Integrated Sports Medicine: A First Investigation of Heart Performance in Opera Singers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Echocardiographic Exam
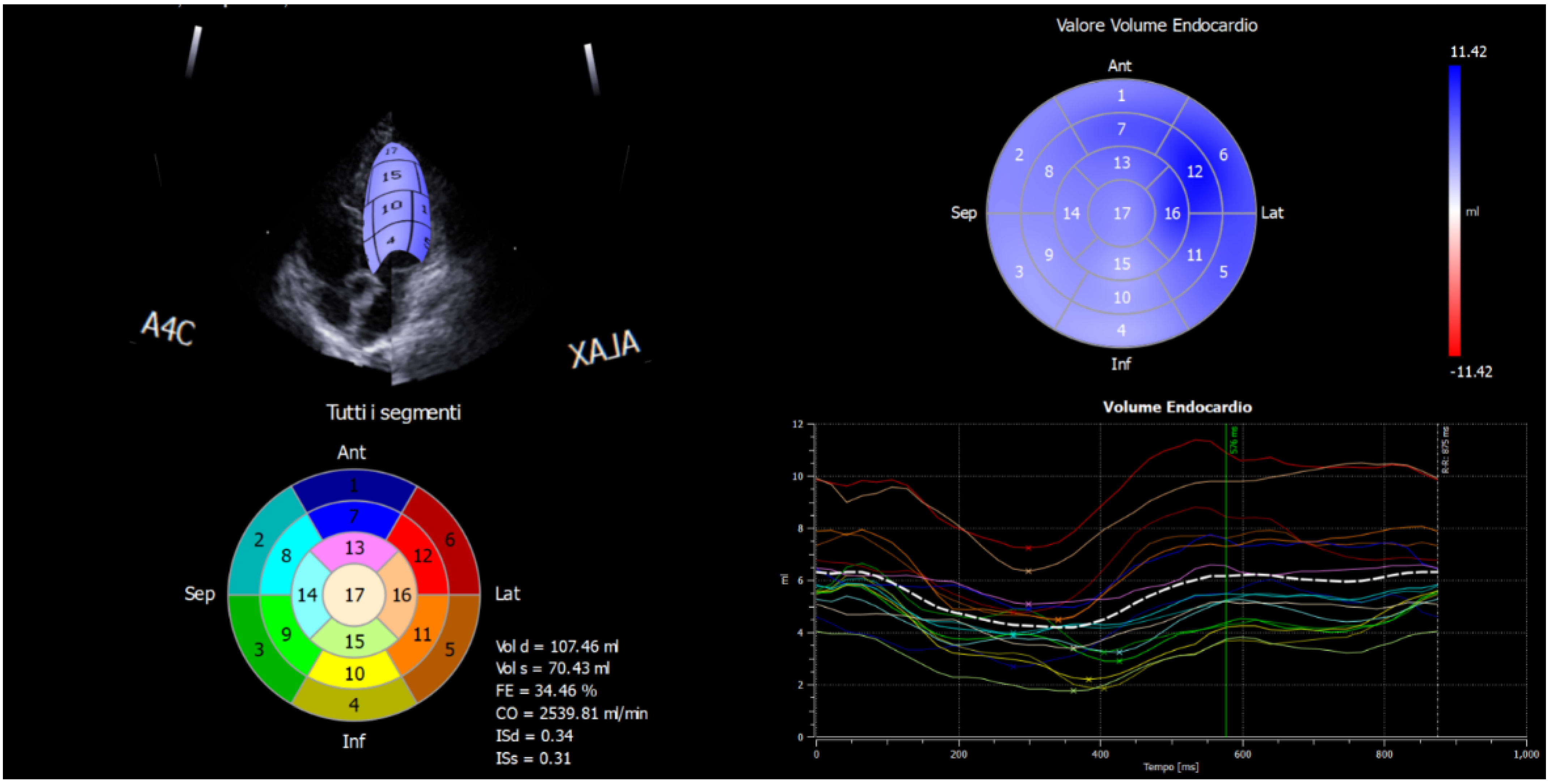
2.2. Strain Analysis by Speckle-Tracking Model
2.3. Statistical Analysis
ECG Parameters
3. Results
4. Conclusions
5. Limits
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koelsch, S.; Jäncke, L. Music and the heart. Eur. Heart J. 2015, 36, 3043–3049. [Google Scholar] [CrossRef] [PubMed]
- Claiborne Ray, C. Singing and Fitness. New York Times, 1 April 2008. Available online: http://www.nytimes.com/2008/04/01/science/01qna.html(accessed on 4 March 2020).
- Vickhoff, B.; Malmgren, H.; Aström, R.; Nyberg, G.; Ekström, S.R.; Engwall, M.; Snygg, J.; Nilsson, M.; Jörnsten, R. Music structure determines heart rate variability of singers. Front. Psychol. 2013, 4, 334. [Google Scholar] [CrossRef] [Green Version]
- Dileo, C.; Bradt, J.; Grocke, D.; Magill, L. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst. Rev. 2011, 8, 1–98. [Google Scholar]
- Kolsch, S.; Remppis, A.; Sammler, D.; Jentschke, S.; Mietchen, D.; Fritz, T.; Bonnemeier, H.; Siebel, W.A. A cardiac signature of emotionality. Eur. J. Neurosci. 2007, 26, 3328–3338. [Google Scholar] [CrossRef]
- Petekkaya, E.; Yücel, A.H.; Sürmelioğlu, Ö. Evaluation of the Supraglottic and Subglottic Activities Including Acoustic Assessment of the Opera-Chant Singers. J. Voice 2019, 33, 255.e1–255.e7. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire sort form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Dyrstad, S.M.; Hansen, B.H.; Holme, I.M.; Anderssen, S.A. Comparison of Self-reported versus Accelerometer-Measured Physical Activity. Med. Sci. Sports Exerc. 2014, 46, 99–106. [Google Scholar] [CrossRef]
- Fogelholm, M.; Malmberg, J.; Suni, J.; Santtila, M.; Kyröläinen, H.; Mäntysaari, M.; Oja, P. International Physical Activity Questionnaire: Validity against fitness. Med. Sci. Sports Exerc. 2006, 38, 753–760. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Voigt, J.U.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- Devereux, R.B. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization and comparison to other methods. Hypertension 1987, 20, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Takeuchi, M.; Tsang, W.; Takigiku, K.; Yasukochi, S.; Patel, A.R.; Mor-Avi, V.; Lang, R.M.; Otsuji, Y. Age-Related Normal Range of Left Ventricular Strain and Torsion Using Three-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bazett, H.C. An analysis of the time-relations of electrocardiograms. Heart 1920, 7, 353–370. [Google Scholar] [CrossRef]
- Auer, R.; Bauer, D.C.; Marques-Vidal, P.; Butler, J.; Min, L.J.; Cornuz, J.; Satterfield, S.; Newman, A.B.; Vittinghoff, E.; Rodondi, N. Health ABCS: Association of major and minor ECG abnormalities with coronary heart disease events. JAMA 2012, 307, 1497–1505. [Google Scholar] [PubMed] [Green Version]
- Grossman, E.; Grossman, A.; Schein, M.; Zimlichman, R.; Gavish, B. Breathing-control lowers blood pressure. J. Hum. Hypertens. 2001, 15, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branca, J.J.V.; Gulisano, M.; Marella, M.; Mascherini, G. Are Opera Singers Fit or Not? Sustainability 2020, 12, 4213. [Google Scholar] [CrossRef]
- Priest, D.-L.; Karageorghis, C.I. A qualitative investigation into the characteristics and effects of music accompanying exercise. Eur. Phys. Educ. Rev. 2008, 14, 347–366. [Google Scholar] [CrossRef]
| Opera Singers | Athletes | p | |||
|---|---|---|---|---|---|
| Median | Quartile Deviation | Median | Quartile Deviation | ||
| Age (y) | 55 | 9.75 | 17 | 2 | <0.001 |
| BMI (kg/m2) | 26.08 | 4.52 | 23.04 | 1.41 | 0.004 |
| LVDd (mm) | 47 | 2.75 | 51 | 1.50 | 0.004 |
| LVSd (mm) | 31 | 3.38 | 32 | 2.50 | 0.486 |
| LV IVS (mm) | 9.40 | 0.89 | 9.70 | 0.50 | 0.190 |
| LV PW (mm) | 9.20 | 0.66 | 9.60 | 0.50 | 0.887 |
| EF% | 66.50 | 5.88 | 62.00 | 2.50 | 0.027 |
| iLV mass (g/m2) | 81.05 | 12.51 | 108.79 | 11.36 | <0.001 |
| MAPSE (mm) | 17.50 | 1.38 | 18 | 1.50 | 0.180 |
| E/A | 1.08 | 0.23 | 2.37 | 0.73 | <0.001 |
| DTc (ms) | 211.50 | 20.25 | 197 | 35 | 0.429 |
| E1 (cm/s) | 9 | 1.56 | 15 | 1.50 | <0.001 |
| A1 (cm/s) | 9.70 | 0.98 | 5 | 1 | <0.001 |
| RV (mm) | |||||
| RVOT Prox | 27 | 3.38 | 25 | 3 | 0.372 |
| RVOT distal | 24 | 2.25 | 20 | 3.50 | 0.062 |
| RV basal | 32.50 | 4.25 | 35 | 2.50 | 0.026 |
| RV medium | 25 | 3.25 | 28 | 3.50 | 0.041 |
| RV long | 66 | 8.13 | 71 | 5.50 | 0.029 |
| RV Free Wall | 7 | 0.96 | 4.40 | 0.65 | <0.001 |
| PP (mmHg) | 8.18 | 1.09 | 10 | 6.05 | 0.025 |
| RV (Area T) (mm2) | 16.54 | 3.16 | 25 | 2.50 | <0.001 |
| RV (Area S) (mm2) | 8.36 | 1.69 | 13 | 2.50 | <0.001 |
| RV TDI S’ (cm/s) | 12 | 0.50 | 13 | 2 | 0.005 |
| Aortic Root (mm) | 24 | 3.88 | 30 | 2 | 0.006 |
| LA Volume (mL) | 31 | 7.25 | 33 | 3 | 0.552 |
| RA Volume (mL) | 23 | 6.38 | 38 | 11.50 | 0.024 |
| Opera Singers | Athletes | p | |||
|---|---|---|---|---|---|
| Median | Quartile Deviation | Median | Quartile Deviation | ||
| 4D Global Strain (%) | −17.71 | 2.74 | −23.30 | 2.36 | 0.003 |
| GLS4c (%) | −18.74 | 2.95 | −20.88 | 2.60 | 0.097 |
| GLS3c (%) | −16.33 | 4.17 | −21.46 | 2.96 | 0.009 |
| GLS2c (%) | −15.39 | 3.95 | −21.22 | 2.24 | 0.002 |
| RV Strain (%) | −18.48 | 3.35 | −23.30 | −2.36 | 0.002 |
| Apical Rotation | 3.24 | 2.13 | 4.60 | 0.95 | 0.199 |
| Basal Rotation | −4.63 | 1.68 | −4.43 | 0.42 | 0.968 |
| Twist | 7.49 | 2.32 | 8.50 | 1.10 | 0.502 |
| Opera Singers | Athletes | p | |||
|---|---|---|---|---|---|
| Median | Quartile Deviation | Median | Quartile Deviation | ||
| HR | 71.50 | 6.38 | 61 | 5.50 | 0.010 |
| P | 101 | 17 | 85 | 16 | 0.177 |
| PR | 158 | 20.75 | 156 | 14 | 0.890 |
| QRS | 91 | 6 | 100 | 9 | 0.065 |
| QTc | 416 | 16.50 | 418 | 22 | 0.635 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsi, M.; Orlandi, G.; Bini, V.; Stefani, L. Integrated Sports Medicine: A First Investigation of Heart Performance in Opera Singers. J. Funct. Morphol. Kinesiol. 2022, 7, 36. https://doi.org/10.3390/jfmk7020036
Corsi M, Orlandi G, Bini V, Stefani L. Integrated Sports Medicine: A First Investigation of Heart Performance in Opera Singers. Journal of Functional Morphology and Kinesiology. 2022; 7(2):36. https://doi.org/10.3390/jfmk7020036
Chicago/Turabian StyleCorsi, Marco, Goffredo Orlandi, Vittorio Bini, and Laura Stefani. 2022. "Integrated Sports Medicine: A First Investigation of Heart Performance in Opera Singers" Journal of Functional Morphology and Kinesiology 7, no. 2: 36. https://doi.org/10.3390/jfmk7020036
APA StyleCorsi, M., Orlandi, G., Bini, V., & Stefani, L. (2022). Integrated Sports Medicine: A First Investigation of Heart Performance in Opera Singers. Journal of Functional Morphology and Kinesiology, 7(2), 36. https://doi.org/10.3390/jfmk7020036







