involved/Advocacy/Burden-Report-Consumer-Report.pdf (accessed on 3 January 2022).
- Treacher, D.F.; Leach, R.M. Oxygen transport-1 basic principles. BMJ 1998, 317, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, R.A.; Ferrari, M.; Natali, A.; Conti, G.; Mega, A.; Gasparetto, A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J. Appl. Physiol. 1994, 76, 1388–1393. [Google Scholar] [CrossRef]
- MedlinePlus Trusted Health Information for You. Available online: https://medlineplus.gov/ency/article/007459.htm (accessed on 2 January 2022).
- Trappe, S.W.; Costill, D.L.; Vukovich, M.D.; Jones, J.; Melham, T. Aging among elite distance runners: A 22-year longitudinal study. J. Appl. Physiol. 1996, 80, 285–290. [Google Scholar] [CrossRef]
- Training Peaks. Available online: https://www.trainingpeaks.com/blog/case-studies-on-training-with-muscle-oxygen-saturation/ (accessed on 20 January 2022).
- Miranda-Fuentes, C.; Chirosa-Ríos, L.J.; Guisado-Requena, I.M.; Delgado-Floody, P.; Jerez-Mayorga, D. Changes in muscle oxygen saturation measured using wireless near-infrared spectroscopy in resistance training: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 4293. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Chung, S.; Rosenberry, R.; Ryan, T.E.; Munson, M.; Dombrowsky, T.; Park, S.; Nasirian, A.; Haykowsky, M.J.; Nelson, M.D. Near-infrared spectroscopy detects age-related differences in skeletal muscle oxidative function: Promising implications for geroscience. Physiol. Rep. 2018, 6, e13588. [Google Scholar] [CrossRef]
- Costes, F.; Denis, C.; Roche, F.; Prieur, F.; Enjolras, F.; Barthélémy, J.C. Age-associated alteration of muscle oxygenation measured by near infrared spectroscopy during exercise. Arch. Physiol. Biochem. 1999, 107, 159–167. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Takagi, S.; Kime, R.; Murase, N.; Niwayama, M.; Sakamoto, S.; Katsumura, T. Skeletal muscle deoxygenation and its relationship to aerobic capacity during early and late stages of aging. Adv. Exp. Med. Biol. 2021, 1269, 77–82. [Google Scholar]
- Takagi, S.; Kime, R.; Murase, N.; Watanabe, T.; Osada, T.; Niwayama, M.; Katsumura, T. Aging affects spatial distribution of leg muscle oxygen saturation during ramp cycling exercise. Adv. Exp. Med. Biol. 2013, 789, 157–162. [Google Scholar]
- De Roia, G.; Pogliaghi, S.; Adami, A.; Papadopoulou, C.; Capelli, C. Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1158–R1166. [Google Scholar] [CrossRef]
- DeLorey, D.S.; Kowalchuk, J.M.; Paterson, D.H. Effects of prior heavy-intensity exercise on pulmonary O2 uptake and muscle deoxygenation kinetics in young and older adult humans. J. Appl. Physiol. 2004, 97, 998–1005. [Google Scholar] [CrossRef]
- Quaresima, V.; Lepanto, R.; Ferrari, M. The use of near infrared spectroscopy in sports medicine. J. Sports Med. Phys. Fit. 2003, 43, 1–13. [Google Scholar]
- De Oliveira, G.V.; Volino-Souza, M.; Barros-Santos, E.; Conte-Junior, C.A.; Alvares, T.S. The influence of cardiovascular risk factors on near-infrared spectroscopy-derived muscle oxygen saturation during exercise recovery in older adults. Sport Sci. Health 2022, 1–8. [Google Scholar] [CrossRef]
- Ichimura, S.; Murase, N.; Osada, T.; Kime, R.; Homma, T.; Ueda, C.; Nagasawa, T.; Motobe, M.; Hamaoka, T.; Katsumura, T. Age and activity status affect muscle reoxygenation time after maximal cycling exercise. Med. Sci. Sports Exerc. 2006, 38, 1277–1281. [Google Scholar] [CrossRef]
- Power, G.A.; Dalton, B.H.; Rice, C.L. Human neuromuscular structure and function in old age: A brief review. J. Sport Health Sci. 2013, 2, 215–226. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef]
- Takagi, S. Skeletal muscle oxygen dynamics and peak aerobic capacity. J. Phys. Fit. Sports Med. 2016, 5, 379–383. [Google Scholar] [CrossRef][Green Version]
- Proctor, D.N.; Shen, P.H.; Dietz, N.M.; Eickhoff, T.J.; Lawler, L.A.; Ebersold, E.J.; Loeffler, D.L.; Joyner, M.J. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J. Appl. Physiol. 1998, 85, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Landers-Ramos, R.Q.; Prior, S.J. The microvasculature and skeletal muscle health in aging. Exerc. Sport Sci. Rev. 2018, 46, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Bielli, A.; Arcuri, G.; Ferlosio, A.; Orlandi, A. Ageing and microvasculature. Vasc. Cell 2014, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C. Vascular ageing and aerobic exercise. Int. J. Environ. Res. Public Health 2021, 18, 10666. [Google Scholar] [CrossRef]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011, 8, 230–242. [Google Scholar]
- Lagerwaard, B.; Nieuwenhuizen, A.G.; De Boer, V.C.J.; Keijer, J. In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. Geroscience 2020, 42, 299–310. [Google Scholar] [CrossRef]
- Willingham, T.B.; McCully, K.K. In vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy. Front. Physiol. 2017, 8, 689. [Google Scholar] [CrossRef]
- Motobe, M.; Murase, N.; Osada, T.; Homma, T.; Ueda, C.; Nagasawa, T.; Kitahara, A.; Ichimura, S.; Kurosawa, Y.; Katsumura, T.; et al. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn. Med. 2004, 3, 2. [Google Scholar] [CrossRef]
- Schöttker, B.; Brenner, H.; Jansen, E.H.J.M.; Gardiner, J.; Peasey, A.; Kubínová, R.; Pająk, A.; Topor-Madry, R.; Tamosiunas, A.; Saum, K.; et al. Evidence for the free radical/oxidative stress theory of ageing from the chances consortium: A meta-analysis of individual participant data. BMC Med. 2015, 13, 300. [Google Scholar] [CrossRef]
- Hamaoka, T.; McCully, K.K. Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J. Physiol. Sci. 2019, 69, 799–811. [Google Scholar] [CrossRef]
- Vierordt, K. The Quantitative Spectral Analysis in Its Application to Physiology, Physics, Chemistry and Technology; Tubingen, H.L., Ed.; Laupp: Tübingen, Germany, 1876. [Google Scholar]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies: I. spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. 1932, 98, 719–733. [Google Scholar] [CrossRef]
- Millikan, G.A. A simple photoelectric colorimeter. J. Physiol. 1933, 79, 152–157. [Google Scholar] [CrossRef]
- Jöbsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Jobsis-Vander, F.F. Discovery of the near-infrared window into the body and the early development of near-infrared spectroscopy. J. Biomed. Opt. 1999, 4, 392–396. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Early development of near-infrared spectroscopy at duke university. J. Biomed. Opt. 2007, 12, 062102. [Google Scholar] [CrossRef]
- Jones, B.; Hesford, C.M.; Cooper, C.E. The use of portable NIRS to measure muscle oxygenation and haemodynamics during a repeated sprint running test. Adv. Exp. Med. Biol. 2013, 789, 185–191. [Google Scholar]
- Hamaoka, T.; Mccully, K.K. Muscle research work with britton chance from in vivo magnetic resonance spectroscopy to near-infrared spectroscopy. J. Innov. Opt. Health Sci. 2011, 4, 227–237. [Google Scholar] [CrossRef]
- Balaban, R.S.; Mootha, V.K.; Arai, A. Spectroscopic determination of cytochrome c oxidase content in tissues containing myoglobin or hemoglobin. Anal. Biochem. 1996, 237, 274–278. [Google Scholar] [CrossRef]
- Davis, M.L.; Barstow, T.J. Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir. Physiol. Neurobiol. 2013, 186, 180–187. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 15, 6–27. [Google Scholar] [CrossRef]
- Boas, D.; Franceschini, M. Near infrared imaging. Sch. J. 2009, 4, 6997. [Google Scholar] [CrossRef]
- Bakker, A.; Smith, B.; Ainslie, P.; Smith, K. Near-infrared spectroscopy. In Applied Aspects of Ultrasonography in Humans; Ainsile, P., Eds.; Intecopen: London, UK, 2012. [Google Scholar]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The missing link—Light induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009, 22, 31–44. [Google Scholar] [CrossRef]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef]
- Chance, B.; Maris, M.B.; Sorge, J.; Zhang, M.Z. Phase Modulation System for Dual Wavelength Difference Spectroscopy of Hemoglobin Deoxygenation in Tissues. In Time-Resolved Laser Spectroscopy in Biochemistry II, Proceedings of the Society of Photo-Optical Instrumentation Engineers Conference, Los Angeles, CA, USA, 1 May 1990; Lakowicz, J., Ed.; SPIE: Bellingham, WA, USA, 1990. [Google Scholar]
- Delpy, D.T.; Cope, M. Quantification in tissue near–infrared spectroscopy. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 649–659. [Google Scholar] [CrossRef]
- Chance, B.; Dait, M.T.; Zhang, C.; Hamaoka, T.; Hagerman, F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am. J. Physiol. 1992, 262, C766–C775. [Google Scholar] [CrossRef]
- Yazdi, S.; Mohammadi, S.; Lahade, S.; Beck, A.; Quang, T.; Lam, J.H.; O’Sullivan, T.D.; Green, M.M. Broad-Bandwidth Frequency-Domain Near-Infrared Spectroscopy System on a Chip. In Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables III, Proceedings of the Society of Photo-Optical Instrumentation Engineers Conference, San Francisco, CA, USA, 2 March 2022; SPIE: Bellingham, WA, USA, 2022. [Google Scholar]
- Chance, B. Optical method. Annu. Rev. Biophys. Chem. 1991, 20, 1–28. [Google Scholar] [CrossRef]
- Torricelli, A.; Quaresima, V.; Pifferi, A.; Biscotti, G.; Spinelli, L.; Taroni, P.; Ferrari, M.; Cubeddu, R. Mapping of calf muscle oxygenation and haemoglobin content during dynamic plantar flexion exercise by multi-channel time-resolved near-infrared spectroscopy. Phys. Med. Biol. 2004, 49, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pirovano, I.; Contini, D.; Spinelli, L.; Torricelli, A. Time domain near infrared spectroscopy device for monitoring muscle oxidative metabolism: Custom probe and in vivo applications. Sensors 2018, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Cope, M.; Gratton, E.; Ramanujam, N.; Tromberg, B. Phase measurement of light absorption and scatter in human tissue. Rev. Sci. Instrum. 1998, 69, 3457–3481. [Google Scholar]
- De Blasi, R.A.; Fantini, S.; Franceschini, M.A.; Ferrari, M.; Gratton, E. Cerebral and muscle oxygen saturation measurement by frequency-domain near-infra-red spectrometer. Med. Biol. Eng. Comput. 1995, 33, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Franceschini, M.A.; Maier, J.S.; Walker, S.A.; Barbieri, B.B.; Gratton, E. Frequency-domain multichannel optical detector for noninvasive tissue spectroscopy and oximetry. Organ Ethic. 1995, 34, 32–42. [Google Scholar] [CrossRef]
- Koga, S.; Rossiter, H.B.; Heinonen, I.; Musch, T.I.; Poole, D.C. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med. Sci. Sports Exerc. 2014, 46, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Mathieu-Costello, O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation 1996, 3, 175–186. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Grassi, B.; Pogliaghi, S.; Rampichini, S.; Quaresima, V.; Ferrari, M.; Marconi, C.; Cerretelli, P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J. Appl. Physiol. 2003, 95, 149–158. [Google Scholar] [CrossRef]
- Krustrup, P.; Jones, A.M.; Wilkerson, D.P.; Calbet, J.A.L.; Bangsbo, J. Muscular and pulmonary O2 uptake kinetics during moderateand high-intensity sub-maximal knee-extensor exercise in humans. J. Physiol. 2009, 587, 1843–1856. [Google Scholar] [CrossRef]
- Wagner, P.D. A theoretical analysis of factors determining VO2max at sea level and altitude. Respir. Physiol. 1996, 106, 329–343. [Google Scholar] [CrossRef]
- Wisen, A.G.M.; Wohlfart, B. Determination of both the time constant of vO2 and ΔvO2/Δw from a single incremental exercise test: Validation and repeatability. Clin. Physiol. Funct. Imaging 2004, 24, 257–265. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brophy, P.; Lin, C.T.; Hickner, R.C.; Neufer, P.D. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: A comparison with in situ measurements. J. Physiol. 2014, 592, 3231–3241. [Google Scholar] [CrossRef]
- Ryan, T.E.; Southern, W.M.; Reynolds, M.A.; McCully, K.K. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J. Appl. Physiol. 2013, 115, 1757–1766. [Google Scholar] [CrossRef]
- Ryan, T.E.; Erickson, M.L.; Verma, A.; Chavez, J.; Rivner, M.H.; Mccully, K.K. Skeletal muscle oxidative capacity in amyotrophic lateral sclerosis. Muscle Nerve 2014, 50, 767–774. [Google Scholar] [CrossRef]
- Hagen, T.M. Oxidative stress, redox imbalance, and the aging process. Antioxid. Redox Signal. 2003, 5, 503–506. [Google Scholar] [CrossRef]
- Richardson, R.S.; Poole, D.C.; Knight, D.R.; Kurdak, S.S.; Hogan, M.C.; Grassi, B.; Johnson, E.C.; Kendrick, K.F.; Erickson, B.K.; Wagner, P.D. High muscle blood flow in man: Is maximal O2 extraction compromised? J. Appl. Physiol. 1993, 75, 1911–1916. [Google Scholar] [CrossRef]
- DeLorey, D.S.; Paterson, D.H.; Kowalchuk, J.M. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 251–1262. [Google Scholar] [CrossRef]
- Proctor, D.N.; Koch, D.W.; Newcomer, S.W.; Le, K.U.; Leuenberger, U.A. Impaired leg vasodilation during dynamic exercise in healthy older women. J. Appl. Physiol. 2003, 95, 1963–1970. [Google Scholar] [CrossRef]
- Poole, J.G.; Lawrenson, L.; Kim, J.; Brown, C.; Richardson, R.S. Vascular and metabolic response to cycle exercise in sedentary humans: Effect of age. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1251–H1259. [Google Scholar] [CrossRef]
- Koch, D.W.; Newcomer, S.C.; Proctor, D.N. Blood flow to exercising limbs varies with age, gender, and training status. Can. J. Appl. Physiol. 2005, 30, 554–575. [Google Scholar] [CrossRef]
- Proctor, D.N.; Koch, D.W.; Newcomer, S.C.; Le, K.U.; Smithmyer, S.L.; Leuenberger, U.A. Leg blood flow and VO2 during peak cycle exercise in younger and older women. Med. Sci. Sports Exerc. 2004, 36, 623–631. [Google Scholar] [CrossRef]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef]
- Hearon, C.M., Jr.; Dinenno, F.A. Regulation of skeletal muscle blood flow during exercise in ageing humans. J. Physiol. 2016, 594, 2261–2273. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Groen, B.B.L.; Hamer, H.M.; Snijders, T.; Van Kranenburg, J.; Frijns, D.; Vink, H.; Van Loon, L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014, 116, 998–1005. [Google Scholar] [CrossRef]
- Coggan, A.R.; Spina, R.J.; King, D.S.; Rogers, M.A.; Brown, M.; Nemeth, P.M.; Holloszy, J.O. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J. Gerontol. 1992, 47, B71–B76. [Google Scholar] [CrossRef]
- Croley, A.N.; Zwetsloot, K.A.; Westerkamp, L.M.; Ryan, N.A.; Pendergast, A.M.; Hickner, R.C.; Pofahl, W.E.; Gavin, T.P. Lower capillarization, VEGF protein, and VEGF MRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J. Appl. Physiol. 2005, 99, 1872–1879. [Google Scholar] [CrossRef]
- Ryan, N.A.; Zwetsloot, K.A.; Westerkamp, L.M.; Hickner, R.C.; Pofahl, W.E.; Gavin, T.P. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J. Appl. Physiol. 2006, 100, 78–185. [Google Scholar] [CrossRef]
- Gavin, T.P.; Stallings, H.W., III; Zwetsloot, K.; Westerkamp, L.M.; Ryan, N.A.; Moore, R.A.; Pofahl, W.E.; Hickner, R.C. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J. Appl. Physiol. 2005, 98, 315–321. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-year longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Gries, K.J.; Raue, U.; Perkins, R.K.; Lavin, K.M.; Overstreet, B.S.; D’Acquisto, L.J.; Graham, B.; Finch, W.H.; Kaminsky, L.A.; Trappe, T.A.; et al. Cardiovascular and skeletal muscle health with lifelong exercise. J. Appl. Physiol. 2018, 125, 636–1645. [Google Scholar] [CrossRef]
- Staiculescu, M.C.; Foote, C.; Meininger, G.A.; Martinez-Lemus, L.A. The role of reactive oxygen species in microvascular remodeling. Int. J. Mol. Sci. 2014, 15, 23792–23835. [Google Scholar] [CrossRef] [PubMed]
- Zafari, A.M.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Shah, A.; Harrison, D.G.; Taylor, W.R.; Griendling, K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Juni, R.P.; Duckers, H.J.; Vanhoutte, P.; Virmani, R.; Moens, A.L. Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 2013, 61, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Jones, S.A.; O’Donnell, V.B.; Wood, J.D.; Broughton, J.P.; Hughes, E.J.; Jones, O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996, 271, H1626–H1634. [Google Scholar] [CrossRef]
- Damico, R.; Zulueta, J.J.; Hassoun, P.M. Pulmonary endothelial cell NOX. Am. J. Respir. Cell Mol. Biol. 2012, 47, 129–139. [Google Scholar] [CrossRef]
- Görlach, A.; Brandes, R.P.; Nguyen, K.; Amidi, M.; Dehghani, F.; Busse, R. A GP91PHOX containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000, 86, 26–32. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise and the aging endothelium. J. Diabetes Res. 2013, 2013, 789607. [Google Scholar] [CrossRef]
- An Introduction to Primary Endothelial Cells. Available online: https://bioscience.lonza.com/lonza_bs/US/en/endothelial-cells (accessed on 12 January 2022).
- Tesauro, M.; Mauriello, A.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Cardillo, C.; Melino, G.; Di Daniele, N. Arterial ageing: From endothelial dysfunction to vascular calcification. J. Intern. Med. 2017, 281, 471–482. [Google Scholar] [CrossRef]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef]
- Foreman, K.E.; Tang, J. Molecular mechanisms of replicative senescence in endothelial cells. Exp. Gerontol. 2003, 38, 1251–1257. [Google Scholar] [CrossRef]
- Matsushita, H.; Chang, E.; Glassford, A.J.; Cooke, J.P.; Chiu, C.P.; Tsao, P.S. eNOS activity is reduced in senescent human endothelial cells: Preservation by HTERT immortalization. Circ. Res. 2001, 89, 793–798. [Google Scholar] [CrossRef]
- Al-Qaisi, M.; Kharbanda, R.K.; Mittal, T.K.; Donald, A.E. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vasc. Health Risk Manag. 2008, 4, 647–652. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994, 24, 471–476. [Google Scholar] [CrossRef]
- Babcock, M.C.; DuBose, L.E.; Witten, T.L.; Brubaker, A.; Stauffer, B.L.; Hildreth, K.L.; Moreau, K.L. Assessment of macrovascular and microvascular function in aging males. J. Appl. Physiol. 2021, 130, 96–103. [Google Scholar] [CrossRef]
- Maruhashi, T.; Kajikawa, M.; Kishimoto, S.; Hashimoto, H.; Takaeko, Y.; Yamaji, T.; Harada, T.; Hashimoto, Y.; Han, Y.; Aibara, Y.; et al. Vascular function is further impaired in subjects aged 80 years or older. Hypertens. Res. 2020, 43, 914–921. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Cockcroft, J.R.; Webb, D.J. Pulse wave analysis and arterial stiffness. J. Cardiovasc. Pharmacol. 1998, 32, S33–S37. [Google Scholar]
- Tschudi, M.R.; Lüscher, T.F. Nitric oxide: The endogenous nitrate in the cardiovascular system. Herz 1996, 21, 50–60. [Google Scholar]
- Alp, N.J.; Channon, K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 413–420. [Google Scholar] [CrossRef]
- McNeill, E.; Channon, K.M. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb. Haemost. 2012, 108, 832–839. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Cohen, R.; Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/l-name (accessed on 14 January 2022).
- Schrage, W.G.; Eisenach, J.H.; Joyner, M.J. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J. Physiol. 2007, 579, 227–236. [Google Scholar] [CrossRef]
- Schrage, W.G.; Joyner, M.J.; Dinenno, F.A. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J. Physiol. 2004, 557, 599–611. [Google Scholar] [CrossRef]
- Feliciani, G.; Peron, C.; La Rocca, A.; Scuppa, M.F.; Malavolta, A.; Bianchini, D.; Corazza, I.; Zannoli, R. Cold pressor test using strain-gauge plethysmography. Adv. Physiol. Educ. 2016, 40, 410–417. [Google Scholar] [CrossRef][Green Version]
- Gerhard, M.; Roddy, M.A.; Creager, S.J.; Creager, M.A. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 1996, 27, 849–853. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of no availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Bui, T.; Duong, H. Muscarinic Agonists. StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31985923 (accessed on 1 February 2022).
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Wadsworth, R.; Stankevicius, E.; Simonsen, U. Physiologically relevant measurements of nitric oxide in cardiovascular research using electrochemical microsensors. J. Vasc. Res. 2006, 43, 70–85. [Google Scholar] [CrossRef]
- Nitric Oxide. Available online: https://www.britannica.com/science/nitric-oxide (accessed on 4 February 2022).
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Soltis, E.E. Effect of age on blood pressure and membrane-dependent vascular responses in the rat. Circ. Res. 1987, 61, 889–897. [Google Scholar] [CrossRef]
- Pie, J.E.; Baek, S.Y.; Kim, H.P.; Ryu, S.D.; Chung, W.G.; Cha, Y.N.; Park, C.S. Age-related decline of inducible nitric oxide synthase gene expression in primary cultured rat hepatocytes. Mol. Cells 2002, 13, 399–406. [Google Scholar]
- Zhou, X.J.; Vaziri, N.D.; Zhang, J.; Wang, H.W.; Wang, X.Q. Association of renal injury with nitric oxide deficiency in aged SHR: Prevention by hypertension control with AT1 blockade. Kidney Int. 2002, 62, 914–921. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Gouspillou, G.; Hepple, R.T. Mitochondria in Skeletal Muscle Health, Aging and Diseases; Frontiers Media SA: Lausanne, Switzerland, 2017. [Google Scholar]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Gaitanos, G.C.; Williams, C.; Boobis, L.H.; Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 1993, 75, 712–719. [Google Scholar] [CrossRef]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. 1999, 277, E890–E900. [Google Scholar] [CrossRef]
- Kemp, G.J.; Ahmad, R.E.; Nicolay, K.; Prompers, J.J. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: A quantitative review. Acta Physiol. 2015, 213, 107–144. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Conley, K.E.; Bajpeyi, S.; Punyanitya, M.; Gallagher, D.; Zhang, Z.; Covington, J.; Smith, S.R.; Ravussin, E. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J. Clin. Endocrinol. Metab. 2012, 97, 242–250. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef]
- Larsen, R.G.; Callahan, D.M.; Foulis, S.A.; Kent-Braun, J.A. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl. Physiol. Nutr. Metab. 2012, 37, 88–99. [Google Scholar] [CrossRef]
- Choi, S.; Reiter, D.A.; Shardell, M.; Simonsick, E.M.; Studenski, S.; Spencer, R.G.; Fishbein, K.W.; Ferrucci, L. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore longitudinal study of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1638–1645. [Google Scholar] [CrossRef]
- Adelnia, F.; Cameron, D.; Bergeron, C.M.; Fishbein, K.W.; Spencer, R.G.; Reiter, D.A.; Ferrucci, L. The role of muscle perfusion in the age-associated decline of mitochondrial function in healthy individuals. Front. Physiol. 2019, 10, 427. [Google Scholar] [CrossRef]
- Porter, C.; Hurren, N.M.; Cotter, M.V.; Bhattarai, N.; Reidy, P.T.; Dillon, E.L.; Durham, W.J.; Tuvdendorj, D.; Sheffield-Moore, M.; Volpi, E.; et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E224–E232. [Google Scholar] [CrossRef]
- Distefano, G.; Standley, R.A.; Zhang, X.; Carnero, E.A.; Yi, F.; Cornnell, H.H.; Coen, P.M. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Haseler, L.J.; Richardson, R.S. Reduced muscle oxidative capacity is independent of O2 availability in elderly people. Age 2013, 35, 1183–1192. [Google Scholar] [CrossRef]
- Hepple, R.T. Mitochondrial involvement and impact in aging skeletal muscle. Front. Aging Neurosci. 2014, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J. Appl. Physiol. 2008, 105, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Brightwell, C.R.; Phalen, D.E.; McKenna, C.F.; Lane, S.J.; Porter, C.; Volpi, E.; Rasmussen, B.B.; Fry, C.S. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp. Gerontol. 2019, 127, 110723. [Google Scholar] [CrossRef]
- Mammucari, C.; Rizzuto, R. Signaling pathways in mitochondrial dysfunction and aging. Mech. Ageing Dev. 2010, 131, 536–543. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Thompson, L.V. Age-related muscle dysfunction. Exp. Gerontol. 2009, 44, 106–111. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, S.S.; Zelenski, K.N.; Perkins, R.K. Age-Related Changes in Skeletal Muscle Oxygen Utilization. J. Funct. Morphol. Kinesiol. 2022, 7, 87. https://doi.org/10.3390/jfmk7040087
Salvatore SS, Zelenski KN, Perkins RK. Age-Related Changes in Skeletal Muscle Oxygen Utilization. Journal of Functional Morphology and Kinesiology. 2022; 7(4):87. https://doi.org/10.3390/jfmk7040087
Chicago/Turabian StyleSalvatore, Sabrina S., Kyle N. Zelenski, and Ryan K. Perkins. 2022. "Age-Related Changes in Skeletal Muscle Oxygen Utilization" Journal of Functional Morphology and Kinesiology 7, no. 4: 87. https://doi.org/10.3390/jfmk7040087
APA StyleSalvatore, S. S., Zelenski, K. N., & Perkins, R. K. (2022). Age-Related Changes in Skeletal Muscle Oxygen Utilization. Journal of Functional Morphology and Kinesiology, 7(4), 87. https://doi.org/10.3390/jfmk7040087





