Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases
Abstract
1. Introduction
2. Literature Search Strategy
3. Effects of Physical Exercise on Brain Health
3.1. Physical Exercise Improves Synaptic Plasticity
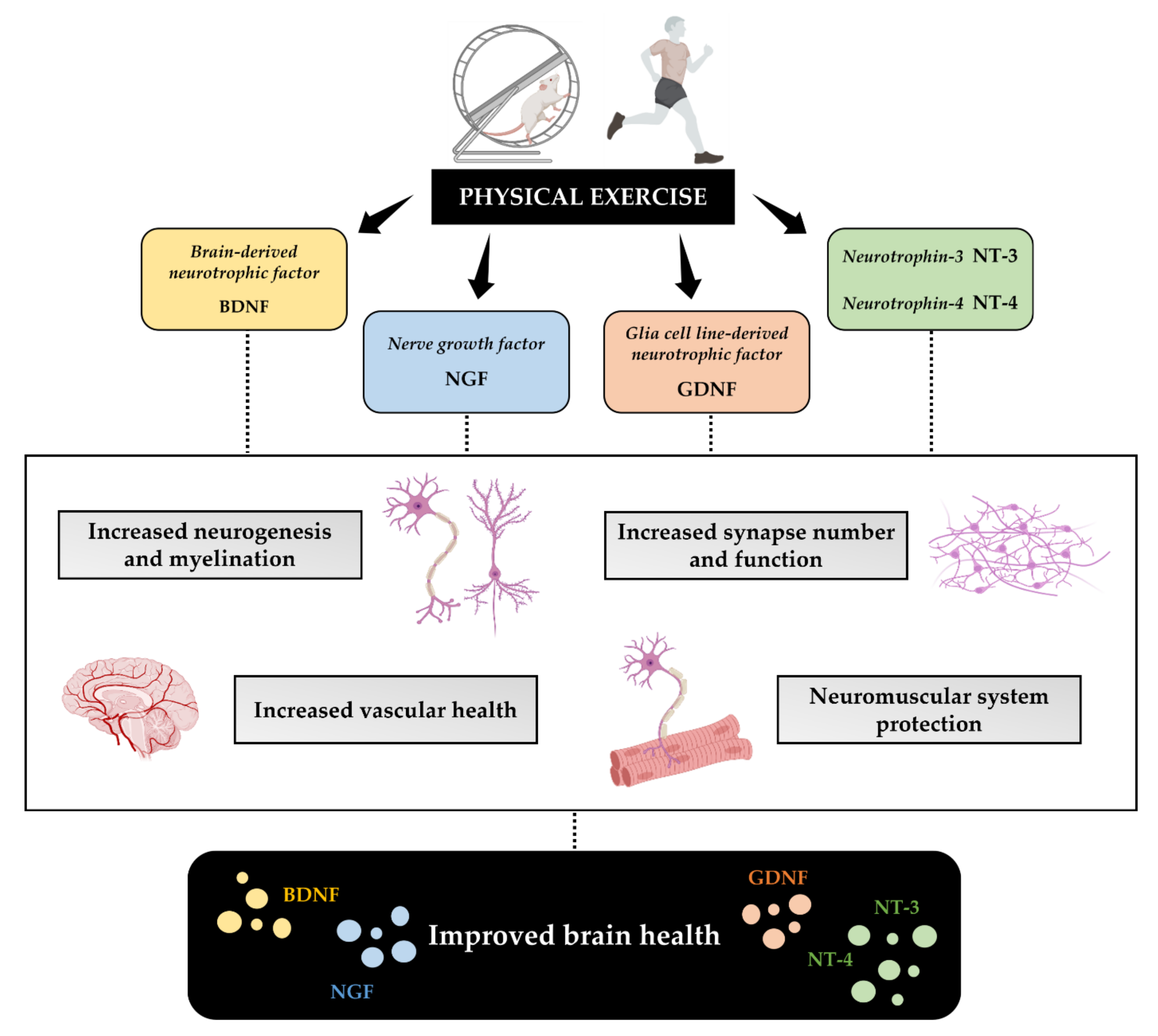
3.2. Physical Exercise Improves Neurotrophins Production
3.2.1. BDNF
3.2.2. NGF
3.2.3. GDNF
3.2.4. NT-3 and NT-4
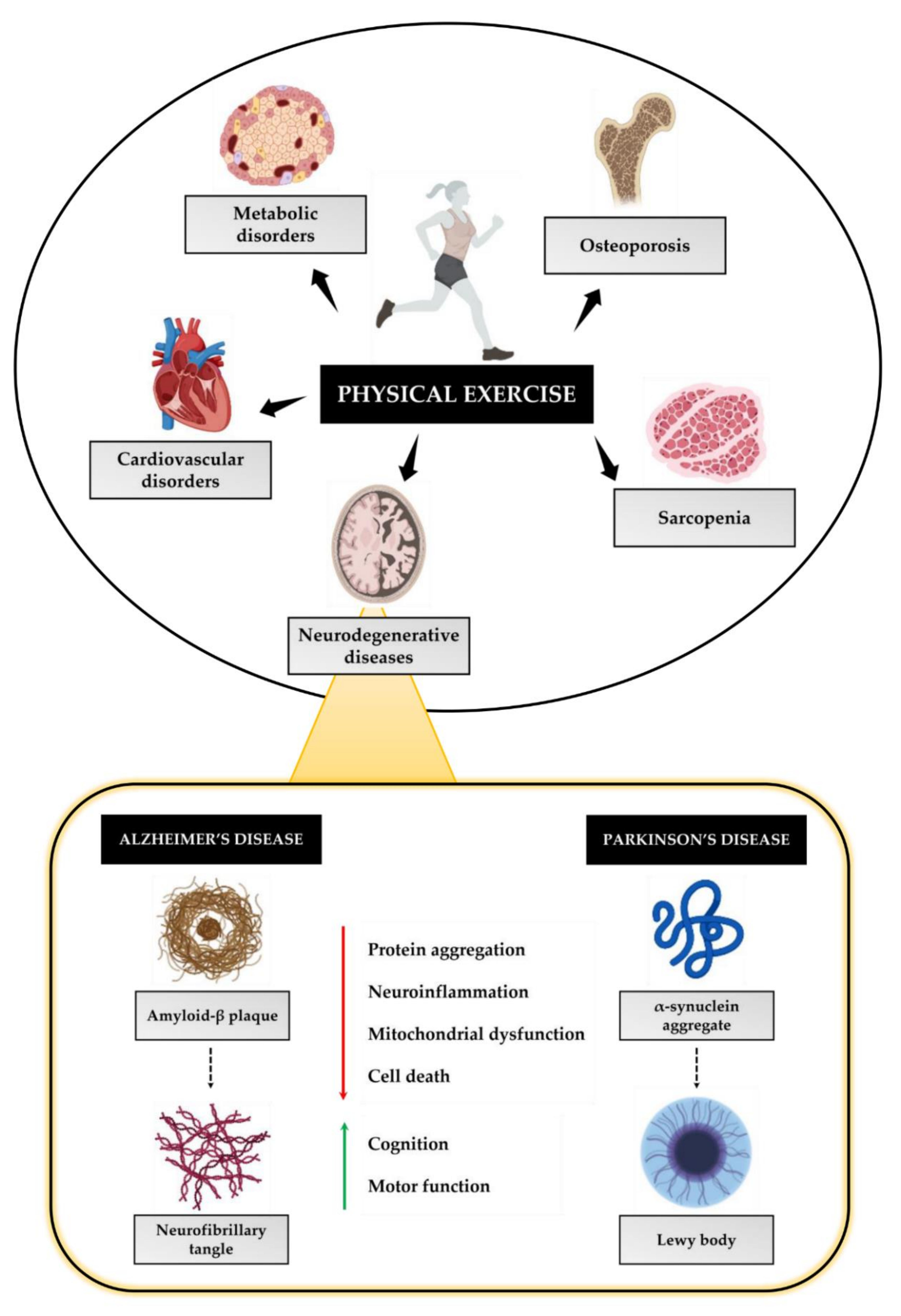
4. Physical Exercise as a Non-Pharmacological Strategy to Prevent Neurodegeneration
4.1. Physical Exercise and AD
4.2. Physical Exercise and PD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ashokan, K. Physical Exercise: An Overview of Benefits From Psychological Level to Genetics and Beyond. Front. Physiol. 2021, 12, 731858. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Cariati, I.; Marini, M.; D’Arcangelo, G.; Tancredi, V.; Primavera, M.; Iundusi, R.; Gasbarra, E.; Scimeca, M. Effects of Simulated Microgravity on Muscle Stem Cells Activity. Cell. Physiol. Biochem. 2020, 54, 736–747. [Google Scholar] [CrossRef]
- Sosner, P.; Guiraud, T.; Gremeaux, V.; Arvisais, D.; Herpin, D.; Bosquet, L. The ambulatory hypotensive effect of aerobic training: A reappraisal through a meta-analysis of selected moderators. Scand. J. Med. Sci. Sports 2017, 27, 327–341. [Google Scholar] [CrossRef]
- Park, W.; Jung, W.-S.; Hong, K.; Kim, Y.-Y.; Kim, S.-W.; Park, H.-Y. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7233. [Google Scholar] [CrossRef]
- Nassef, Y.; Lee, K.-J.; Nfor, O.N.; Tantoh, D.M.; Chou, M.-C.; Liaw, Y.-P. The Impact of Aerobic Exercise and Badminton on HDL Cholesterol Levels in Taiwanese Adults. Nutrients 2020, 12, 1204. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, T.K.; Qiu, W.; Zhang, X.; Zhou, S.; Liu, Y.; He, Y. Comparable Effects of High-Intensity Interval Training and Prolonged Continuous Exercise Training on Abdominal Visceral Fat Reduction in Obese Young Women. J. Diabetes Res. 2017, 2017, 5071740. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Rêgo, M.L.; Cabral, D.A.; Costa, E.C.; Fontes, E.B. Physical Exercise for Individuals with Hypertension: It Is Time to Emphasize its Benefits on the Brain and Cognition. Clin. Med. Insights. Cardiol. 2019, 13, 1179546819839411. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J. Appl. Physiol. 2020, 128, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.; Soligard, T.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Onorato, F.; Mastrogregori, A.; Rossi, D.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Role of Physical Activity in Bone-Muscle Crosstalk: Biological Aspects and Clinical Implications. J. Funct. Morphol. Kinesiol. 2021, 6, 55. [Google Scholar] [CrossRef]
- Harris, J.E.; Baer, L.A.; Stanford, K.I. Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends Endocrinol. Metab. 2018, 29, 164–177. [Google Scholar] [CrossRef]
- Labonte-Lemoyne, E.; Curnier, D.; Ellemberg, D. Exercise during pregnancy enhances cerebral maturation in the newborn: A randomized controlled trial. J. Clin. Exp. Neuropsychol. 2017, 39, 347–354. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Lawlor, D.A.; Juhl, M.; Owe, K.M.; Lewis, B.; Liu, J.; Wilcox, A.J.; Longnecker, M.P. Physical activity during pregnancy and language development in the offspring. Paediatr. Perinat. Epidemiol. 2013, 27, 283–293. [Google Scholar] [CrossRef]
- Klein, C.P.; Hoppe, J.B.; Saccomori, A.B.; Dos Santos, B.G.; Sagini, J.P.; Crestani, M.S.; August, P.M.; Hözer, R.M.; Grings, M.; Parmeggiani, B.; et al. Physical Exercise During Pregnancy Prevents Cognitive Impairment Induced by Amyloid-β in Adult Offspring Rats. Mol. Neurobiol. 2019, 56, 2022–2038. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. Calcium Dynamics and Synaptic Plasticity. Adv. Exp. Med. Biol. 2020, 1131, 965–984. [Google Scholar] [CrossRef]
- Bettio, L.; Thacker, J.S.; Hutton, C.; Christie, B.R. Modulation of synaptic plasticity by exercise. Int. Rev. Neurobiol. 2019, 147, 295–322. [Google Scholar] [CrossRef]
- Cheyne, J.E.; Montgomery, J.M. The cellular and molecular basis of in vivo synaptic plasticity in rodents. Am. J. Physiol. Cell Physiol. 2020, 318, C1264–C1283. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- De Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- De Domenico, E.; D’Arcangelo, G.; Faraoni, I.; Palmieri, M.; Tancredi, V.; Graziani, G.; Grimaldi, P.; Tentori, L. Modulation of GDF11 expression and synaptic plasticity by age and training. Oncotarget 2017, 8, 57991–58002. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Pallone, G.; Scimeca, M.; Frank, C.; Tancredi, V.; D’Arcangelo, G. Hippocampal Adaptations to Continuous Aerobic Training: A Functional and Ultrastructural Evaluation in a Young Murine Model. J. Funct. Morphol. Kinesiol. 2021, 6, 101. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Ku, N.-W.; Wang, T.-F.; Yang, Y.-H.; Shih, Y.-H.; Wu, S.-Y.; Lee, C.-W.; Yu, M.; Yang, T.-T.; Kuo, Y.-M. Long-Term Moderate Exercise Rescues Age-Related Decline in Hippocampal Neuronal Complexity and Memory. Gerontology 2018, 64, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, M.; Shen, B.; Li, M.; Gao, Q.; Wei, S.-G. Moderate exercise prevents neurodegeneration in D-galactose-induced aging mice. Neural Regen. Res. 2016, 11, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Pallone, G.; Annino, G.; Tancredi, V.; D’Arcangelo, G. Modulation of Synaptic Plasticity by Vibratory Training in Young and Old Mice. Brain Sci. 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Annino, G.; Scimeca, M.; Bonanno, E.; D’Arcangelo, G.; Tancredi, V. Dose-Response Effect of Vibratory Stimulus on Synaptic and Muscle Plasticity in a Middle-Aged Murine Model. Front. Physiol. 2021, 12, 678449. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Law, C.-K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Petrigna, L.; Pereira, F.C.; Muscella, A.; Bianco, A.; Tavares, P. The Impact of Physical Exercise on the Circulating Levels of BDNF and NT 4/5: A Review. Int. J. Mol. Sci. 2021, 22, 8814. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-L.; Ma, X.-T.; Wang, J.-J.; Liu, H.; Chen, Y.-F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- De Azevedo, K.P.M.; de Oliveira, V.H.; de Medeiros, G.C.B.S.; Mata, Á.N.D.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef]
- Coelho, F.G.D.M.; Gobbi, S.; Andreatto, C.A.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduróz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.-M.; Chen, Y.-H.; Chang, J.-K.; Luo, J.J.; Dun, N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef]
- Hashemi, M.-S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Katanosaka, K.; Murase, S.; Kashio, M.; Tominaga, M.; Mizumura, K. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS ONE 2013, 8, e65751. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shi, X.; Luo, M.; Li, K.; Zhang, M.; Ma, J.; Li, Y.; Liu, Y.; Zhang, C.; Liu, X.; et al. Taurine inhibits neuron apoptosis in hippocampus of diabetic rats and high glucose exposed HT-22 cells via the NGF-Akt/Bad pathway. Amino Acids 2020, 52, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Liao, X.-M.; Liu, D.; Hu, J.; Yin, Y.-Y.; Wang, J.-Z.; Zhu, L.-Q. NGF promotes long-term memory formation by activating poly(ADP-ribose)polymerase-1. Neuropharmacology 2012, 63, 1085–1092. [Google Scholar] [CrossRef]
- Chae, C.-H.; Kim, H.-T. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem. Int. 2009, 55, 208–213. [Google Scholar] [CrossRef]
- Hong, Y.-P.; Lee, H.-C.; Kim, H.-T. Treadmill exercise after social isolation increases the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J. Exerc. Nutr. Biochem. 2015, 19, 11–18. [Google Scholar] [CrossRef]
- Bonini, M.; Fioretti, D.; Sargentini, V.; Del Giacco, S.; Rinaldi, M.; Tranquilli, C.; Bonini, S. Increased nerve growth factor serum levels in top athletes. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2013, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; So, W.Y.; Roh, H.T. Effects of aerobic exercise training and cranial electrotherapy stimulation on the stress-related hormone, the neurotrophic factor, and mood states in obese middle-aged women: A pilot clinical trial. Salud Ment. 2016, 39, 249–256. [Google Scholar] [CrossRef]
- Roh, H.-T.; Cho, S.-Y.; Yoon, H.-G.; So, W.-Y. Effect of Exercise Intensity on Neurotrophic Factors and Blood-Brain Barrier Permeability Induced by Oxidative-Nitrosative Stress in Male College Students. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 239–246. [Google Scholar] [CrossRef]
- Cintrón-Colón, A.F.; Almeida-Alves, G.; Boynton, A.M.; Spitsbergen, J.M. GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020, 382, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, A.M.; McCullough, M.J.; Spitsbergen, J.M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 2014, 257, 111–118. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.J.; Gyorkos, A.M.; Spitsbergen, J.M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 2013, 240, 258–268. [Google Scholar] [CrossRef]
- Gyorkos, A.M.; Spitsbergen, J.M. GDNF content and NMJ morphology are altered in recruited muscles following high-speed and resistance wheel training. Physiol. Rep. 2014, 2, e00235. [Google Scholar] [CrossRef]
- Peake, J.M.; Roberts, L.A.; Figueiredo, V.C.; Egner, I.; Krog, S.; Aas, S.N.; Suzuki, K.; Markworth, J.F.; Coombes, J.S.; Cameron-Smith, D.; et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J. Physiol. 2017, 595, 695–711. [Google Scholar] [CrossRef]
- Zahavi, E.E.; Ionescu, A.; Gluska, S.; Gradus, T.; Ben-Yaakov, K.; Perlson, E. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J. Cell Sci. 2015, 128, 1241–1252. [Google Scholar] [CrossRef]
- Haase, G.; Dessaud, E.; Garcès, A.; de Bovis, B.; Birling, M.; Filippi, P.; Schmalbruch, H.; Arber, S.; de Lapeyrière, O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron 2002, 35, 893–905. [Google Scholar] [CrossRef]
- Shimazu, K.; Zhao, M.; Sakata, K.; Akbarian, S.; Bates, B.; Jaenisch, R.; Lu, B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 2006, 13, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gómez-Pinilla, F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003, 987, 93–99. [Google Scholar] [CrossRef]
- Koo, H.M.; Lee, S.M.; Kim, M.H. Spontaneous Wheel Running Exercise Induces Brain Recovery via Neurotrophin-3 Expression Following Experimental Traumatic Brain Injury in Rats. J. Phys. Ther. Sci. 2013, 25, 1103–1107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, D.; Shadike, S.; Deng, J.; Liu, J.; Hu, Z.; Zhou, J.; Zhou, L.; Liu, Y. Effect of willed movement therapy on the expression of neurotrophin 3 and growth-associated protein 43 in rats with cerebral ischemia reperfusion. Nan Fang Yi Ke Da Xue Xue Bao 2011, 31, 1401–1404. [Google Scholar]
- Chung, J.-Y.; Kim, M.-W.; Bang, M.-S.; Kim, M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS ONE 2013, 8, e52461. [Google Scholar] [CrossRef]
- Domínguez-Sanchéz, M.A.; Bustos-Cruz, R.H.; Velasco-Orjuela, G.P.; Quintero, A.P.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Triana-Reina, H.R.; García-Hermoso, A.; González-Ruíz, K.; Peña-Guzmán, C.A.; et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018, 9, 741. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef]
- Cariati, I.; Masuelli, L.; Bei, R.; Tancredi, V.; Frank, C.; D’Arcangelo, G. Neurodegeneration in Niemann-Pick Type C Disease: An Updated Review on Pharmacological and Non-Pharmacological Approaches to Counteract Brain and Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 6600. [Google Scholar] [CrossRef]
- Diociaiuti, M.; Bonanni, R.; Cariati, I.; Frank, C.; D’Arcangelo, G. Amyloid Prefibrillar Oligomers: The Surprising Commonalities in Their Structure and Activity. Int. J. Mol. Sci. 2021, 22, 6435. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Villain, N.; Dubois, B. Alzheimer’s Disease Including Focal Presentations. Semin. Neurol. 2019, 39, 213–226. [Google Scholar] [CrossRef]
- Cass, S.P. Alzheimer’s Disease and Exercise: A Literature Review. Curr. Sports Med. Rep. 2017, 16, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-J.; Choi, D.-H.; Kwon, K.-C.; Kim, E.-H.; Kim, T.-K.; Koo, J.-H.; Cho, J.-Y. Exercise Reverses Amyloid Beta-Peptide-mediated Cognitive Deficits in Alzheimer’s Disease Mice Expressing Mutant Presenilin-2. Med. Sci. Sports Exerc. 2022, 54, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- De Freitas, G.B.; Lourenco, M.V.; De Felice, F.G. Protective actions of exercise-related FNDC5/Irisin in memory and Alzheimer’s disease. J. Neurochem. 2020, 155, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Silva, A.J. Molecular and cellular mechanisms of memory allocation in neuronetworks. Neurobiol. Learn. Mem. 2008, 89, 285–292. [Google Scholar] [CrossRef][Green Version]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Cuello, A.C.; Pentz, R.; Hall, H. The Brain NGF Metabolic Pathway in Health and in Alzheimer’s Pathology. Front. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef]
- Triaca, V.; Ruberti, F.; Canu, N. NGF and the Amyloid Precursor Protein in Alzheimer’s Disease: From Molecular Players to Neuronal Circuits. Adv. Exp. Med. Biol. 2021, 1331, 145–165. [Google Scholar] [CrossRef]
- Xu, C.-J.; Wang, J.-L.; Jin, W.-L. The Emerging Therapeutic Role of NGF in Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1211–1218. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Ni, X.; Li, N.; Zhang, B.; Fang, X. Enhancement of the nonamyloidogenic pathway by exogenous NGF in an Alzheimer transgenic mouse model. Neuropeptides 2014, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Noroozian, M.; Hashemian, F. Do serum GDNF levels correlate with severity of Alzheimer’s disease? Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 2865–2872. [Google Scholar] [CrossRef]
- Revilla, S.; Ursulet, S.; Álvarez-López, M.J.; Castro-Freire, M.; Perpiñá, U.; García-Mesa, Y.; Bortolozzi, A.; Giménez-Llort, L.; Kaliman, P.; Cristòfol, R.; et al. Lenti-GDNF gene therapy protects against Alzheimer’s disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci. Ther. 2014, 20, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, X.; Wang, H.; Si, C.; Liu, Q.; Du, Y. Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 629356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Li, J.-T.; Zhu, Y.-H.; Zhou, H.-L.; Liu, S.; Wang, T.-H. Effects of NT-4 gene modified fibroblasts transplanted into AD rats. Neurosci. Lett. 2009, 466, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet. Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet. Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Jang, Y.; Koo, J.-H.; Kwon, I.; Kang, E.-B.; Um, H.-S.; Soya, H.; Lee, Y.; Cho, J.-Y. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017, 1655, 186–193. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Kohno, R.; Sawada, H.; Kawamoto, Y.; Uemura, K.; Shibasaki, H.; Shimohama, S. BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem. Biophys. Res. Commun. 2004, 318, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Cai, Q.; Xie, Y.; Sheng, Z.-H. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Rep. 2012, 2, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Gamble, K.L.; Schultheiss, C.E.; Riddle, D.M.; West, A.B.; Lee, V.M.-Y. Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol. Biol. Cell 2014, 25, 4010–4023. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Wysocka, A.; Stepniewska, A.; Niewiadomska, G. Exercise-Induced Neuroprotection and Recovery of Motor Function in Animal Models of Parkinson’s Disease. Front. Neurol. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Lorigados Pedre, L.; Pavón Fuentes, N.; Alvarez González, L.; McRae, A.; Serrano Sánchez, T.; Blanco Lescano, L.; Macías González, R. Nerve growth factor levels in Parkinson disease and experimental parkinsonian rats. Brain Res. 2002, 952, 122–127. [Google Scholar] [CrossRef]
- Wu, D.-D.; Huang, L.; Zhang, L.; Wu, L.-Y.; Li, Y.-C.; Feng, L. LLDT-67 attenuates MPTP-induced neurotoxicity in mice by up-regulating NGF expression. Acta Pharmacol. Sin. 2012, 33, 1187–1194. [Google Scholar] [CrossRef]
- Luo, D.; Zhao, J.; Cheng, Y.; Lee, S.M.-Y.; Rong, J. N-Propargyl Caffeamide (PACA) Ameliorates Dopaminergic Neuronal Loss and Motor Dysfunctions in MPTP Mouse Model of Parkinson’s Disease and in MPP(+)-Induced Neurons via Promoting the Conversion of proNGF to NGF. Mol. Neurobiol. 2018, 55, 2258–2267. [Google Scholar] [CrossRef]
- Barker, R.A.; Björklund, A.; Gash, D.M.; Whone, A.; Van Laar, A.; Kordower, J.H.; Bankiewicz, K.; Kieburtz, K.; Saarma, M.; Booms, S.; et al. GDNF and Parkinson’s Disease: Where Next? A Summary from a Recent Workshop. J. Parkinsons. Dis. 2020, 10, 875–891. [Google Scholar] [CrossRef]
- Penn, R.D.; Dalvi, A.; Slevin, J.; Young, B.; Gash, D.; Gerhardt, G.; Hutchinson, M. GDNF in treatment of Parkinson’s disease: Response to editorial. Lancet. Neurol. 2006, 5, 202–203. [Google Scholar] [CrossRef]
- Gottschalk, C.G.; Jana, M.; Roy, A.; Patel, D.R.; Pahan, K. Gemfibrozil Protects Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease via PPARα-Dependent Astrocytic GDNF Pathway. J. Neurosci. 2021, 41, 2287–2300. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Huang, H.; Bi, J.; Yao, Y.; Wen, T. Combined treatment of neurotrophin-3 gene and neural stem cells is ameliorative to behavior recovery of Parkinson’s disease rat model. Brain Res. 2009, 1257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lingor, P.; Unsicker, K.; Krieglstein, K. GDNF and NT-4 protect midbrain dopaminergic neurons from toxic damage by iron and nitric oxide. Exp. Neurol. 2000, 163, 55–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. https://doi.org/10.3390/jfmk7020038
Bonanni R, Cariati I, Tarantino U, D’Arcangelo G, Tancredi V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. Journal of Functional Morphology and Kinesiology. 2022; 7(2):38. https://doi.org/10.3390/jfmk7020038
Chicago/Turabian StyleBonanni, Roberto, Ida Cariati, Umberto Tarantino, Giovanna D’Arcangelo, and Virginia Tancredi. 2022. "Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases" Journal of Functional Morphology and Kinesiology 7, no. 2: 38. https://doi.org/10.3390/jfmk7020038
APA StyleBonanni, R., Cariati, I., Tarantino, U., D’Arcangelo, G., & Tancredi, V. (2022). Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. Journal of Functional Morphology and Kinesiology, 7(2), 38. https://doi.org/10.3390/jfmk7020038






