Trunk Angle Modulates Feedforward and Feedback Control during Single-Limb Squatting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
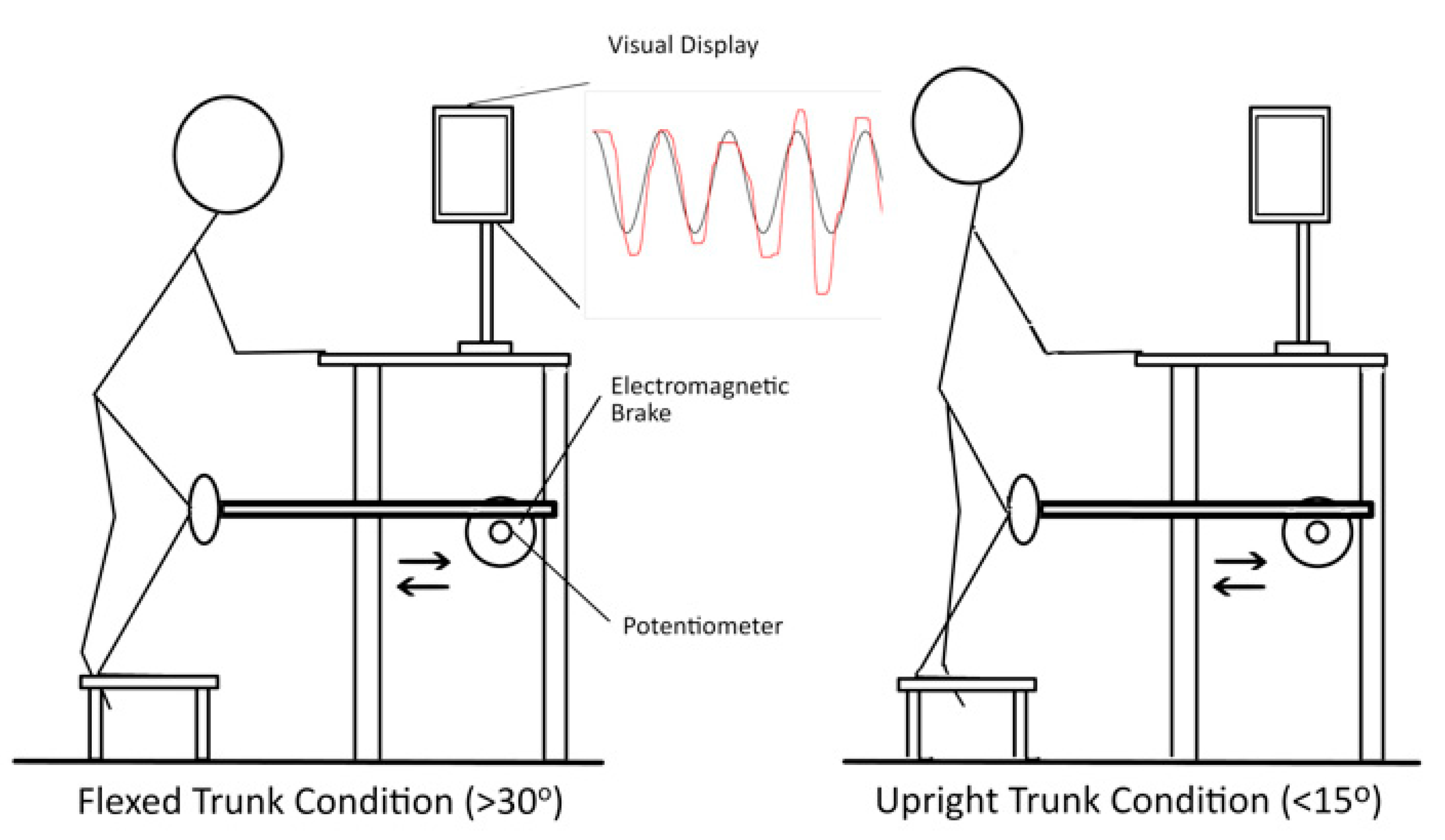
2.3. SLS Task
2.4. Electromyography (EMG)
2.5. Kinematics
2.6. Statistical Analyses
3. Results
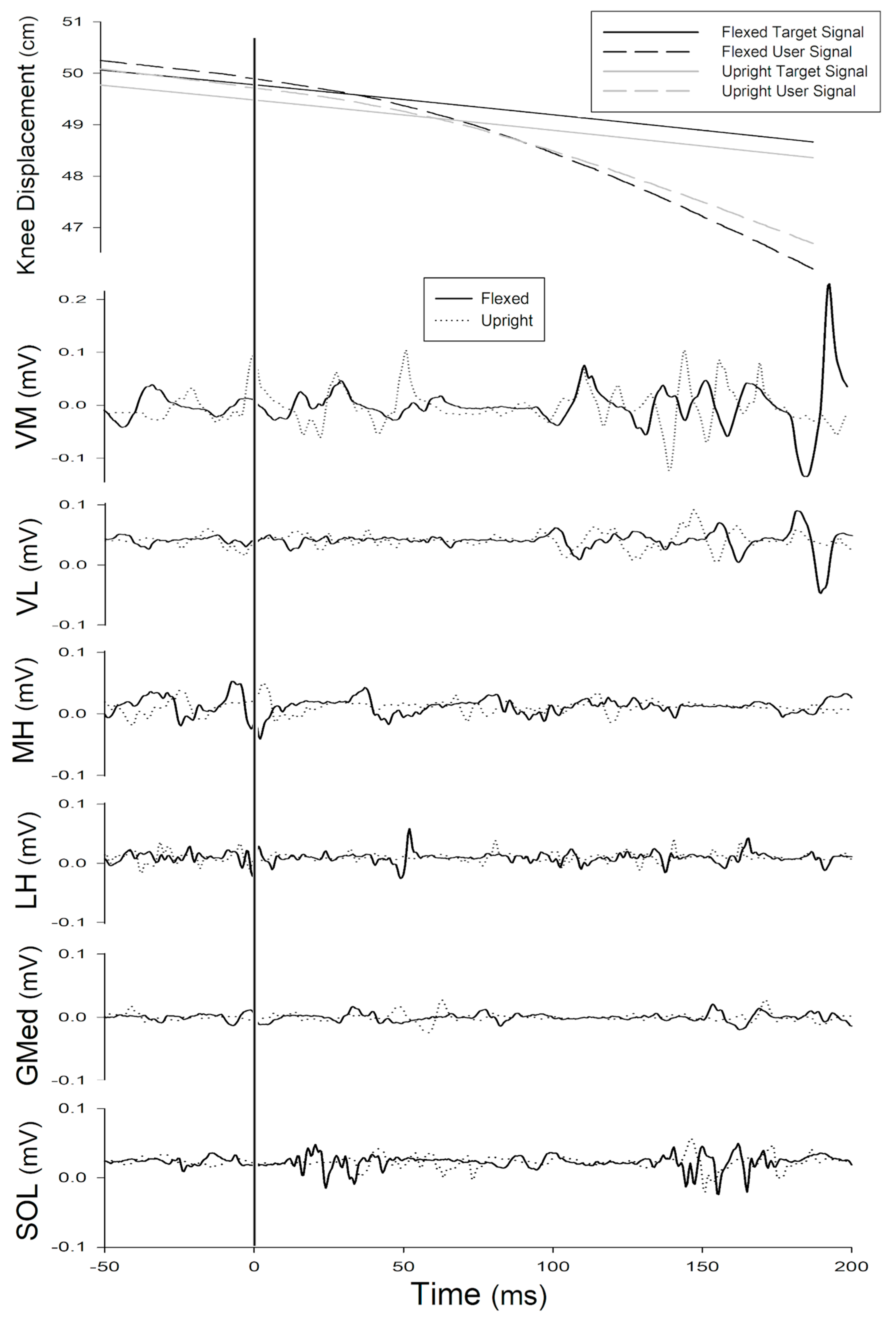
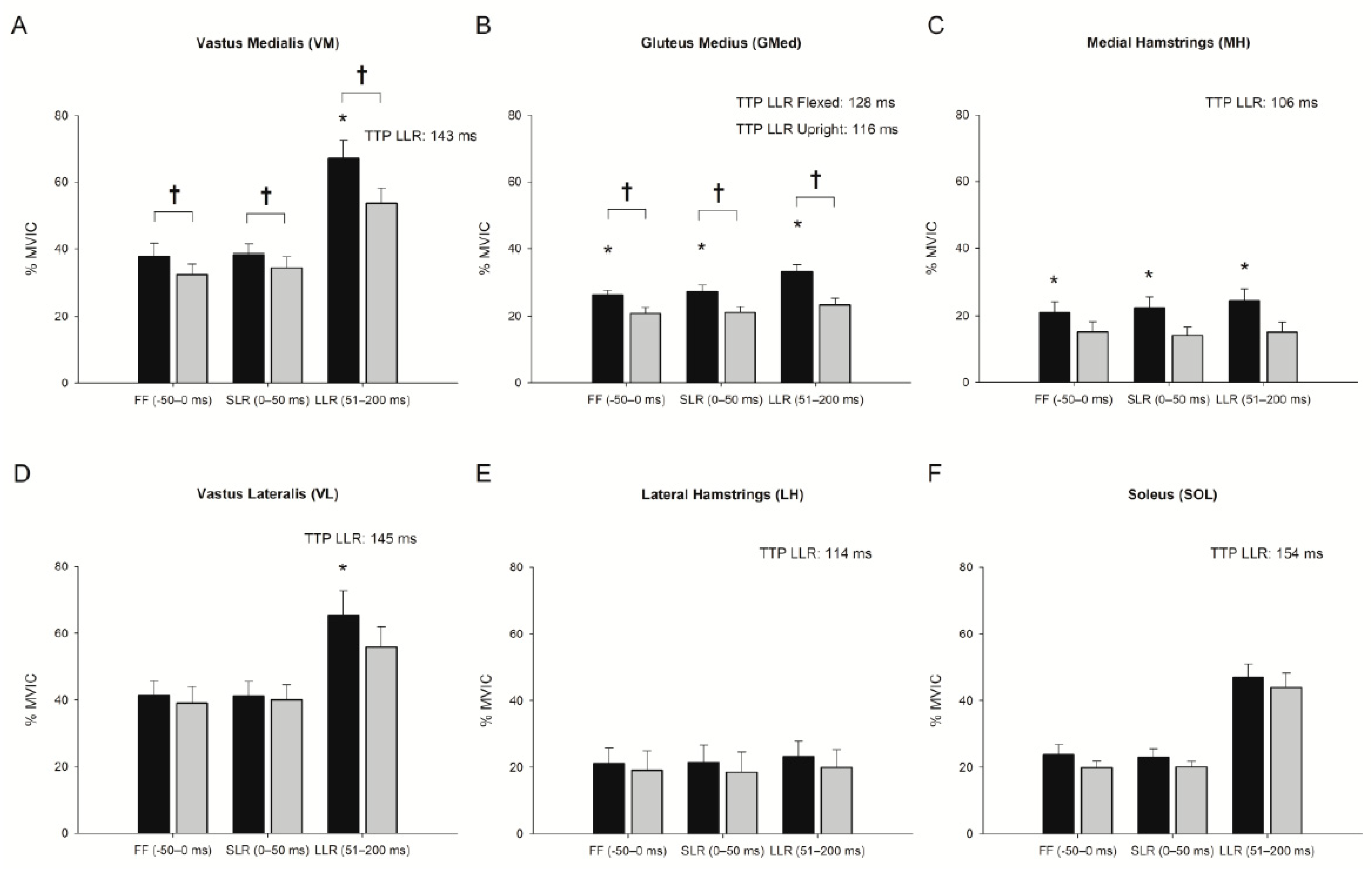
3.1. Effect of Trunk Position on Feedforward Control (−50–0 ms)
3.2. Effect of Trunk Position on Short-Latency Reflex (0–50 ms)
3.3. Effect of Trunk Position on Long-Latency Reflex (51–200 ms)
3.4. SLS Performance
4. Discussion
4.1. Sex Differences
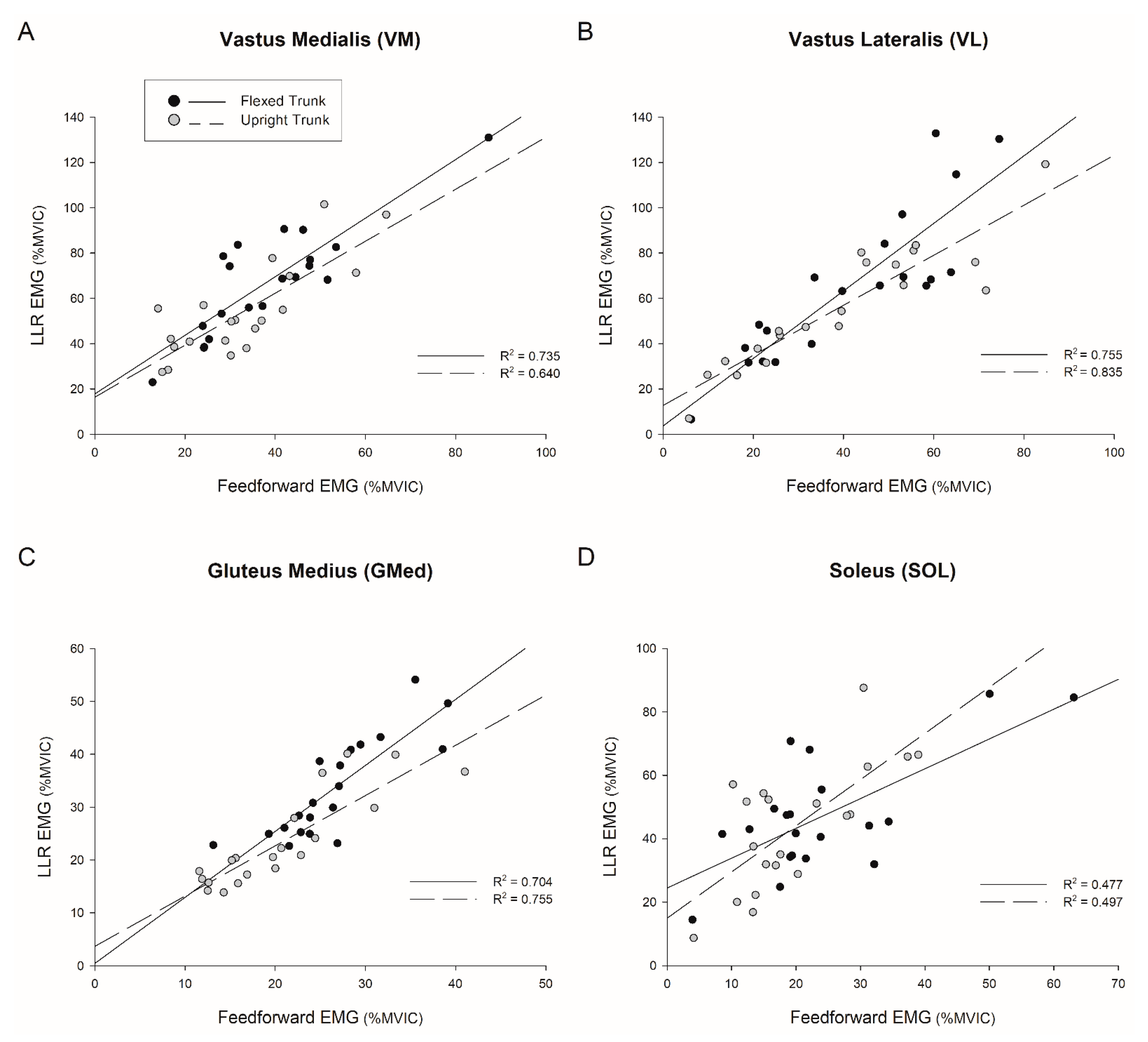
4.2. Feedforward Activation Predicts Long-Latency Response
4.3. Trunk Flexion Elicits Novel Activation Patterns
4.4. Functional Relevance of Observed Motor Responses
4.5. Effect of Trunk Flexion on Task Performance
4.6. Study Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Herzog, M.M.; Marshall, S.W.; Lund, J.L.; Pate, V.; Spang, J.T. Cost of Outpatient Arthroscopic Anterior Cruciate Ligament Reconstruction among Commercially Insured Patients in the United States, 2005–2013. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Agel, J.; Albohm, M.J.; Arendt, E.A.; Dick, R.W.; Garrett, W.E.; Garrick, J.G.; Hewett, T.E.; Huston, L.; Ireland, M.L.; et al. Noncontact Anterior Cruciate Ligament Injuries: Risk Factors and Prevention Strategies. J. Am. Acad. Orthop. Surg. 2000, 8, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Alentorn-Geli, E.; Myer, G.D.; Silvers, H.J.; Samitier, G.; Romero, D.; Lázaro-Haro, C.; Cugat, R. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: Mechanisms of injury and underlying risk factors. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 705–729. [Google Scholar] [CrossRef] [PubMed]
- Carlson, V.R.; Sheehan, F.T.; Boden, B.P. Video Analysis of Anterior Cruciate Ligament (ACL) Injuries: A Systematic Review. JBJS Rev. 2016, 4, e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahr, R. Why screening tests to predict injury do not work-and probably never will: A critical review. Br. J. Sports Med. 2016, 50, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losciale, J.; Zdeb, R.M.; Ledbetter, L.; Reiman, M.P.; Sell, T.C. The Association Between Passing Return-to-Sport Criteria and Second Anterior Cruciate Ligament Injury Risk: A Systematic Review with Meta-analysis. J. Orthop. Sports Phys. Ther. 2019, 49, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agel, J.; Rockwood, T.; Klossner, D. Collegiate ACL Injury Rates Across 15 Sports: National Collegiate Athletic Association Injury Surveillance System Data Update (2004–2005 Through 2012–2013). Clin. J. Sport Med. 2016, 26, 518–523. [Google Scholar] [CrossRef]
- Ashigbi, E.Y.K.; Banzer, W.; Niederer, D. Return to Sport Tests’ Prognostic Value for Reinjury Risk after Anterior Cruciate Ligament Reconstruction: A Systematic Review. Med. Sci. Sports Exerc. 2019, 52, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, A.; Lloyd, D.G.; Bryant, A.L.; Headrick, J.; Sayer, T.A.; Saxby, D.J. Mechanism of Anterior Cruciate Ligament Loading during Dynamic Motor Tasks. Med. Sci. Sports Exerc. 2021, 53, 1235–1244. [Google Scholar] [CrossRef]
- Ritzmann, R.; Lee, K.; Krause, A.; Gollhofer, A.; Freyler, K. Stimulus Prediction and Postural Reaction: Phase-Specific Modulation of Soleus H-Reflexes Is Related to Changes in Joint Kinematics and Segmental Strategy in Perturbed Upright Stance. Front. Integr. Neurosci. 2018, 12, 62. [Google Scholar] [CrossRef]
- Matthews, P.B. The human stretch reflex and the motor cortex. Trends Neurosci. 1991, 14, 87–91. [Google Scholar] [CrossRef]
- Pruszynski, J.A.; Kurtzer, I.; Scott, S.H. The long-latency reflex is composed of at least two functionally independent processes. J. Neurophysiol. 2011, 106, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B.; Diener, H.C.; Nashner, L.M. Influence of central set on human postural responses. J. Neurophysiol. 1989, 62, 841–853. [Google Scholar] [CrossRef]
- Prochazka, A.; Clarac, F.; Loeb, G.; Rothwell, J.; Wolpaw, J. What do reflex and voluntary mean? Modern views on an ancient debate. Exp. Brain Res. 2000, 130, 417–432. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L. Neuromuscular Performance in Normal and Anterior Cruciate Ligament-Deficient Lower Extremities. Am. J. Sports Med. 1994, 22, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, S.; Shields, R.K. Neuromuscular responses in individuals with anterior cruciate ligament repair. Clin. Neurophysiol. 2011, 122, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.K.; Madhavan, S.; Gregg, E.; Leitch, J.; Petersen, B.; Salata, S.; Wallerich, S. Neuromuscular Control of the Knee during a Resisted Single-Limb Squat Exercise. Am. J. Sports Med. 2005, 33, 1520–1526. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Cole, K.; Shaffer, M.A.; Petrie, M.A.; Yen, C.-L.; Shields, R.K. Speed, resistance, and unexpected accelerations modulate feed forward and feedback control during a novel weight bearing task. Gait Posture 2017, 52, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, F.T.; Sipprell, W.H., III; Boden, B.P. Dynamic sagittal plane trunk control during anterior cruciate ligament injury. Am. J. Sports Med. 2012, 40, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, B.P.; Torg, J.S.; Knowles, S.B.; Hewett, T.E. Video analysis of anterior cruciate ligament injury: Abnormalities in hip and ankle kinematics. Am. J. Sports Med. 2009, 37, 252–259. [Google Scholar] [CrossRef]
- Sasaki, S.; Nagano, Y.; Kaneko, S.; Imamura, S.; Koabayshi, T.; Fukubayashi, T. The Relationships Between the Center of Mass Position and the Trunk, Hip, and Knee Kinematics in the Sagittal Plane: A Pilot Study on Field-Based Video Analysis for Female Soccer Players. J. Hum. Kinet. 2015, 45, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Hewett, T.E.; Myer, G.D. The Mechanistic Connection Between the Trunk, Hip, Knee, and Anterior Cruciate Ligament Injury. Exerc. Sport Sci. Rev. 2011, 39, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Kulas, A.S.; Hortobágyi, T.; DeVita, P. Trunk position modulates anterior cruciate ligament forces and strains during a single-leg squat. Clin. Biomech. 2012, 27, 16–21. [Google Scholar] [CrossRef]
- Blackburn, J.T.; Padua, D.A. Sagittal-Plane Trunk Position, Landing Forces, and Quadriceps Electromyographic Activity. J. Athl. Train. 2009, 44, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Shimokochi, Y.; Ambegaonkar, J.P.; Meyer, E.G.; Lee, S.Y.; Shultz, S.J. Changing sagittal plane body position during single-leg landings influences the risk of non-contact anterior cruciate ligament injury. Knee Surg. Sports Traumatol. Arthrosc. 2012, 21, 888–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, J.T.; Padua, D. Influence of trunk flexion on hip and knee joint kinematics during a controlled drop landing. Clin. Biomech. 2008, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-C.; Ko, Y.-A.; Hammond, K.E.; Xerogeanes, J.W.; Warren, G.; Powers, C.M. Increasing hip and knee flexion during a drop-jump task reduces tibiofemoral shear and compressive forces: Implications for ACL injury prevention training. J. Sports Sci. 2016, 35, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Demorat, G.; Weinhold, P.; Blackburn, T.; Chudik, S.; Garrett, W. Aggressive Quadriceps Loading Can Induce Noncontact Anterior Cruciate Ligament Injury. Am. J. Sports Med. 2004, 32, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Ashton-Miller, J.; Huston, L. A Gender-Related Difference in the Contribution of the Knee Musculature to Sagittal-Plane Shear Stiffness in Subjects with Similar Knee Laxity. J. Bone Jt. Surg. Am. Vol. 2002, 84, 10–16. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Hoogenboom, B.J.; Myer, G.D. Understanding and preventing acl injuries: Current biomechanical and epidemiologic considerations—Update 2010. N. Am. J. sports Phys. Ther. NAJSPT 2010, 5, 234–251. [Google Scholar]
- Huston, L.; Wojtys, E.M. Neuromuscular Performance Characteristics in Elite Female Athletes. Am. J. Sports Med. 1996, 24, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Nashner, L.M.; Diener, H.C. Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 1990, 82, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.B. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J. Physiol. 1986, 374, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Maniar, N.; Schache, A.G.; Sritharan, P.; Opar, D. Non-knee-spanning muscles contribute to tibiofemoral shear as well as valgus and rotational joint reaction moments during unanticipated sidestep cutting. Sci. Rep. 2018, 8, 2501. [Google Scholar] [CrossRef] [Green Version]
- Mokhtarzadeh, H.; Yeow, C.-H.; Goh, J.C.H.; Oetomo, D.; Malekipour, F.; Lee, P.V.S. Contributions of the Soleus and Gastrocnemius muscles to the anterior cruciate ligament loading during single-leg landing. J. Biomech. 2013, 46, 1913–1920. [Google Scholar] [CrossRef]
- Bates, N.A.; Schilaty, N.; Krych, A.J.; Hewett, T.E. Variation in ACL and MCL Strain Before Initial Contact Is Dependent on Injury Risk Level During Simulated Landings. Orthop. J. Sports Med. 2019, 7, 2325967119884906. [Google Scholar] [CrossRef]
- Englander, Z.A.; Baldwin, E.L.; Smith, W.A.; Garrett, W.E.; Spritzer, C.E.; DeFrate, L.E. In Vivo Anterior Cruciate Ligament Deformation During a Single-Legged Jump Measured by Magnetic Resonance Imaging and High-Speed Biplanar Radiography. Am. J. Sports Med. 2019, 47, 3166–3172. [Google Scholar] [CrossRef]
- Chen, J.; Kim, J.; Shao, W.; Schlecht, S.H.; Baek, S.Y.; Jones, A.K.; Ahn, T.; Ashton-Miller, J.A.; Holl, M.M.B.; Wojtys, E.M. An Anterior Cruciate Ligament Failure Mechanism. Am. J. Sports Med. 2019, 47, 2067–2076. [Google Scholar] [CrossRef]
- Serpell, B.G.; Scarvell, J.M.; Pickering, M.R.; Ball, N.B.; Newman, P.; Perriman, D.; Warmenhoven, J.; Smith, P.N. Medial and lateral hamstrings and quadriceps co-activation affects knee joint kinematics and ACL elongation: A pilot study. BMC Musculoskelet. Disord. 2015, 16, 348. [Google Scholar] [CrossRef] [Green Version]
- Palmieri-Smith, R.M.; McLean, S.G.; Ashton-Miller, J.; Wojtys, E.M. Association of Quadriceps and Hamstrings Cocontraction Patterns with Knee Joint Loading. J. Athl. Train. 2009, 44, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Garrett, W.E.; Gross, M.T.; Padua, D.A.; Queen, R.M.; Yu, B. The effect of performance demands on lower extremity biomechanics during landing and cutting tasks. J. Sport Health Sci. 2016, 8, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, K.R.; Shields, R.K. Age and Cognitive Stress Influences Motor Skill Acquisition, Consolidation, and Dual-Task Effect in Humans. J. Mot. Behav. 2019, 51, 622–639. [Google Scholar] [CrossRef] [PubMed]

| Variable | Males (n = 10) | Females (n = 10) | p-Value |
|---|---|---|---|
| Age (years) | 24.7 (4.1) | 23.0 (0.7) | 0.210 |
| Height (cm) | 176.5 (7.1) | 172.0 (7.4) | 0.174 |
| Weight (kg) | 80.9 (16.1) | 61.5 (10.9) | 0.006 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.A.; Nozu, S.; Shields, R.K. Trunk Angle Modulates Feedforward and Feedback Control during Single-Limb Squatting. J. Funct. Morphol. Kinesiol. 2021, 6, 82. https://doi.org/10.3390/jfmk6040082
Johnson KA, Nozu S, Shields RK. Trunk Angle Modulates Feedforward and Feedback Control during Single-Limb Squatting. Journal of Functional Morphology and Kinesiology. 2021; 6(4):82. https://doi.org/10.3390/jfmk6040082
Chicago/Turabian StyleJohnson, Kristin A., Shojiro Nozu, and Richard K. Shields. 2021. "Trunk Angle Modulates Feedforward and Feedback Control during Single-Limb Squatting" Journal of Functional Morphology and Kinesiology 6, no. 4: 82. https://doi.org/10.3390/jfmk6040082
APA StyleJohnson, K. A., Nozu, S., & Shields, R. K. (2021). Trunk Angle Modulates Feedforward and Feedback Control during Single-Limb Squatting. Journal of Functional Morphology and Kinesiology, 6(4), 82. https://doi.org/10.3390/jfmk6040082






