Abstract
Huntington’s disease (HD) is a rare, hereditary, and progressive neurodegenerative disease, characterized by involuntary choreatic movements with cognitive and behavioral disturbances. In order to mitigate impairments in motor function, physical exercise was integrated in HD rehabilitative interventions, showing to be a powerful tool to ameliorate the quality of life of HD-affected patients. This review aims to describe the effects of physical exercise on HD-related skeletal muscle disorders in both murine and human models. We performed a literature search using PubMed, Scopus, and Web of Science databases on the role of physical activity in mouse models of HD and human patients. Fifteen publications fulfilled the criteria and were included in the review. Studies performed on mouse models showed a controversial role played by exercise, whereas in HD-affected patients, physical activity appeared to have positive effects on gait, motor function, UHDMRS scale, cognitive function, quality of life, postural stability, total body mass, fatty acid oxidative capacity, and VO2 max. Physical activity seems to be feasible, safe, and effective for HD patients. However, further studies with longer follow-up and larger cohorts of patients will be needed to draw firm conclusions on the positive effects of exercise for HD patients.
1. Introduction
Huntington’s disease (HD) is a hereditary neurodegenerative disorder characterized by progressive motor dysfunction, psychiatry disturbances, and cognitive deficit [1,2]. The genetic basis of the disease is an abnormal expansion of the cytosine-adenine-guanine (CAG) trinucleotide repeat in the IT15 gene on chromosome 4 [3]. Nowadays, a genetic test can identify individuals at risk of inheriting the expanded CAG nucleotide before the onset of clinical symptoms. The mean age of onset is around 40 years, and the disease progression leads to death in 15 to 20 years [4]. Biological evidence indicates that an increasing number of CAG repeats promote the functioning and survival of brain neurons which are crucial for embryonic development [5]. In the healthy population, CAG repeat lengths in the HTT gene vary significantly from 6 to 36. An expansion above 39 CAG repeats causes the manifestation of the pathology [6]. This mutation leads to an unusually long expansion of the polyglutamine tract in the protein that causes toxicity, subsequent dysfunction, and death of the striatal and cortical neurons [7]. The complex mechanisms of the pathophysiology behind Huntington’s disease are not fully understood yet. In a recent study on individuals that carry the mutation that causes HD, it was found that CAG repeats of 37 to 42 were associated with higher cognitive skill development, while longer repeats were associated with lower skill levels [8]. When the pathology manifests itself, the most characteristic neuropathological abnormalities are neuronal loss in the basal ganglia and cerebral cortex [9]. The neurological degeneration leads to psychiatric symptoms like apathy, depression, irritability, aggressive behavior, anxiety, and also cognitive symptoms that affect attention, memory, and language [10]. The impact of cortical degeneration and dysfunction also give a significant contribution to impairments in motor functions [11,12]. Motor abnormalities include involuntary movements such as chorea and dystonia as well as disturbances such as bradykinesia [13,14]. Even if chorea is prominent in the early stages of the disease, later progressive bradykinesia, rigidity, and incoordination become functionally more disabling [15]. Moreover, many patients often have substantial cognitive or behavioral disturbances before the onset of motor symptoms [16]. Another symptom that leads to the damaging of motor functions is dystonia occurring in more than 90% of HD [17]. Choreatic symptoms decrease in later stages of HD, instead, dystonia tends to increase with the progression of the disease, probably due to direct pathway dysfunction [18].
Huntingtin (HTT) protein is widely distributed in a large variety of tissues, including brain, heart, skeletal muscles, kidneys, and liver [19]. Many studies on HD throughout the years have investigated the role of HTT protein in the brain but, recently, the importance of understanding the molecular mechanisms that lead to a deterioration of the skeletal muscles is growing. The pathogenetic mechanisms involved in muscle dysfunction are not fully understood yet. However, the pathological effects of HD on skeletal muscles have been demonstrated both in animal models and humans [20,21,22]. Studies performed on muscle of HD transgenic mice and on muscle cell cultures from HD patients showed mitochondrial dysfunction, decreased levels of ATP, oxidative stress, and inflammation [23,24]. Braubach et al. [25] found that the skeletal muscles of HD mice exhibit severely disturbed Ca2+ homeostasis with a significant reduction of Ca2+ entry, release, and removal. Romer et al. [26], found that the t-tubule network of HD mice was intact but the diameter of the individual t-tubules was reduced, causing disrupted Ca2+ signaling that may explain the symptoms of weakness and fatigue in HD. In a study evaluating the symptomatic HD patients with a 31P magnetic resonance spectroscopy, a reduction in phosphocreatine to inorganic phosphate ratio at rest was found [27]. The authors reported also that ATP/phosphocreatine and inorganic phosphate levels in muscles were significantly reduced in HD patients compared to control. Furthermore, in another study, Ciammola et al. [28], showed that pre-symptomatic HD subjects have a lower anaerobic threshold and increased level of plasma lactate compared to control. Gehrig et al. [29] showed that the percentage of type 1 muscles fibers is in proportion, significantly higher in HD patients compared to control. Moreover, the mitochondrial respiratory capacity specific to complex I and the capacity of maximal oxidative phosphorylation were marginally lower in HD patients [30]. The lower percentage of type 2 fibers found in HD patients could help the interpretation of the findings by Busse et al. [30], whose HD patients exhibited nearly half the isometric strength of healthy control when different muscle groups were evaluated with a handheld dynamometer. At a clinical level, the most typical motor symptom of HD is chorea, which is characterized by abnormal involuntary movement and brief, irregular contractions that appear to flow from one muscle to the other. Moreover, chorea often comes along with athetosis that causes also twisting and writhing movements [31]. During the progression of the disease, dystonia also occurs and the facial muscles are affected until, in later stages, dysarthria and dysphagia become a serious issue. Furthermore, HD patients also develop hypokinesia, bradykinesia, rigidity, and akinesia [32], (Figure 1). The impairments affecting the musculoskeletal systems also lead to gait and balance impairments in HD patients. Premanifest HD patients show slower gait velocity and cadence, shorter stride length, and poor dynamic balance control, compared to healthy people [33]. Patients with manifest HD have even worse gait cadence and velocity compared to premanifest HD and increased amplitude and velocity of mediolateral trunk’s sway. They also manifest a wider base of support, poor balance, and difficulties in dual-tasking [33].

Figure 1.
Motor symptoms related to disease progression.
The management of HD is currently based on symptomatic treatment, and widely directed at the chorea and neurobehavioral problems [34]. However, none of these treatments has a long-term disease-modifying effect. Physical exercise in the treatment of HD-affected patients was investigated throughout the years [35]. This review aims to describe HD-related skeletal muscles disturbances and highlight the effect of physical exercise and multidisciplinary rehabilitation on motor functions in persons with HD.
2. Materials and Methods
In this narrative review, we provided an overview on the impact of HD on the musculoskeletal system and on the effects of physical activity both in mouse models and human patients. Keywords for literature included “Huntington’s disease”, “muscle HD pathophysiology”; “muscle wasting HD”, “mouse models HD”, “Huntington mouse exercise” “motor function HD”, “gait HD”, “physical exercise HD”, “rehabilitation HD”, “physical therapy HD”. The searches were limited to studies published in English language that included mouse models or humans HD patients. The study design included narrative, systematic reviews, and original articles. We started the literature search from July 2021 to January 2022 on PubMed, Scopus, and Web of science databases. Twenty-one sources met the eligibility criteria, considered appropriate for the purpose of the review. All the included studies were original article, presented in Table 1. Considering the great variability present in physical exercise protocols for the treatment of HD, we evaluated that a narrative review was the most appropriate form for our study.

Table 1.
Characteristics of included studies.
3. The Effects of Physical Activity in Mouse Models and Patients with HD
3.1. The Effects of Physical Activity in HD Rodent Models
Studies throughout the years have shown the positive association between physical exercise and a lower risk of developing neurodegenerative diseases [57,58]. In the mouse models, running improves hippocampal neurogenesis, spine density, vascularization, neurotrophins levels, learning, and long-term potentiation [59,60]. Moreover, acute treadmill exercise induced in mouse skeletal muscle, the formation of new intracellular junctions, known as calcium entry units (CEUs). CEUs, containing proteins used to promote Ca2+ entry and storage, represent an important element of muscle adaptation during fatigue [61]. Exercise-induced CEUs, increased Orai1-dependent Ca2+ entry to favor myoplasmic Ca2+ dynamics, reducing muscle force decline during sustained activation [62,63]. Noteworthy, exercise supports the production and secretion of myokines by skeletal muscles [64], such as BDNF, which promotes HD mice’s motor functions and survival rate, by counteracting brain atrophy [65,66]. However, the effects of physical exercise on HD animal models reported controversial data. In HD rodent models, treadmill exercise improved motor coordination, spatial learning, and short-term memory [50,51]. On the contrary, Wood et al. [52] showed that physical exercise on the rotarod did not significantly improve motor coordination of R6/2 mice, although it induced no deleterious effects. Exercise on a motorized treadmill and wheel-running improved cognition and prevented depressive-like behaviors [53,54,55]. Moreover, voluntary exercise postponed the onset of pathology, by ameliorating cognitive ability [67]. Exercise improved hindlimb clasping symptoms and prevented the alteration in mitochondrial content and function occurring in the late stage of HD [68]. In accord, Caldwell et al. showed that treadmill training was effective in enhancing mitochondrial function in the CAG140 KI HD mouse model, resulting in improved motor performance [49]. Moreover, Van Dellen et colleagues demonstrated that wheel running from a juvenile age can delay the onset of some, but not all, motor deficits in a mouse model of HD [37].
On the other hand, it has been shown that running accelerates the age of onset and increases the severity of HD symptoms in N171-82Q transgenic HD mouse models [38]. Moreover, endurance training was detrimental for HD mice, inducing in skeletal muscle the activation of AMPK [56], whose increase is known to promote neuronal death by reducing HD mice’s lifespan [69].
3.2. The Effects of Physical Activity in HD Patients
Nowadays the negative impact of HD on motor functions and cognition in humans is well-known. The evaluation of motor function is based on the UHDRS-TMS scale, which permits the evaluation of movements, gait, hand movements, dystonia, and chorea [70]. There are two phases in HD regarding the motor functions, the hyperkinetic one, with chorea, and the hypokinetic one, characterized by dystonia, bradykinesia, gait, and balance perturbations [71]. The employment of physical activity intervention programs to improve motor functions in HD patients seems to be effective, reducing the effects of the natural disease progression [72] (Figure 2). Table 2 gives an overview description of physical activity interventions. Different authors investigated the integration of physical activity in multidisciplinary rehabilitation to ameliorate the quality of life in Huntington’s disease patients [36,39,40,43,44,48]. A study performed on 40 HD-affected patients, showed that a rehabilitation program that includes respiratory exercises, speech therapy, physical/occupational therapy, and cognitive rehabilitation can help the maintenance of functional and motor performance in patients with early to moderate HD [36]. An improvement in UHDRS-TMS score and quality of life, along with a reduction of motor and postural stability deterioration, was found by Thompson et al. [39], in early to middle stage of HD patients which performed nine months of multidisciplinary rehabilitation. The protocol used by the authors consisted of 9 months of 40 min aerobic training once a week supervised in an exercise clinic, occupational therapy 1 h every two weeks for six months, and a tailored, home-based, self-monitored exercise program three times per week for 6 months. Piira et al. [40], showed that a multidisciplinary approach, comprehending physical exercise, social activities, and group/teaching sessions, is associated with improved balance, gait function, and quality of life in patients with early to middle stage HD. The duration of the intervention was one-year with 3 admissions of 3 weeks each, and 5 days of evaluation approximately 3 months after the last rehabilitation admission. The patients underwent 8 h of activities including physiotherapy, occupational therapy, speech therapy, training in gym/swimming pool, and group discussions. Cruickshank et al. [44], employed a 9-months program of supervised clinical exercise 1 time per week, self-directed home-based exercises three times per week, and occupational therapy once every 2 weeks. The supervised exercise program consisted of 1 h of aerobic and resistance training while the home-based program was focused on strengthening and fine motor exercises for 1 h. The findings from this study showed that multidisciplinary rehabilitation can give positive results in terms of increasing grey matter volume in the right caudate and both sides of the dorsolateral prefrontal cortex, leading to an improvement in verbal learning and memory. The positive effects of multidisciplinary rehabilitation with physical exercise on brain structure for Huntington’s disease patients were also seen by Bartlett et al. [48]. In their study, 18 HD patients (10 premanifest and 8 prodromal) underwent 9 months of aerobic and resistance training two times per week, bilingual exercise, dual-task training one time per week for 1 h, computerized cognitive training three times per week for 1 h, and social activities. The authors reported that the intervention groups showed significantly less right hypothalamic grey matter volume loss than the control group and maintained higher concentrations of brain-derived neurotrophic factors, indicating that this kind of intervention can be beneficial for HD patients.
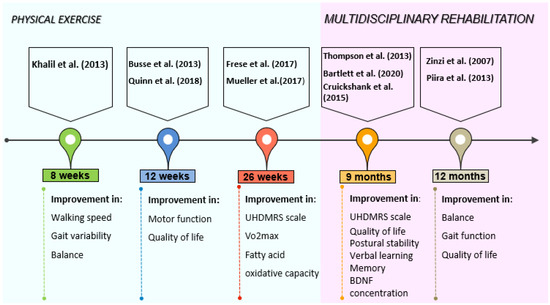
Figure 2.
Intriguing timeline of physical exercise and multidisciplinary rehabilitation interventions with their relative outcomes.

Table 2.
Overview description of physical activity interventions. CG: control group; IG: intervention group; n/a= not available.
The effects of physical exercise alone were investigated by several authors [41,42,45,46,47]. An exercise protocol of 12 weeks of walking and cycling aerobic training (55–75% of predicted HRmax) and resistance exercises with leg press, leg extension, lat pulldown, hamstring curl, calf raises (2 × 8–12 reps at 60–70% of 1RM) maintained motor function stable and gave improvements on the quality of life [41]. In a randomized controlled pilot trial of Khalil et al. [42], on 25 early to mid-stage HD patients, a home-based program of 8 weeks of gradual progressive walking exercise three times per week resulted in an improvement of walking speed, gait variability, and balance measured with the Berg balance scale. Improvements in motor function measured with UHDRS motor score, and fitness measured with predicted VO2max, were found by Quinn et al. [45], in HD-affected patients that underwent 12 weeks of 30 min cycling training (65–85% of age predicted HRmax) and 10–15 min of strengthening exercises like chair stand, seated wood chop, plank, and chair lunges (2 × 10–12 reps). The study of Frese et al. [46], demonstrated the feasibility of aerobic training, high-intensity interval training (HIIT), and endurance training for HD patients. The intervention from the authors consisted of 10 weeks of 30 min cycling (65%VO2peak) 3 times per week, 8 weeks of HIIT (4 × 4 min at 90–95% of HRpeak with 3 min low-intensity rest intervals at 70% of HRpeak) three times per week, and 6 final weeks of endurance training three times per week [46]. This 26-weeks program resulted in an improvement of VO2peak, peak cycling power, and cycling time to exhaustion. Moreover, the UHDRS motor score remained stable indicating that the exercise could be beneficial for these patients. Another study of Mueller et al. [47], with the same protocol showed improvement of citrate synthase, complex III + V activity, and fatty acid oxidative capacity. Based on these studies, physical exercise exerted beneficial effects on HD-affected patients. However, all exercise training interventions have to be tailor-made, in terms of frequency, intensity, and specificity and accompanied by frequent assessments to avoid the acceleration and/or worsening of symptoms [72,73]. In fact, excessive training performed by a marathon runner provoked progressive myopathy many years before the first signs of chorea were detected [74].
The promising results from these studies indicate that exercise may be beneficial and feasible for HD patients and can enter the management of HD whether included in a multidisciplinary rehabilitation approach or alone.
4. Limitations
The current review presents different limitations: (1) the included studies are based on a small number of patients, with different disease stages and heterogeneity of disability. Works performed on larger cohorts might clarify the individual alterations in variables; (2) the differences in the training protocol used and the lack of control groups in some of these studies; (3) the multidisciplinary approach used in some works, which does not allow us to say with certainty that the positive effects are mainly due to physical exercise.
5. Conclusions
Several studies over the past few decades investigated how HD affects motor and cognitive functions. The effects of physical activity interventions in both mouse models and humans were also studied. To date, the treatment of HD relies mainly on pharmaceutical interventions to reduce the symptoms. However, recent studies are highlighting the positive effects that structured programs of physical exercise and multidisciplinary rehabilitation have on HD patients’ cognitive, motor functions, and quality of life. To the best of our knowledge, this is the first narrative review that summarizes the effects of HD on skeletal muscles and the positive role played by physical exercise and multidisciplinary rehabilitation. A major part of the reported studies sustains the beneficial role of physical activity for HD patients indicating that it should be prescribed, when possible, to ameliorate their quality of life. However, the exercise protocol has to be tailor-made to avoid the acceleration and/or worsening of symptoms.
Author Contributions
Conceptualization, B.T. and G.M. (Giuseppe Musumeci); methodology, B.M., A.C. and V.D.; investigation, B.T., B.M., G.M. (Grazia Maugeri); resources, G.M. (Giuseppe Musumeci); writing—original draft preparation, B.T. and B.M.; writing—review and editing, A.C., G.M. (Grazia Maugeri) and G.M. (Giuseppe Musumeci); visualization, B.T. and B.M.; supervision G.M. (Giuseppe Musumeci); project administration, G.M. (Giuseppe Musumeci); funding acquisition, G.M. (Giuseppe Musumeci). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the University Research Project Grant (PIACERI Found-NATURE-OA-2020–2022), Department of Biomedical and Biotechnological Sciences (BIOMETEC), University of Catania, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mühlau, M.; Winkelmann, J.; Rujescu, D.; Giegling, I.; Koutsouleris, N.; Gaser, C.; Arsic, M.; Weindl, A.; Reiser, M.; Meisenzahl, E.M. Variation within the Huntington’s disease gene influences normal brain structure. PLoS ONE 2012, 7, e29809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Cattaneo, E.; Zuccato, C.; Tartari, M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat. Rev. Neurosci. 2005, 6, 919–930. [Google Scholar] [CrossRef]
- Hamilton, J.M.; Salmon, D.P.; Corey-Bloom, J.; Gamst, A.; Paulsen, J.S.; Jerkins, S.; Jacobson, M.W.; Peavy, G. Behavioural abnormalities contribute to functional decline in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Andrew, S.E.; Goldberg, Y.P.; Kremer, B.; Telenius, H.; Theilmann, J.; Adam, S.; Starr, E.; Squitieri, F.; Lin, B.; Kalchman, M.A.; et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 1993, 4, 398–403. [Google Scholar] [CrossRef]
- Schultz, J.L.; van der Plas, E.; Langbehn, D.R.; Conrad, A.L.; Nopoulos, P.C. Age-Related Cognitive Changes as a Function of CAG Repeat in Child and Adolescent Carriers of Mutant Huntingtin. Ann. Neurol. 2021, 89, 1036–1040. [Google Scholar] [CrossRef]
- Nana, A.L.; Kim, E.H.; Thu, D.C.; Oorschot, D.E.; Tippett, L.J.; Hogg, V.M.; Synek, B.J.; Roxburgh, R.; Waldvogel, H.J.; Faull, R.L. Widespread heterogeneous neuronal loss across the cerebral cortex in Huntington’s disease. J. Huntingt. Dis. 2014, 3, 45–64. [Google Scholar] [CrossRef]
- Weydt, P.; Dupuis, L.; Petersen, Å. Thermoregulatory disorders in Huntington disease. Handb. Clin. Neurol. 2018, 157, 761–775. [Google Scholar] [CrossRef]
- Reiner, A.; Albin, R.L.; Anderson, K.D.; D’Amato, C.J.; Penney, J.B.; Young, A.B. Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5733–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P., Jr. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Beste, C.; Konrad, C.; Saft, C.; Ukas, T.; Andrich, J.; Pfleiderer, B.; Hausmann, M.; Falkenstein, M. Alterations in voluntary movement execution in Huntington’s disease are related to the dominant motor system: Evidence from event-related potentials. Exp. Neurol. 2009, 216, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Pantelyat, A.; Kogan, J.; Brandt, J. Determinants of functional disability in Huntington’s disease: Role of cognitive and motor dysfunction. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblatt, A.; Abbott, M.H.; Gourley, L.M.; Troncoso, J.C.; Margolis, R.L.; Brandt, J.; Ross, C.A. Predictors of neuropathological severity in 100 patients with Huntington’s disease. Ann. Neurol. 2003, 54, 488–493. [Google Scholar] [CrossRef]
- Marder, K.; Zhao, H.; Myers, R.H.; Cudkowicz, M.; Kayson, E.; Kieburtz, K.; Orme, C.; Paulsen, J.; Penney, J.B., Jr.; Siemers, E.; et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology 2000, 54, 452–458. [Google Scholar] [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Orekhov, A.N.; Deykin, A.V. Modern approaches for modelling dystonia and Huntington’s disease in vitro and in vivo. Int. J. Exp. Pathol. 2019, 100, 64–71. [Google Scholar] [CrossRef]
- Gibson, J.S.; Claassen, D.O. State-of-the-art pharmacological approaches to reduce chorea in Huntington’s disease. Expert Opin. Pharmacother. 2021, 22, 1015–1024. [Google Scholar] [CrossRef]
- Sassone, J.; Colciago, C.; Cislaghi, G.; Silani, V.; Ciammola, A. Huntington’s disease: The current state of research with peripheral tissues. Exp. Neurol. 2009, 219, 385–397. [Google Scholar] [CrossRef]
- Miranda, D.R.; Reed, E.; Jama, A.; Bottomley, M.; Ren, H.; Rich, M.M.; Voss, A.A. Mechanisms of altered skeletal muscle action potentials in the R6/2 mouse model of Huntington’s disease. Am. J. Physiol. Cell Physiol. 2020, 319, C218–C232. [Google Scholar] [CrossRef]
- Gizatullina, Z.Z.; Lindenberg, K.S.; Harjes, P.; Chen, Y.; Kosinski, C.M.; Landwehrmeyer, B.G.; Ludolph, A.C.; Striggow, F.; Zierz, S.; Gellerich, F.N. Low stability of Huntington muscle mitochondria against Ca2+ in R6/2 mice. Ann. Neurol. 2006, 59, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, M.; Sciandra, F. Molecular Mechanisms Underlying Muscle Wasting in Huntington’s Disease. Int. J. Mol. Sci. 2020, 21, 8314. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Adhihetty, P.; Shukla, S.; Hennessy, T.; Calingasan, N.; Yang, L.; Starkov, A.; Kiaei, M.; Cannella, M.; Sassone, J.; et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum. Mol. Genet. 2009, 18, 3048–3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciammola, A.; Sassone, J.; Alberti, L.; Meola, G.; Mancinelli, E.; Russo, M.A.; Squitieri, F.; Silani, V. Increased apoptosis, Huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington’s disease subjects. Cell Death Differ. 2006, 13, 2068–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braubach, P.; Orynbayev, M.; Andronache, Z.; Hering, T.; Landwehrmeyer, G.B.; Lindenberg, K.S.; Melzer, W. Altered Ca2+ signaling in skeletal muscle fibers of the R6/2 mouse, a model of Huntington’s disease. J. Gen. Physiol. 2014, 144, 393–413. [Google Scholar] [CrossRef]
- Romer, S.H.; Metzger, S.; Peraza, K.; Wright, M.C.; Jobe, D.S.; Song, L.S.; Rich, M.M.; Foy, B.D.; Talmadge, R.J.; Voss, A.A. A mouse model of Huntington’s disease shows altered ultrastructure of transverse tubules in skeletal muscle fibers. J. Gen. Physiol. 2021, 153, e202012637. [Google Scholar] [CrossRef]
- Lodi, R.; Schapira, A.H.; Manners, D.; Styles, P.; Wood, N.W.; Taylor, D.J.; Warner, T.T. Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Ann. Neurol. 2000, 48, 72–76. [Google Scholar] [CrossRef]
- Ciammola, A.; Sassone, J.; Sciacco, M.; Mencacci, N.E.; Ripolone, M.; Bizzi, C.; Colciago, C.; Moggio, M.; Parati, G.; Silani, V.; et al. Low anaerobic threshold and increased skeletal muscle lactate production in subjects with Huntington’s disease. Mov. Disord. 2011, 26, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Gehrig, S.M.; Petersen, J.A.; Frese, S.; Mueller, S.M.; Mihaylova, V.; Ligon-Auer, M.; Lundby, C.; Toigo, M.; Jung, H.H. Skeletal muscle characteristics and mitochondrial function in Huntington’s disease patients. Mov. Disord. 2017, 32, 1258–1259. [Google Scholar] [CrossRef]
- Busse, M.E.; Hughes, G.; Wiles, C.M.; Rosser, A.E. Use of hand-held dynamometry in the evaluation of lower limb muscle strength in people with Huntington’s disease. J. Neurol. 2008, 255, 1534–1540. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F. Huntington disease and other choreas. Neurol. Clin. 2009, 27, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Roos, R.A. Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, K.; Canning, C.G.; Menant, J.C.; Loy, C.T. Gait, balance, and falls in Huntington disease. Handb. Clin. Neurol. 2018, 159, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s disease: Diagnosis and management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fritz, N.E.; Rao, A.K.; Kegelmeyer, D.; Kloos, A.; Busse, M.; Hartel, L.; Carrier, J.; Quinn, L. Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review. J. Huntingt. Dis. 2017, 6, 217–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinzi, P.; Salmaso, D.; De Grandis, R.; Graziani, G.; Maceroni, S.; Bentivoglio, A.; Zappata, P.; Frontali, M.; Jacopini, G. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: A pilot study. Clin. Rehabil. 2007, 21, 603–613. [Google Scholar] [CrossRef]
- Van Dellen, A.; Cordery, P.M.; Spires, T.L.; Blakemore, C.; Hannan, A.J. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington’s disease. BMC Neurosci. 2008, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.C.; Yuan, C.; Ottenritter, C.; Mughal, M.; van Praag, H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington’s disease. PLoS Curr. 2010, 2, RRN1201. [Google Scholar] [CrossRef]
- Thompson, J.A.; Cruickshank, T.M.; Penailillo, L.E.; Lee, J.W.; Newton, R.U.; Barker, R.A.; Ziman, M.R. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: A pilot study. Eur. J. Neurol. 2013, 20, 1325–1329. [Google Scholar] [CrossRef]
- Piira, A.; van Walsem, M.R.; Mikalsen, G.; Nilsen, K.H.; Knutsen, S.; Frich, J.C. Effects of a One Year Intensive Multidisciplinary Rehabilitation Program for Patients with Huntington’s Disease: A Prospective Intervention Study. PLoS Curr. 2013, 5, ecurrents.hd.9504af71e0d1f87830c25c394be47027. [Google Scholar] [CrossRef]
- Busse, M.; Quinn, L.; Debono, K.; Jones, K.; Collett, J.; Playle, R.; Kelly, M.; Simpson, S.; Backx, K.; Wasley, D.; et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J. Neurol. Phys. Ther. 2013, 37, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Quinn, L.; van Deursen, R.; Dawes, H.; Playle, R.; Rosser, A.; Busse, M. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin. Rehabil. 2013, 27, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Piira, A.; van Walsem, M.R.; Mikalsen, G.; Øie, L.; Frich, J.C.; Knutsen, S. Effects of a Two-Year Intensive Multidisciplinary Rehabilitation Program for Patients with Huntington’s Disease: A Prospective Intervention Study. PLoS Curr. 2014, 6, ecurrents.hd.2c56ceef7f9f8e239a59ecf2d94cddac. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, T.M.; Thompson, J.A.; Domínguez, D.J.; Reyes, A.P.; Bynevelt, M.; Georgiou-Karistianis, N.; Barker, R.A.; Ziman, M.R. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain Behav. 2015, 5, e00312. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Hamana, K.; Kelson, M.; Dawes, H.; Collett, J.; Townson, J.; Roos, R.; van der Plas, A.A.; Reilmann, R.; Frich, J.C.; et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat. Disord. 2016, 31, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Frese, S.; Petersen, J.A.; Ligon-Auer, M.; Mueller, S.M.; Mihaylova, V.; Gehrig, S.M.; Kana, V.; Rushing, E.J.; Unterburger, E.; Kägi, G.; et al. Exercise effects in Huntington disease. J. Neurol. 2017, 264, 32–39. [Google Scholar] [CrossRef]
- Mueller, S.M.; Gehrig, S.M.; Petersen, J.A.; Frese, S.; Mihaylova, V.; Ligon-Auer, M.; Khmara, N.; Nuoffer, J.M.; Schaller, A.; Lundby, C.; et al. Effects of endurance training on skeletal muscle mitochondrial function in Huntington disease patients. Orphanet J. Rare Dis. 2017, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, D.M.; Dominguez, D.J.; Lazar, A.S.; Kordsachia, C.C.; Rankin, T.J.; Lo, J.; Govus, A.D.; Power, B.D.; Lampit, A.; Eastwood, P.R.; et al. Multidisciplinary rehabilitation reduces hypothalamic grey matter volume loss in individuals with preclinical Huntington’s disease: A nine-month pilot study. J. Neurol. Sci. 2020, 408, 116522. [Google Scholar] [CrossRef]
- Caldwell, C.C.; Petzinger, G.M.; Jakowec, M.W.; Cadenas, E. Treadmill exercise rescues mitochondrial function and motor behavior in the CAG(140) knock-in mouse model of Huntington’s disease. Chem. Biol. Interact. 2020, 315, 108907. [Google Scholar] [CrossRef]
- Ji, E.S.; Kim, Y.M.; Shin, M.S.; Kim, C.J.; Lee, K.S.; Kim, K.; Ha, J.; Chung, Y.R. Treadmill exercise enhances spatial learning ability through suppressing hippocampal apoptosis in Huntington’s disease rats. J. Exerc. Rehabil. 2015, 11, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Ji, E.S.; Kim, S.H.; Kim, T.W.; Ko, I.G.; Jin, J.J.; Kim, C.J.; Kim, T.W.; Kim, D.H. Treadmill exercise improves short-term memory by enhancing hippocampal cell proliferation in quinolinic acid-induced Huntington’s disease rats. J. Exerc. Rehabil. 2015, 11, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.I.; Glynn, D.; Morton, A.J. “Brain training” improves cognitive performance and survival in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2011, 42, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Renoir, T.; Pang, T.Y.; Zajac, M.S.; Chan, G.; Du, X.; Leang, L.; Chevarin, C.; Lanfumey, L.; Hannan, A.J. Treatment of depressive-like behaviour in Huntington’s disease mice by chronic sertraline and exercise. Br. J. Pharmacol. 2012, 165, 1375–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanko, D.P.; Shah, V.D.; Yamasaki, W.K.; Petzinger, G.M.; Jakowec, M.W. Treadmill exercise delays the onset of non-motor behaviors and striatal pathology in the CAG(140) knock-in mouse model of Huntington’s disease. Neurobiol. Dis. 2017, 105, 15–32. [Google Scholar] [CrossRef]
- Harrison, D.J.; Busse, M.; Openshaw, R.; Rosser, A.E.; Dunnett, S.B.; Brooks, S.P. Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington’s disease mouse model. Exp. Neurol. 2013, 248, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Corrochano, S.; Blanco, G.; Williams, D.; Wettstein, J.; Simon, M.; Kumar, S.; Moir, L.; Agnew, T.; Stewart, M.; Landman, A.; et al. A genetic modifier suggests that endurance exercise exacerbates Huntington’s disease. Hum. Mol. Genet. 2018, 27, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Kautiainen, H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.; Pitkälä, K.H. Effects of Exercise on Cognition: The Finnish Alzheimer Disease Exercise Trial: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 731–738. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.L.; Grover, L.M.; Schwartzkroin, P.A.; Bothwell, M. Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron 1992, 9, 1081–1088. [Google Scholar] [CrossRef]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 14286. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; García-Castañeda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse tubule remodeling enhances Orai1-dependent Ca2+ entry in skeletal muscle. Elife 2019, 8, e47576. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2021, 42, 233–249. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Park, Y.; Won, S.Y.; Cho, K.S. Physical Exercise-Induced Myokines in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 5795. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hayden, M.R.; Xu, B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J. Neurosci. 2010, 30, 14708–14718. [Google Scholar] [CrossRef]
- Giralt, A.; Carretón, O.; Lao-Peregrin, C.; Martín, E.D.; Alberch, J. Conditional BDNF release under pathological conditions improves Huntington’s disease pathology by delaying neuronal dysfunction. Mol. Neurodegener. 2011, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Pang, T.Y.C.; Stam, N.C.; Nithianantharajah, J.; Howard, M.L.; Hannan, A.J. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 2006, 141, 569–584. [Google Scholar] [CrossRef]
- Herbst, E.A.; Holloway, G.P. Exercise training normalizes mitochondrial respiratory capacity within the striatum of the R6/1 model of Huntington’s disease. Neuroscience 2015, 303, 515–523. [Google Scholar] [CrossRef]
- Ju, T.C.; Chen, H.M.; Lin, J.T.; Chang, C.P.; Chang, W.C.; Kang, J.J.; Sun, C.P.; Tao, M.H.; Tu, P.H.; Chang, C.; et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J. Cell Biol. 2011, 194, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Unified Huntington’s Disease Rating Scale: Reliability and consistency. Huntington Study Group. Mov. Disord. 1996, 11, 136–142. [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; Petersen, J.A.; Jung, H.H. Exercise in Huntington’s Disease: Current State and Clinical Significance. Tremor Other Hyperkinet. Mov. 2019, 9, 601. [Google Scholar] [CrossRef]
- Quinn, L.; Debono, K.; Dawes, H.; Rosser, A.E.; Nemeth, A.H.; Rickards, H.; Tabrizi, S.J.; Quarrell, O.; Trender-Gerhard, I.; Kelson, M.J.; et al. Task-specific training in Huntington disease: A randomized controlled feasibility trial. Phys. Ther. 2014, 94, 1555–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosinski, C.M.; Schlangen, C.; Gellerich, F.N.; Gizatullina, Z.; Deschauer, M.; Schiefer, J.; Young, A.B.; Landwehrmeyer, G.B.; Toyka, K.V.; Sellhaus, B.; et al. Myopathy as a first symptom of Huntington’s disease in a Marathon runner. Mov. Disord. 2007, 22, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).