Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characteristics of Subjects
2.2. Histochemical Research
2.3. Immunohistochemical (IHC) Analysis
2.4. Statistical Analysis
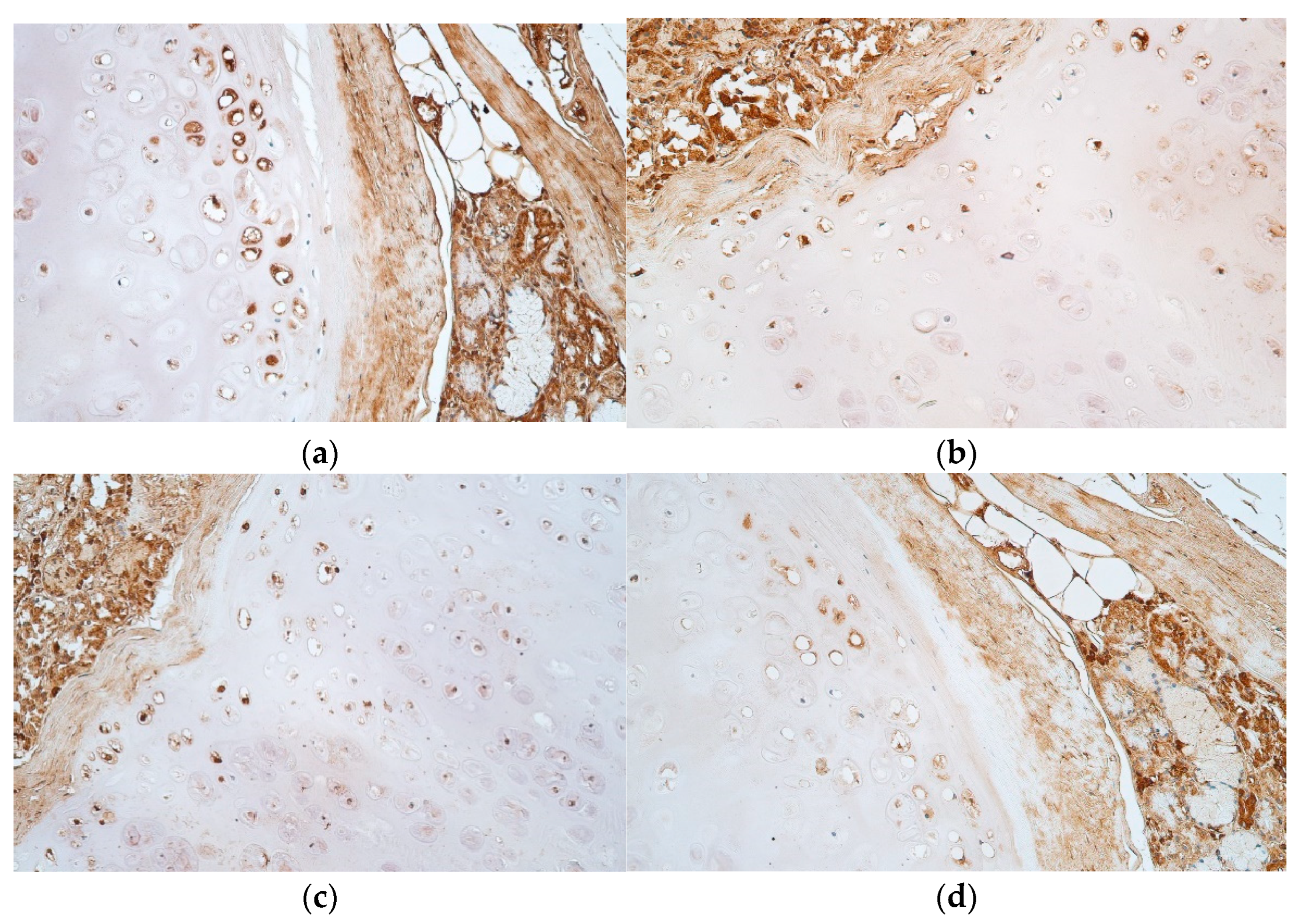
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brand-Saberi, B.; Schäfer, T. Trachea: Anatomy and physiology. Thorac. Surg. Clin. 2014, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadi, M.O.; Artenstein, A.W.; Braman, S.S. The “forgotten zone”: Acquired disorders of the trachea in adults. Respir. Med. 2013, 107, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Tsitouras, K.; Paderewska, J.; Munker, D.; Walcher, S.; Neurohr, C.; Kneidinger, N. Tracheal stenosis in prolonged mechanically ventilated patients: Prevalence, risk factors, and bronchoscopic management. BMC Pulm. Med. 2022, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Schibilsky, D.; Driessen, A.; White, W.J.; Lefering, R.; Paffrath, T.; Bouillon, B.; Walker, T.; Schlensak, C.; Mutschler, M. Traumatic tracheobronchial injuries: Incidence and outcome of 136.389 patients derived from the DGU traumaregister. Sci. Rep. 2020, 10, 20555. [Google Scholar] [CrossRef] [PubMed]
- Etienne, H.; Fabre, D.; Gomez Caro, A.; Kolb, F.; Mussot, S.; Mercier, O.; Mitilian, D.; Stephan, F.; Fadel, E.; Dartevelle, P. Tracheal replacement. Eur. Respir. J. 2018, 51, 1702211. [Google Scholar] [CrossRef]
- Ferreirinha, J.; Caviezel, C.; Weder, W.; Opitz, I.; Inci, I. Postoperative outcome of tracheal resection in benign and malignant tracheal stenosis. Swiss. Med. Wkly. 2020, 150, w20383. [Google Scholar] [CrossRef]
- Haykal, S.; Salna, M.; Waddell, T.K.; Hofer, S.O. Advances in tracheal reconstruction. Plast. Reconstr. Surg. Glob. Open 2014, 2, e178. [Google Scholar] [CrossRef]
- Damiano, G.; Palumbo, V.D.; Fazzotta, S.; Curione, F.; Lo Monte, G.; Brucato, V.; Lo Monte, A.I. Current Strategies for Tracheal Replacement: A Review. Life 2021, 11, 618. [Google Scholar] [CrossRef]
- Mescher, A.L. (Ed.) Cartilage. In Junqueira’s Basic Histology Text and Atlas, 16th ed.; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Rains, J.K.; Bert, J.L.; Roberts, C.R.; Paré, P.D. Mechanical properties of human tracheal cartilage. J. Appl. Physiol. 1992, 72, 219–225. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef]
- Binette, J.P.; Burgi, W.; Ohishi, H.; Grundboeck-Jusko, J.; Burki, R.; Maekawa, Y.; Tschopp, F.A.; Kimura, A.; Schmid, K. The glycosaminoglycan composition of human tracheas and the changes observed during aging and in disease. Clin. Chim. Acta 1994, 225, 179–185. [Google Scholar] [CrossRef]
- Roberts, C.R.; Paré, P.D. Composition changes in human tracheal cartilage in growth and aging, including changes in proteoglycan structure. Am. J. Physiol. 1991, 261 Pt 1, L92–L101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wood, L.D.; Richardson, J.B.; Roberts, S.; Kuiper, N.J. Glycosaminoglycan profiles of repair tissue formed following autologous chondrocyte implantation differ from control cartilage. Arthritis Res. Ther. 2007, 9, R79. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, R.; Scott, J. Distribution of Acid Glycosaminoglycans in Human Articular Cartilage. Nature 1967, 215, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Riedler, K.L.; Shokrani, A.; Markarian, A.; Fisher, L.M.; Pepper, J.P. Age-related histologic and biochemical changes in auricular and septal cartilage. Laryngoscope 2017, 127, E399–E407. [Google Scholar] [CrossRef]
- Jansen, N.W.; Roosendaal, G.; Hooiveld, M.J.; Bijlsma, J.W.; van Roon, J.A.; Theobald, M.; Lafeber, F.P. Interleukin-10 protects against blood-induced joint damage. Br. J. Haematol. 2008, 142, 953–961. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin. Med. J. 2001, 114, 723–725. [Google Scholar]
- Olvera, D.P.R.; Gutiérrez, C.C. Multifunctional Activity of the β-Defensin-2 during Respiratory Infections. In Immune Response Activation and Immunomodulation; Tyagi, R.K., Bisen, P.S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Machado, L.R.; Ottolini, B. An evolutionary history of defensins: A role for copy number variation in maximizing host innate and adaptive immune responses. Front. Immunol. 2015, 6, 115. [Google Scholar] [CrossRef]
- Harimurti, K.; Djauzi, S.; Witarto, A.B.; Dewiasty, E. Human β-defensin 2 concentration of respiratory tract mucosa in elderly patients with pneumonia and its associated factors. Acta Med. Indones. 2011, 43, 218–223. [Google Scholar]
- Liu, S.; He, L.R.; Wang, W.; Wang, G.H.; He, Z.Y. Prognostic value of plasma human β-defensin 2 level on short-term clinical outcomes in patients with community-acquired pneumonia: A preliminary study. Respir. Care 2013, 58, 655–661. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Ogawa, H. The role of human β-defensins in allergic diseases. Clin. Exp. Allergy 2016, 46, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Jo, E.K. Antimicrobial Peptides in Innate Immunity against Mycobacteria. Immune Netw. 2011, 11, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, L.; Yuan, Q.; Gu, P.; You, Z.; Zhuang, A.; Bi, X. Effect of bifunctional beta defensin 2- modified scaffold on bone defect re-construction. ACS Omega 2020, 5, 4302–4312. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, J.; Zou, Z.; Yan, G.; Dong, S.; Deng, J.; Ran, X.; Feng, Y.; Luo, C.; Wang, Y.; et al. Transplantation of BMSCs expressing hPDGF-A/HBD-2 promotes wound healing in rats with combined radiation-wound injury. Gene Ther. 2009, 16, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Varoga, D.; Paulsen, F.P.; Kohrs, S.; Grohmann, S.; Lippross, S.; Mentlein, R.; Tillmann, B.N.; Goldring, M.B.; Besch, L.; Pufe, T. Expression and regulation of human beta-defensin-2 in osteoarthritic cartilage. J. Pathol. 2006, 209, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Varoga, D.; Klostermeier, E.; Paulsen, F.; Wruck, C.; Lippross, S.; Brandenburg, L.O.; Tohidnezhad, M.; Seekamp, A.; Tillmann, B.; Pufe, T. The antimicrobial peptide HBD-2 and the Toll-like receptors-2 and -4 are induced in synovial membranes in case of septic arthritis. Virchows Arch. 2009, 454, 685–694. [Google Scholar] [CrossRef] [PubMed]
- McGlasson, S.L.; Semple, F.; MacPherson, H.; Gray, M.; Davidson, D.J.; Dorin, J.R. Human β-defensin 3 increases the TLR9-dependent response to bacterial DNA. Eur. J. Immunol. 2017, 47, 658–664. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 2006, 1758, 1499–1512. [Google Scholar] [CrossRef]
- Ferris, L.K.; Mburu, Y.K.; Mathers, A.R.; Fluharty, E.R.; Larregina, A.T.; Ferris, R.L.; Falo, L.D., Jr. Human beta-defensin 3 induces maturation of human langerhans cell-like dendritic cells: An antimicrobial peptide that functions as an endogenous adjuvant. J. Investig. Dermatol. 2013, 133, 460–468. [Google Scholar] [CrossRef]
- Bedran, T.B.; Mayer, M.P.; Spolidorio, D.P.; Grenier, D. Synergistic anti-inflammatory activity of the antimicrobial peptides human beta-defensin-3 (HBD-3) and cathelicidin (LL-37) in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. PLoS ONE 2014, 9, e106766. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, B.; Liao, C.; Zhang, W.; Wang, W.; Chang, Y.; Shao, Y. Human beta-defensin 3 contributes to the carcinogenesis of cervical cancer via activation of NF-κB signaling. Oncotarget 2016, 7, 75902–75913. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Neumann, H.M.; Brand, S.; Schrader, H.; Schmidt, W.E.; Schmitz, F. Expression of beta-defensin 4 is increased in human gastritis. Eur. J. Clin. Investig. 2009, 39, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Smiley, A.K.; Gardner, J.; Klingenberg, J.M.; Neely, A.N.; Supp, D.M. Expression of human beta defensin 4 in genetically modified keratinocytes enhances antimicrobial activity. J. Burn Care Res. 2007, 28, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, D.; Yang, J.; Wang, P.; Han, B.; Zhao, L.; Jepsen, S.; Dommisch, H.; Winter, J.; Xu, Y. The expression of human β-defensins (HBD-1, HBD-2, HBD-3, HBD-4) in gingival epithelia. Arch. Oral Biol. 2016, 66, 15–21. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1077. [Google Scholar] [CrossRef]
- Musumeci, G.; Carnazza, M.L.; Loreto, C.; Leonardi, R.; Loreto, C. β-Defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem. 2012, 114, 805–812. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Deng, Y.; Tsao, B.P. Genetics of Human SLE. In Dubois’ Lupus Erythematosus and Related Syndromes, 8th ed.; Wallace, D.J., Hahn, B.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 35–40. [Google Scholar]
- Wong, H.R.; Nowak, J.E.; Standage, S.W.; Oliveira, C.F. Sepsis and septic shock. In Pediatric Critical Care, 4th ed.; Fuhrman, B., Zimmerman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1413–1429. [Google Scholar]
- Agier, J.; Efenberger, M.; Brzezińska-Błaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczyńska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. BioFactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Tomchuck, S.L.; Zwezdaryk, K.J.; Danka, E.S.; Scandurro, A.B. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol. Cancer Res. 2009, 7, 907–915. [Google Scholar] [CrossRef]
- Weber, G.; Chamorro, C.I.; Granath, F.; Liljegren, A.; Zreika, S.; Saidak, Z.; Sandstedt, B.; Rotstein, S.; Mentaverri, R.; Sánchez, F.; et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. BCR 2009, 11, R6. [Google Scholar] [CrossRef]
- Büchau, A.S.; Morizane, S.; Trowbridge, J.; Schauber, J.; Kotol, P.; Bui, J.D.; Gallo, R.L. The host defense peptide cathelicidin is required for NK cell-mediated suppression of tumor growth. J. Immunol. 2010, 184, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch. Biochem. 1948, 16, 131–141. [Google Scholar]
- McManus, J.F. The periodic acid routing applied to the kidney. Am. J. Pathol. 1948, 24, 643–653. [Google Scholar]
- Cook, H.C. Human Tissue Mucins; Laboratory Aid Series; Butterworths: London, UK, 1972. [Google Scholar]
- Spatz, M. Bismarck brown as a selective stain for mast cells. Tech. Bull. Regist. Med. Technol. 1960, 30, 141–143. [Google Scholar] [CrossRef]
- Cohen, A.H. Masson’s trichrome stain in the evaluation of renal biopsies. An appraisal. Am. J. Clin. Pathol. 1976, 65, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Guesdon, J.L.; Ternynck, T.; Avrameas, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cyto-Chem. 1979, 27, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Pilmane, M.; Sidhoma, E.; Akota, I.; Kazoka, D. Characterization of Cytokines and Proliferation Marker Ki67 in Cleft Affected Lip Tissue. Medicina 2019, 55, 518. [Google Scholar] [CrossRef]
- Ferringer, T.; Ko, C.J. The basics: Diagnostic terms, skin anatomy, and stains. In Dermatopathology, 3rd ed.; Elston, D.M., Ferringer, T., Ko, C.J., Peckham, S., High, W.A., DiCaudo, D.J., Bhuta, S., Eds.; Elsevier Limited: Amsterdam, The Netherlands, 2019; Chapter 1; pp. 1–35. [Google Scholar]
- Moog, F.; Wenger, E.L. The occurrence of a neutral mucopolysaccharide at sites of high alkaline phosphatase activity. Am. J. Anat. 1952, 90, 339–377. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.R.; Davidson, D.J.; Dorin, J.R. The Dichotomous Responses Driven by β-Defensins. Front. Immunol. 2020, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Man, W.; de Steenhuijsen Piters, W.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tiriveedhi, V.; Banan, B.; Deepti, S.; Nataraju, A.; Hachem, R.; Trulock, E.; Alexander, P.G.; Thalachallour, M. Role of defensins in the pathogenesis of chronic lung allograft rejection. Hum. Immunol. 2014, 75, 370–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef]
- Semple, F.; Webb, S.; Li, H.N.; Patel, H.B.; Perretti, M.; Jackson, I.J.; Gray, M.; Davidson, D.J.; Dorin, J.R. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 2010, 40, 1073–1078. [Google Scholar] [CrossRef]
- Sheng, Q.; Lv, Z.; Cai, W.; Song, H.; Qian, L.; Mu, H.; Shi, J.; Wang, X. Human β-defensin-3 promotes intestinal epithelial cell migration and reduces the development of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2014, 76, 269–279. [Google Scholar] [CrossRef]
- García, J.R.; Krause, A.; Schulz, S.; Rodríguez-Jiménez, F.J.; Klüver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human beta-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001, 15, 1819–1821. [Google Scholar] [CrossRef]
- Yanagi, S.; Ashitani, J.; Ishimoto, H.; Date, Y.; Mukae, H.; Chino, N.; Nakazato, M. Isolation of human beta-defensin-4 in lung tissue and its increase in lower respiratory tract infection. Respir. Res. 2005, 6, 130. [Google Scholar] [CrossRef]
- Al-Bayatee, N.T.; Ad’hiah, A.H. Human beta-defensins 2 and 4 are dysregulated in patients with coronavirus disease 19. Microb. Pathog. 2021, 160, 105205. [Google Scholar] [CrossRef]
- Huang, L.C.; Petkova, T.D.; Reins, R.Y.; Proske, R.J.; McDermott, A.M. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Hiemstra, P.S.; Ward, C.; Forrest, I.A.; Murphy, D.; Proud, D.; Lordan, J.; Corris, P.A.; Fisher, A.J. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Eur. Respir. J. 2008, 32, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ashoor, G.A.; Shamma, T.; Alanazi, F.; Altuhami, A.; Kazmi, S.; Ahmed, H.A.; Mohammed Assiri, A.; Clemens Broering, D. IL-10 Mediated Immunomodulation Limits Subepithelial Fibrosis and Repairs Airway Epithelium in Rejecting Airway Allografts. Cells 2021, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Van Meegeren, M.E.; Roosendaal, G.; Jansen, N.W.; Wenting, M.J.; van Wesel, A.C.; van Roon, J.A.; Lafeber, F.P. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthr. Cartil. 2012, 20, 764–772. [Google Scholar] [CrossRef]
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef]
- Waly, N.E.; Refaiy, A.; Aborehab, N.M. IL-10 and TGF-β: Roles in chondroprotective effects of Glucosamine in experimental Osteoarthritis? Pathophysiology 2017, 24, 45–49. [Google Scholar] [CrossRef]
- John, T.; Müller, R.D.; Oberholzer, A.; Zreiqat, H.; Kohl, B.; Ertel, W.; Hostmann, A.; Tschoeke, S.K.; Schulze-Tanzil, G. Interleukin-10 modulates pro-apoptotic effects of TNF-alpha in human articular chondrocytes in vitro. Cytokine 2007, 40, 226–234. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- Barańska-Rybak, W.; Sonesson, A.; Nowicki, R.; Schmidtchen, A. Glycosaminoglycans inhibit the antibacterial activity of LL-37 in biological fluids. J. Antimicrob. Chemother. 2006, 57, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Reeves, E.P.; McNally, P.; Chotirmall, S.H.; Greene, C.M.; Greally, P.; Murphy, P.; O’Neill, S.J.; McElvaney, N.G. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 2009, 183, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Savage, J.R.; Zhang, J.; Jia, W.; Oottamasathien, S.; Prestwich, G.D. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan. PLoS ONE 2013, 8, e77854. [Google Scholar] [CrossRef]
- Varoga, D.; Pufe, T.; Harder, J.; Schröder, J.M.; Mentlein, R.; Meyer-Hoffert, U.; Goldring, M.B.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Human beta-defensin 3 mediates tissue remodeling processes in articular cartilage by increasing levels of metallopro-teinases and reducing levels of their endogenous inhibitors. Arthritis Rheum. 2005, 52, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Balode, E.; Pilmane, M. Characteristics of Neuropeptide-Containing Innervation, Tissue Remodeling, Growth, and Vascularity in Noses of Patients With Cleft Lip and Palate. Cleft Palate Craniofac. J. 2020, 57, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Russo, P.A.; Hopfenziz, M.; Kurz, B.; Becker, S.T.; Sherry, E.; Springer, I.; Sivananthan, S. Antimicrobial Peptide immunity protects human nasal and auricular cartilage against infection. J. Craniofac. Surg. 2010, 21, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Acevedo Rua, L.; Mumme, M.; Manferdini, C.; Darwiche, S.; Khalil, A.; Hilpert, M.; Buchner, D.A.; Lisignoli, G.; Occhetta, P.; von Rechenberg, B.; et al. Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Sci. Transl. Med. 2021, 13, eaaz4499. [Google Scholar] [CrossRef]
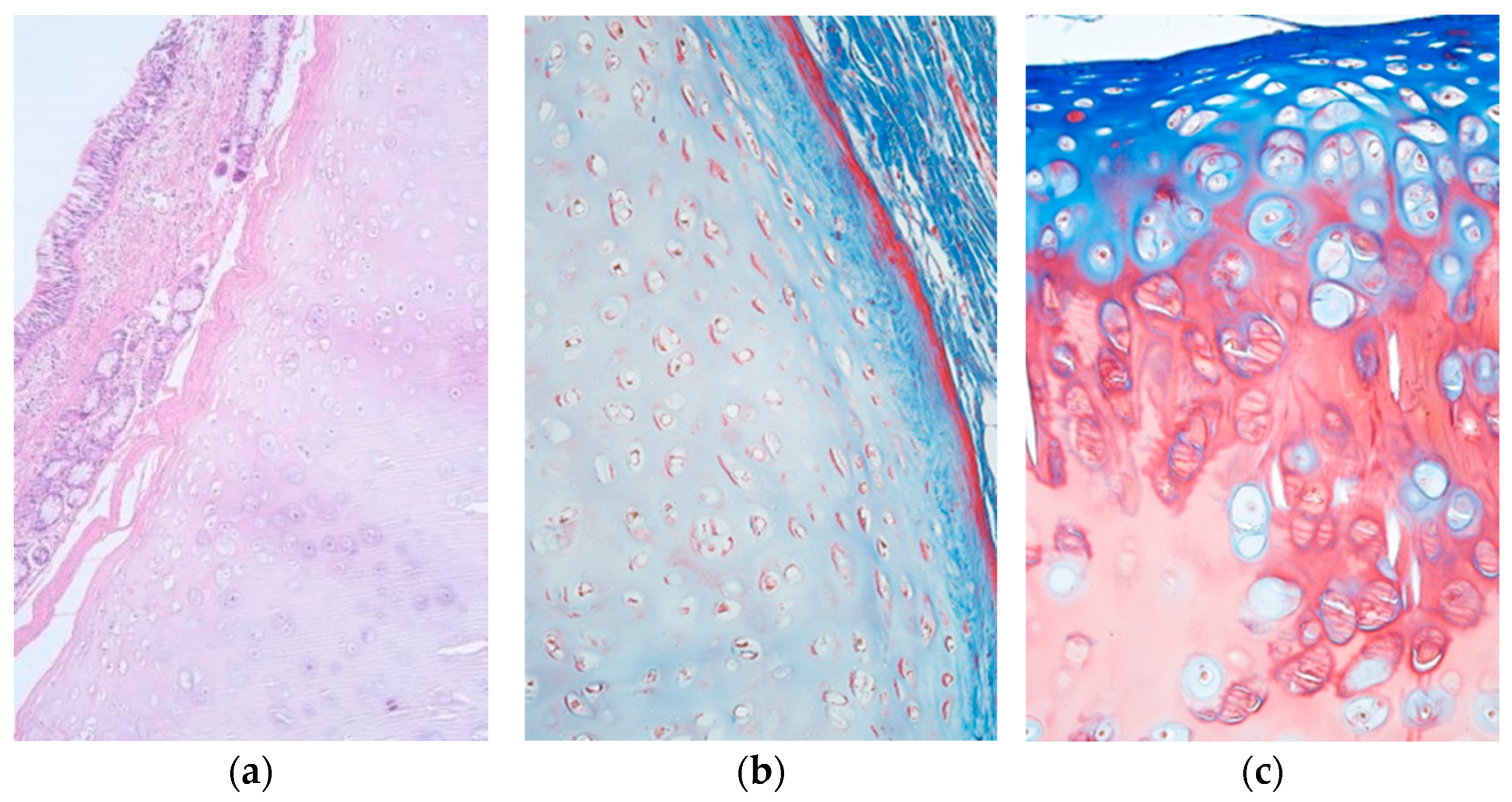
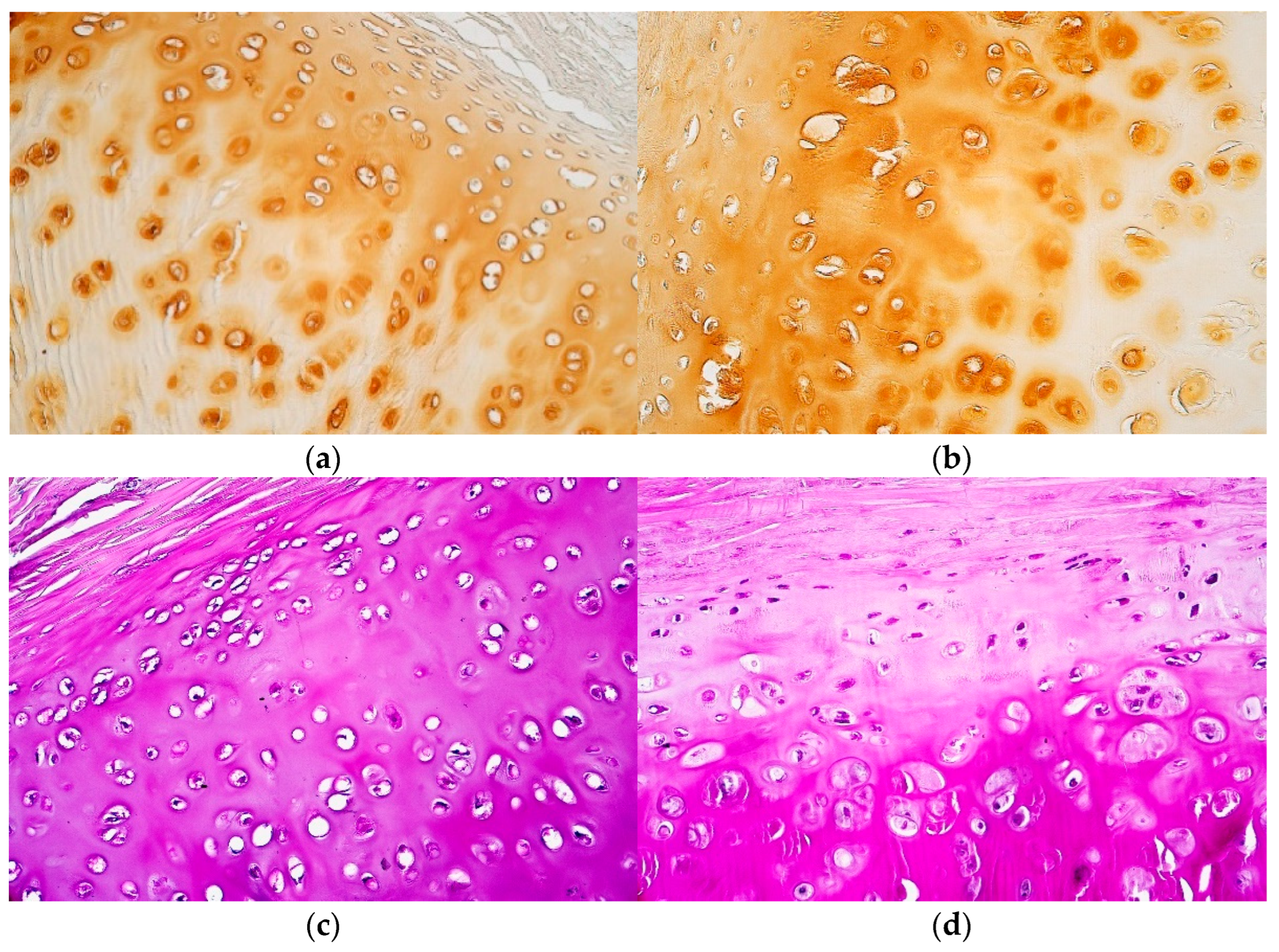
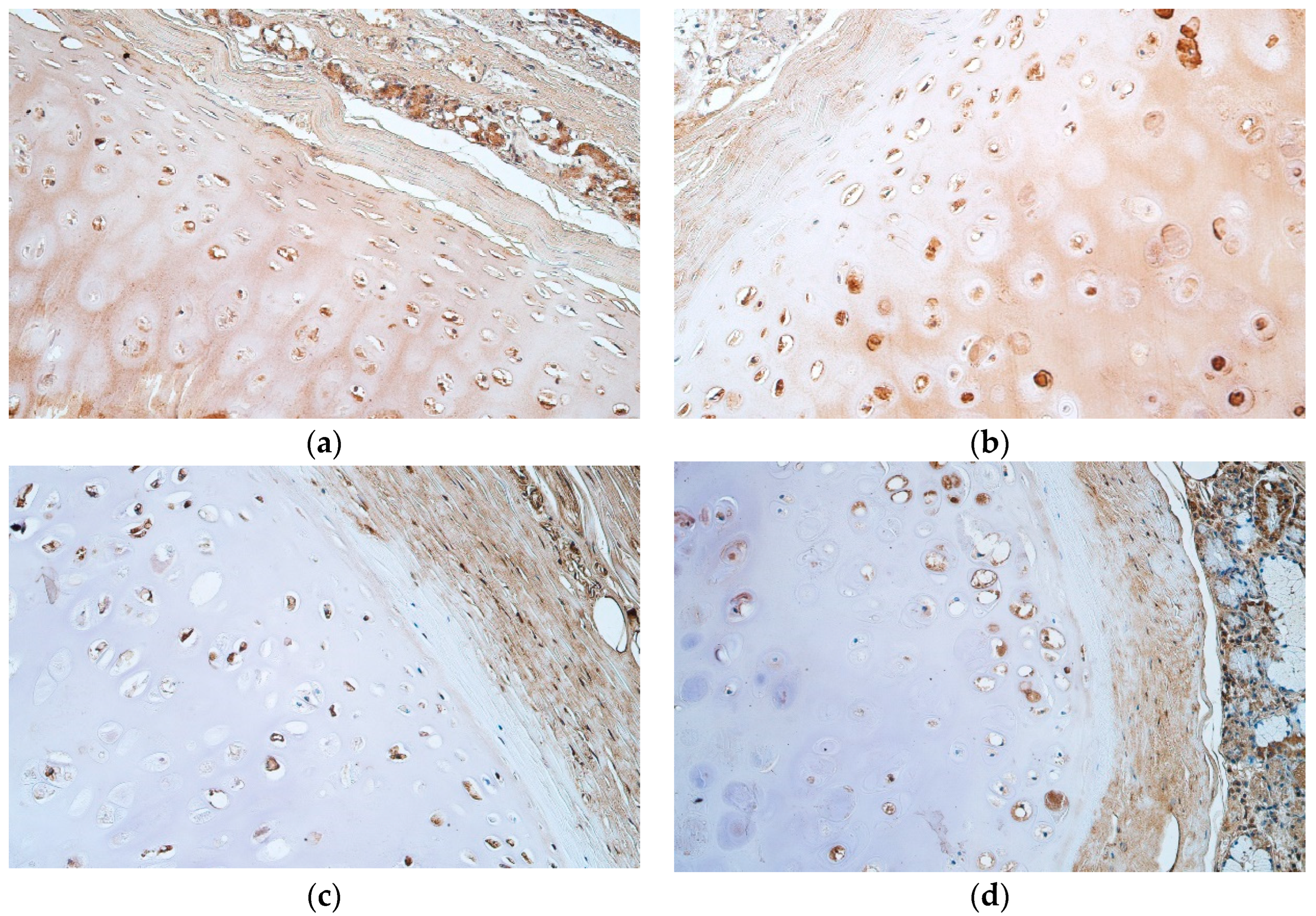
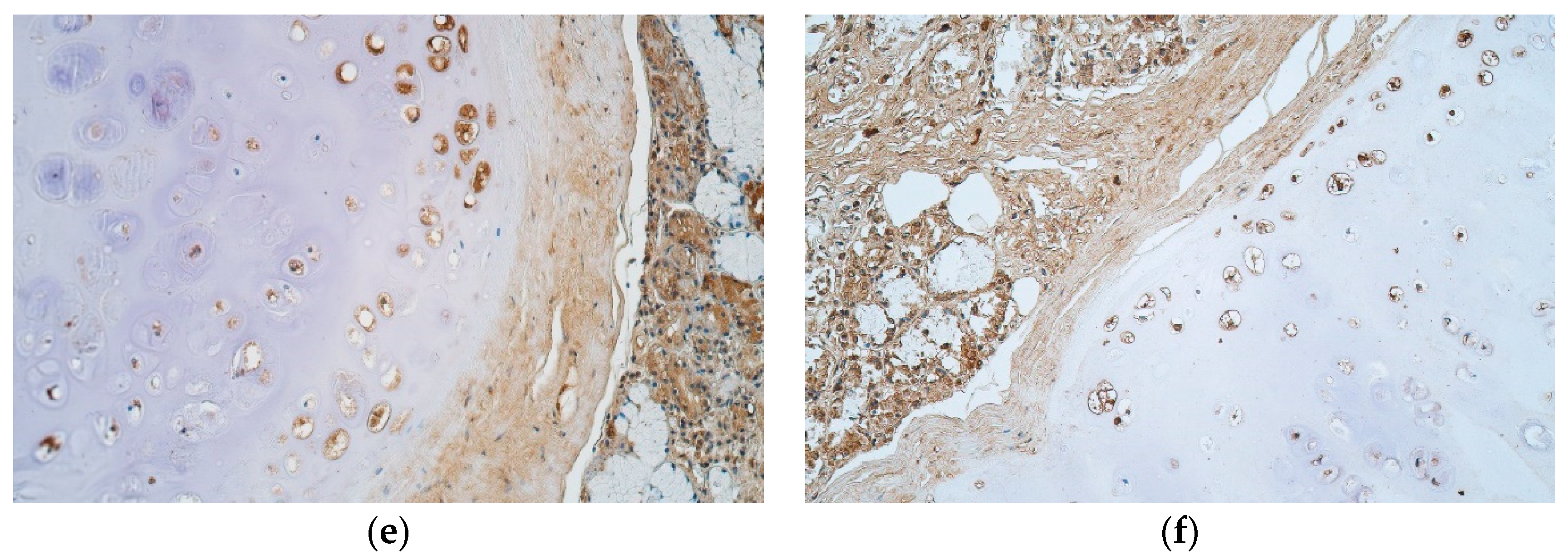
| Patient | Cartilage Nr. | Part of Cartilage | BB | PAS | ||||
|---|---|---|---|---|---|---|---|---|
| PZ | GZ | MCZ | PZ | GZ | MCZ | |||
| 1 | 1 | A | + | + | - | ± | ± | + |
| 2 | A | + | + | - | ± | ± | + | |
| 3 | A | + | + | - | + | + | + | |
| B | + | + | - | + | + | + | ||
| C | + | + | - | - | ± | + | ||
| 2 | 1 | A | + | + | ± | + | + | + |
| C | + | + | - | + | + | + | ||
| 3 | A | + | + | ± | + | + | + | |
| 3 | 1 | A | + | + | - | - | ± | + |
| 3 | A | + | + | - | + | + | + | |
| B | + | + | ± | + | + | + | ||
| 4 | 1 | A | + | + | ± | + | + | + |
| C | + | + | ± | + | + | + | ||
| 5 | 1 | A | + | + | - | + | + | + |
| B | + | + | - | + | + | + | ||
| 2 | A | + | + | - | + | + | + | |
| 6 | 3 | A | + | + | + | + | + | + |
| 7 | 2 | A | + | + | + | ± | ± | + |
| B | + | + | + | + | + | ± | ||
| 8 | 1 | A | + | + | - | ± | ± | ± |
| B | + | + | ± | ± | ± | + | ||
| 9 | 1 | A | + | + | - | + | + | + |
| 2 | A | + | + | - | ± | ± | + | |
| B | + | + | - | ± | ± | + | ||
| 10 | 1 | A | + | + | - | ± | ± | + |
| B | + | + | - | + | + | + | ||
| C | + | + | - | + | + | + | ||
| Median value | + | + | - | + | + | + | ||
| Patient | Cartilage Nr. | Part of Cartilage | BB | PAS | ||||
|---|---|---|---|---|---|---|---|---|
| PZ | GZ | MCZ | PZ | GZ | MCZ | |||
| 1 | 1 | A | +++ | +++ | +++ | + | ++ | ++++ |
| 2 | A | +++ | +++ | +++ | +++ | +++ | ++++ | |
| 3 | A | +++ | +++ | ++++ | +++ | +++ | +++ | |
| B | ++ | +++ | +++ | +++ | +++ | +++ | ||
| C | ++/+++ | +++ | +++ | +++ | +++/++++ | +++ | ||
| 2 | 1 | A | +++ | ++++ | ++++ | +++ | +++ | +++ |
| C | +++ | ++++ | ++++ | ++++ | ++++ | ++++ | ||
| 3 | A | ++++ | ++++ | ++++ | +++ | +++ | +++ | |
| 3 | 1 | A | ++/+++ | ++++ | +++ | ++ | ++ | ++/+++ |
| 3 | A | ++/+++ | +++ | ++/+++ | ++ | ++ | ++/+++ | |
| B | +++/++++ | ++++ | +++/++++ | +++ | +++ | +++ | ||
| 4 | 1 | A | +++ | +++ | +++ | +++ | +++ | +++ |
| C | +++ | +++ | +++ | +++ | +++ | +++ | ||
| 5 | 1 | A | +++ | +++ | +++ | +++ | +++ | +++/++++ |
| B | ++/+++ | +++ | ++++ | +++ | ++++ | ++++ | ||
| 2 | A | ++/+++ | +++ | +++ | +++ | +++ | ++++ | |
| 6 | 3 | A | +++ | +++ | +++ | +++ | +++ | +++ |
| 7 | 2 | A | +++ | +++ | +++ | +++ | +++ | +++ |
| B | ++ | ++ | ++ | +++ | +++ | +++ | ||
| 8 | 1 | A | +++ | +++ | +++/++++ | +++ | +++ | +++ |
| B | +++ | +++ | +++ | +++ | +++ | +++ | ||
| 9 | 1 | A | +++ | +++ | +++ | ++ | +++ | +++ |
| 2 | A | ++ | +++ | +++ | +++ | +++ | +++ | |
| B | +++ | ++++ | ++++ | ++ | ++ | +++ | ||
| 10 | 1 | A | +++ | +++ | +++ | ++ | +++ | +++ |
| B | +++ | +++/++++ | +++ | +++ | +++ | +++ | ||
| C | +++ | +++ | +++ | +++ | +++ | +++ | ||
| Median value | +++ | +++ | +++ | +++ | +++ | +++ | ||
| Patient | Cartilage Nr. | Part of Cartilage | HBD-2 | HBD-3 | HBD-4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PZ | GZ | MCZ | PZ | GZ | MCZ | PZ | GZ | MCZ | |||
| 1 | 1 | A | +/++ | ++ | ++ | +/++ | ++ | 0/+ | ++ | ++ | + |
| 2 | A | ++/+++ | +++/++++ | ++ | ++ | ++ | + | ++ | ++ | + | |
| 3 | A | ++ | ++/+++ | ++ | + | ++ | 0/+ | +/++ | ++ | + | |
| B | ++ | ++ | ++ | ++ | ++ | + | + | +/++ | 0/+ | ||
| C | ++/+++ | ++/+++ | ++ | +/++ | ++ | 0/+ | 0/+ | +/++ | 0/+ | ||
| 2 | 1 | A | ++ | ++/+++ | ++/+++ | ++/+++ | +++/++++ | ++ | + | ++ | ++ |
| C | ++ | ++ | ++ | ++ | +++ | +/++ | + | +/++ | +/++ | ||
| 3 | A | ++ | ++/+++ | ++/+++ | +/++ | +++ | +/++ | +/++ | ++ | ++ | |
| 3 | 1 | A | ++ | ++/+++ | ++ | +/++ | +/++ | 0/+ | +/++ | ++ | 0/+ |
| 3 | A | ++/+++ | ++/+++ | ++ | ++ | ++ | + | ++ | ++ | + | |
| B | ++ | +++ | ++/+++ | ++ | ++ | 0/+ | +/++ | ++ | + | ||
| 4 | 1 | A | ++ | ++ | ++ | +/++ | ++ | + | + | +/++ | + |
| C | ++ | ++ | ++ | + | +/++ | + | + | +/++ | + | ||
| 5 | 1 | A | N | N | N | + | + | 0/+ | +/++ | ++ | + |
| B | ++ | ++/+++ | ++ | +/++ | ++ | + | +/++ | ++ | + | ||
| 2 | A | +/++ | ++ | +/++ | 0/+ | + | 0 | +/++ | +/++ | + | |
| 6 | 3 | A | ++/+++ | ++/+++ | ++ | + | + | 0 | +/++ | +/++ | 0/+ |
| 7 | 2 | A | +++ | +++/++++ | +++ | +/++ | ++ | + | ++ | ++ | +/++ |
| B | +++ | +++ | +++ | ++ | ++ | + | +/++ | ++ | +/++ | ||
| 8 | 1 | A | ++/+++ | ++/+++ | ++ | + | +/++ | 0 | +/++ | +/++ | + |
| B | +/++ | ++ | +/++ | + | +/++ | + | +/++ | +/++ | 0/+ | ||
| 9 | 1 | A | ++ | ++ | +/++ | ++ | ++/+++ | + | ++ | ++/+++ | + |
| 2 | A | ++ | ++ | +/++ | ++ | ++ | + | ++ | ++ | + | |
| B | ++ | ++/+++ | ++ | N | N | N | ++ | ++/+++ | + | ||
| 10 | 1 | A | +/++ | ++ | ++ | +/++ | +/++ | 0/+ | + | + | 0/+ |
| B | +/++ | ++ | ++ | +/++ | +/++ | 0/+ | +/++ | + | 0/+ | ||
| C | ++ | ++/+++ | ++ | ++ | + | 0/+ | ++ | +/++ | 0/+ | ||
| Median value | ++ | ++/+++ | ++ | +/++ | ++ | + | +/++ | ++ | + | ||
| Patient | Cartilage Nr. | Part of Cartilage | IL-10 | LL-37 | ||||
|---|---|---|---|---|---|---|---|---|
| PZ | GZ | MCZ | PZ | GZ | MCZ | |||
| 1 | 1 | A | +/++ | ++ | 0 | ++ | ++ | + |
| 2 | A | ++ | + | 0/+ | ++ | ++ | 00/+ | |
| 3 | A | +/++ | ++ | +/++ | ++ | ++ | 0 | |
| B | + | ++ | + | +/++ | ++ | 00/+ | ||
| C | + | ++ | +/++ | + | +/++ | 0 | ||
| 2 | 1 | A | +/++ | ++ | ++ | +/++ | ++/+++ | ++ |
| A | +/++ | ++ | ++ | +/++ | +/++ | + | ||
| 3 | B | + | ++ | +/++ | ++ | +++ | +/++ | |
| 3 | 1 | A | +/++ | + | 0/+ | ++ | ++ | 00/+ |
| 3 | A | + | + | 0 | ++ | ++ | 0/+ | |
| B | +/++ | ++ | + | ++ | ++/+++ | 0/+ | ||
| 4 | 1 | A | +/++ | ++ | + | ++ | ++ | + |
| C | +/++ | ++ | + | +/++ | +/++ | + | ||
| 5 | 1 | A | N | N | N | N | N | N |
| B | +/++ | +/++ | 0/+ | + | + | 00/+ | ||
| 2 | A | + | + | 0/+ | + | + | 00/+ | |
| 6 | 3 | A | + | + | 0 | +/++ | +/++ | 0 |
| 7 | 2 | A | +/++ | ++ | + | ++/+++ | ++ | +/++ |
| B | + | ++ | + | +/++ | +/++ | 0/+ | ||
| 8 | 1 | A | + | +/++ | 0/+ | + | + | 0 |
| B | + | + | 0/+ | +/++ | +/++ | 00/+ | ||
| 9 | 1 | A | ++ | ++ | + | ++/+++ | +++ | 0 |
| 2 | A | ++ | ++ | +/++ | ++ | ++ | 0/+ | |
| B | NS | NS | NS | ++/+++ | +++ | 0 | ||
| 10 | 1 | A | + | + | 0/+ | +/++ | +/++ | 0 |
| B | + | +/++ | + | ++ | ++ | 0/+ | ||
| C | +/++ | + | 0/+ | ++ | +/++ | 00/+ | ||
| Median value | +/++ | ++ | + | ++ | ++ | 0/+ | ||
| Factor 1 | Factor 2 | R | p-Value |
|---|---|---|---|
| A strong correlation (0.6–0.8) | |||
| HBD3 | IL-10 | 0.623 | 0.001 |
| HBD4 | LL-37 | 0.639 | <0.001 |
| A moderate correlation (0.4–0.6) | |||
| HBD3 | HBD4 | 0.534 | 0.005 |
| HBD3 | LL-37 | 0.593 | 0.002 |
| IL-10 | LL-37 | 0.521 | 0.008 |
| PAS | LL-37 | −0.410 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deņisova, A.; Pilmane, M.; Fedirko, P. Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans. J. Funct. Morphol. Kinesiol. 2022, 7, 55. https://doi.org/10.3390/jfmk7030055
Deņisova A, Pilmane M, Fedirko P. Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans. Journal of Functional Morphology and Kinesiology. 2022; 7(3):55. https://doi.org/10.3390/jfmk7030055
Chicago/Turabian StyleDeņisova, Arina, Māra Pilmane, and Pavlo Fedirko. 2022. "Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans" Journal of Functional Morphology and Kinesiology 7, no. 3: 55. https://doi.org/10.3390/jfmk7030055
APA StyleDeņisova, A., Pilmane, M., & Fedirko, P. (2022). Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans. Journal of Functional Morphology and Kinesiology, 7(3), 55. https://doi.org/10.3390/jfmk7030055







