Effects of Activity Tracker-Based Counselling and Live-Web Exercise on Breast Cancer Survivors during Italy COVID-19 Lockdown
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Recording and Control of Daily Physical Activity, Sedentary, and Sleep Time
2.4. Dietary Habits
2.5. Live Online Physical Exercise Sessions
2.6. Statistical Analysis
3. Results
3.1. Basal Characteristics of the Sample
3.2. Sedentary Time
3.3. Time Spent in Light- to Vigorous-Intensity Physical Activities
3.4. Time Spent in Light-Intensity Physical Activities
3.5. Time Spent in Moderate-Intensity Physical Activities
3.6. Time Spent in Vigorous-Intensity Physical Activities
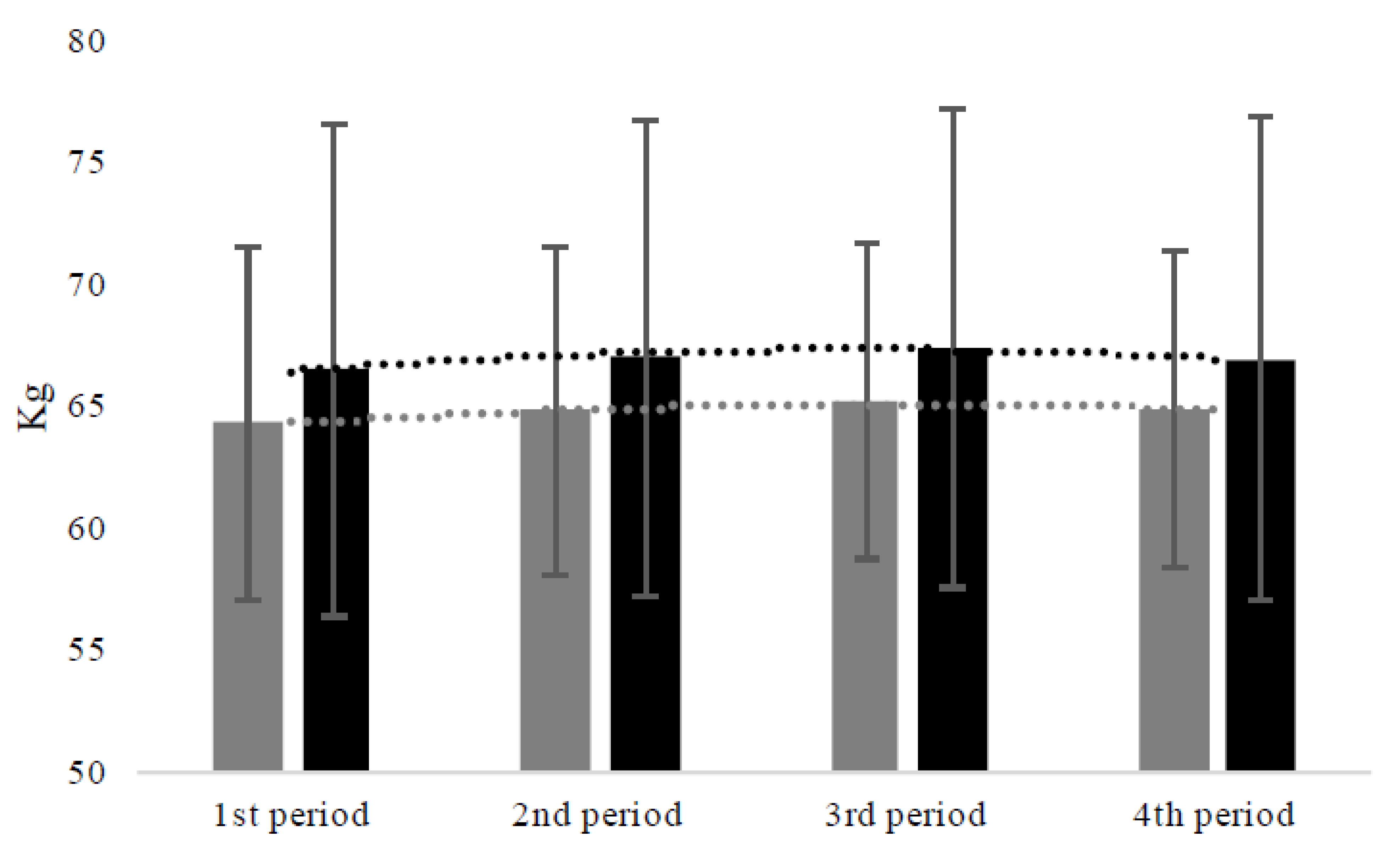
3.7. Body Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrawal, S. Late effects of cancer treatment in breast cancer survivors. S. Asian J. Cancer 2014, 3, 112–115. [Google Scholar] [CrossRef]
- Wang, X.S.; Woodruff, J.F. Cancer-related and treatment-related fatigue. Gynecol. Oncol. 2015, 136, 446–452. [Google Scholar] [CrossRef]
- Duregon, F.; Vendramin, B.; Bullo, V.; Gobbo, S.; Cugusi, L.; Di Blasio, A.; Neunhaeuserer, D.; Zaccaria, M.; Bergamin, M.; Ermolao, A. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: A systematic review. Crit. Rev. Oncol. 2018, 121, 90–100. [Google Scholar] [CrossRef]
- Fassier, P.; Zelek, L.; Partula, V.; Srour, B.; Bachmann, P.; Touillaud, M.; Druesne-Pecollo, N.; Galan, P.; Cohen, P.; Hoarau, H.; et al. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Santé cohort. Medicine 2016, 95, e4629. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Geraerts, I.; Demeyer, H.; Van der Gucht, E.; Dams, L.; de Kinkelder, C.; Dukers-van Althuis, S.; Van Kampen, M.; Devoogdt, N. Physical activity levels after treatment for breast cancer: Two-year follow-up. Breast 2018, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Monninkhof, E.M.; Peeters, P.H.M.; Van Gils, C.H.; Van Den Bongard, D.H.J.G.; Wendel-Vos, G.C.W.; Zuithoff, N.P.A.; Verkooijen, H.M.; May, A.M. Physical activity levels of women with breast cancer during and after treatment, a comparison with the Dutch female population. Acta Oncol. 2019, 58, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.A.; Ehlers, D.; Gavin, K.L.; Aguinaga, S.; Cottrell, A.; Nielsen, A.; Solk, P.; McAuley, E.; Phillips, S.M. Effects of reallocating sedentary time with physical activity on quality of life indicators in breast cancer survivors. Psychooncology 2019, 28, 1430–1437. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Alam, K.; Dunn, J.; Gow, J. The burden of chronic diseases among Australian cancer patients: Evidence from a longitudinal exploration, 2007–2017. PLoS ONE 2020, 15, e0228744. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Cianchetti, E.; Gallina, S.; Bucci, I.; Di Santo, S.; Tinari, C.; Di Donato, F.; Izzicupo, P.; Di Baldassarre, A.; et al. Psychophysical health status of breast cancer survivors and effects of 12 weeks of aerobic training. Complement Ther. Clin. Pract. 2017, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Di Blasio, A.; Morano, T.; Napolitano, G.; Bucci, I.; Di Santo, S.; Gallina, S.; Cugusi, L.; Di Donato, F.; D’Arielli, A.; Cianchetti, E. Nordic walking and the Isa method for breast cancer survivors: Effects on upper limb circumferences and total body extracellular water—A pilot study. Breast Care 2016, 11, 428–431. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Bucci, I.; Di Santo, S.; D’Arielli, A.; Castro, C.G.; Cugusi, L.; Cianchetti, E.; Napolitano, G. Physical exercises for breast cancer survivors: Effects of 10 weeks of training on upper limb circumferences. J. Phys. Ther. Sci. 2016, 28, 2778–2784. [Google Scholar] [CrossRef]
- Hartman, S.J.; Nelson, S.H.; Weiner, L.S. Patterns of Fitbit use and activity levels throughout a physical activity intervention: Exploratory analysis from a randomized controlled trial. JMIR Mhealth Uhealth 2018, 6, e29. [Google Scholar] [CrossRef]
- Valle, C.G.; Deal, A.M.; Tate, D.F. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: A randomized controlled pilot study. J. Cancer Surviv. 2017, 11, 133–148. [Google Scholar] [CrossRef]
- Lloyd, G.R.; Oza, S.; Kozey-Keadle, S.; Pellegrini, C.A.; Conroy, D.E.; Penedo, F.J.; Spring, B.J.; Phillips, S.M. Breast cancer survivors’ beliefs and preferences regarding technology-supported sedentary behavior reduction interventions. AIMS Public Health 2016, 3, 592–614. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Hadgraft, N.T.; Moore, M.M.; Rosenberg, D.E.; Lynch, C.; Reeves, M.M.; Lynch, B.M. A qualitative evaluation of breast cancer survivors’ acceptance of and preferences for consumer wearable technology activity trackers. Support. Care Cancer 2017, 25, 3375–3384. [Google Scholar] [CrossRef]
- Rosenberg, D.; Kadokura, E.; Bouldin, E.; Miyawaki, C.; Higano, C.; Hartzler, A. Acceptability of Fitbit for physical activity tracking within clinical care among men with prostate cancer. In Proceedings of the AMIA Annual Symposium, Chicago, IL, USA, 12–16 November 2016; pp. 1050–1059. [Google Scholar]
- Wu, H.S.; Gal, R.; van Sleeuwen, N.C.; Brombacher, A.C.; IJsselsteijn, W.A.; May, A.M.; Monninkhof, E.M. Breast cancer survivors’ experience with an activity tracker integrated into a supervised exercise program: Qualitative study. JMIR Mhealth Uhealth 2020, 7, e10820. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.R. What’s happening in the “quantified self” movement? ICLS 2014 Proceedings. In Proceedings of the International Conference of the Learning Sciences, Boulder, CO, USA, 23–27 June 2014; pp. 1032–1036. [Google Scholar]
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in physical activity and sedentary behavior in response to COVID-19 and their associations with mental health in 3052 US adults. Int. J. Environ. Res. Public Health 2020, 17, 6469. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B.; Coca, A. Physical activity change during COVID-19 confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef]
- Trabelsi, K.; Ammar, A.; Masmoudi, L.; Boukhris, O.; Chtourou, H.; Bouaziz, B.; Brach, M.; Bentlage, E.; How, D.; Ahmed, M.; et al. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biol. Sport 2021, 38, 495–506. [Google Scholar]
- Ammar, A.; Trabelsi, K.; Brach, M.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of home confinement on mental health and lifestyle behaviours during the COVID-19 outbreak: Insight from the ECLB-COVID19 multicenter study. Biol. Sport 2021, 38, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bentlage, E.; Ammar, A.; How, D.; Ahmed, M.; Trabelsi, K.; Chtourou, H.; Brach, M. Practical Recommendations for Maintaining Active Lifestyle during the COVID-19 Pandemic: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6265. [Google Scholar] [CrossRef]
- Swainston, J.; Chapman, B.; Grunfeld, E.A.; Derakshan, N. COVID-19 lockdown and its adverse impact on psychological health in breast cancer. Front. Psychol. 2020, 11, 2033. [Google Scholar] [CrossRef]
- Vanni, G.; Materazzo, M.; Pellicciaro, M.; Ingallinella, S.; Rho, M.; Santori, F.; Cotesta, M.; Caspi, J.; Makarova, A.; Pistolese, C.A.; et al. Breast cancer and COVID-19: The effect of fear on patients’ decision-making process. In Vivo 2020, 34, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Buman, M.P.; Youngstedt, S.D. Physical activity, sleep, and biobehavioral synergies for health. In Sleep and Affect; Elsevier: Amsterdam, The Nehterlands, 2015; pp. 321–337. [Google Scholar]
- A Creasy, S.; E Crane, T.; O Garcia, D.; A Thomson, C.; Kohler, L.N.; Wertheim, B.C.; Baker, L.D.; Coday, M.; Hale, L.; Womack, C.R.; et al. Higher amounts of sedentary time are associated with short sleep duration and poor sleep quality in postmenopausal women. Sleep 2019, 42, zsz093. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Galliott, M.; Lynch, B.M.; Nguyen, N.H.; Cohen, P.A.; Mohan, G.R.; Johansen, N.J.; Saunders, C. Acceptability and utility of, and preference for wearable activity tracker amongst non-metropolitan cancer survivors. PLoS ONE 2018, 13, e0210039. [Google Scholar] [CrossRef]
- Wright, S.P.; Brown, T.S.H.; Collier, S.R.; Sandberg, K. How consumer physical activity monitors could transform human physiology research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R358–R367. [Google Scholar] [CrossRef]
- Brooke, S.M.; An, H.-S.; Kang, S.-K.; Noble, J.M.; Berg, K.E.; Lee, J.-M. Concurrent validity of wearable activity tracker under free-living conditions. J. Strength Cond. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Sedentary Behaviour Research Network. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1988; p. 104. [Google Scholar]
- Newton, R.U.; Hart, N.H.; Clay, T. Keeping patients with cancer exercising in the age of COVID-19. JCO Oncol. Pract. 2020, 16, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Wessel, P.A.; Tinius, R.A.; Schafer, M.A.; Maples, J. Validity of activity trackers in estimating energy expenditure during high-intensity functional training. Res. Q. Exerc. Sport 2019, 90, 377–384. [Google Scholar] [CrossRef]
- Gardner, B.; Lally, P.; Rebar, A. Does habit weaken the relationship between intention and behavior? Revisiting the habit-intention interaction hypothesis. Soc. Personal. Psychol. Compass 2020, 14, e12553. [Google Scholar] [CrossRef]
- Shah, D. Healthy worker effect phenomenon. Indian J. Occup. Environ. Med. 2009, 13, 77–79. [Google Scholar] [CrossRef]
| N = 51 | E− (n = 27) | E+ (n = 24) | E− vs. E+ p | |
|---|---|---|---|---|
| Age (years) | 50.98 ± 6.17 | 50.62 ± 3.71 | 51.37 ± 8.18 | 0.67 |
| Time from surgery (months) | 13.68 ± 7.03 | 14.14 ± 6.72 | 13.16 ± 7.46 | 0.62 |
| Chemotherapy (y/n) | 22/29 | 12/15 | 10/14 | 0.49 |
| Radiation therapy (y/n) | 33/18 | 18/9 | 15/9 | 0.28 |
| Blood pressure-lowering drugs (y/n) | 37/14 | 6/21 | 8/16 | 0.28 |
| Lipid-lowering drugs (y/n) | 29/22 | 9/18 | 7/17 | 0.49 |
| Sedentary time (min) | 457.62 ± 101.36 | 465.67 ± 97.36 | 448.56 ± 105.36 | 0.53 |
| Light-intensity physical activities (min) | 327.42 ± 90.00 | 351.77 ± 87.77 | 300.01 ± 84.77 | 0.02 |
| Moderate-intensity physical activities (min) | 62.15 ± 41.96 | 60.53 ± 37.96 | 63.98 ± 46.15 | 0.81 |
| Vigorous-intensity physical activities (min) | 10.51 ± 16.81 | 6.08 ± 10.78 | 15.48 ± 20.62 | 0.04 |
| Model A Unconditional Means Model | Model B Unconditional Growth Model | Model C Personal Level Covariate | |||
|---|---|---|---|---|---|
| Initial status | Intercept | γ00 | 468.25 ± 14.64 *** | 482.50 ± 14.31 *** | 456.18 ± 20.66 *** |
| Intervention | γ01 | 49.56 ± 28.39 * | |||
| Rate of change | Intercept (time) | γ10-1 | −24.90 ± 5.60 *** | −8.18 ± 8.03 | |
| γ10-2 | Reference | Reference | |||
| γ10-3 | −10.92 ± 5.60 * | −17.88 ± 8.05 * | |||
| γ10-4 | −26.59 ± 7.56 *** | −35.05 ± 10.78 ** | |||
| Interaction | Time * intervention | ||||
| γ11-1 | −31.53 ± 11.04 ** | ||||
| γ11-2 | Reference | ||||
| γ11-3 | 13.09 ± 11.06 | ||||
| γ11-4 | 15.89 ± 14.82 | ||||
| Level 1 | Within-person | δ2e | 3547.98 ± 170.41 *** | 2417.51 ± 119.84 *** | 2415.17 ± 119.91 *** |
| Level 2 | In initial status | δ20 | 10,730.01 ± 2185.46 *** | 9582.94 ± 2015.14 *** | 9730.47 ± 2064.95 *** |
| In rate of change | δ21 | 35.68 ± 8.09 ** | 31.89 ± 7.39 *** | ||
| Covariance | δ01 | −81.03 ± 91.87 | −99.58 ± 89.64 | ||
| ρ | 0.75 | ||||
| R2y,y1 | 0.02 | 0.06 | |||
| R2e | 0.30 | ||||
| R20 | 0.01 | ||||
| R21 | 0.13 | ||||
| AIC | 10,309 | 10,063 | 10,025 | ||
| BIC | 10,313 | 10,071 | 10,032 |
| Model A Unconditional Means Model | Model B Unconditional Growth Model | Model C Personal Level Covariate | |||
|---|---|---|---|---|---|
| Initial status | Intercept | γ00 | 371.32 ± 11.98 *** | 341.75 ± 12.23 *** | 338.78 ± 17.97 *** |
| Intervention | γ01 | 5.86 ± 24.70 | |||
| Rate of change | Intercept (time) | γ10-1 | 54.13 ± 5.90 *** | 33.30 ± 11.63 ** | |
| γ10-2 | Reference | Reference | |||
| γ10-3 | 24.69 ± 5.88 *** | −16.89 ± 11.61 | |||
| γ10-4 | 54.78 ± 8.02 *** | −35.86 ± 15.73 * | |||
| Interaction | Time * intervention | ||||
| γ11-1 | 19.67 ± 9.53 * | ||||
| γ11-2 | Reference | ||||
| γ11-3 | 5.27 ± 9.94 | ||||
| γ11-4 | 47.55 ± 11.76 *** | ||||
| Level 1 | Within-person | δ2e | 4411.31 ± 211.87 *** | 2568.43 ± 127.38 *** | 2561.44 ± 127.20 *** |
| Level 2 | In initial status | δ20 | 7071.39 ± 1463.34 *** | 10,265 ± 2159.66 *** | 9984.46 ± 2120.10 *** |
| In rate of change | δ21 | 43.94 ± 9.85 *** | 39.55 ± 9.01 *** | ||
| Covariance | δ01 | −368.56 ± 118.64 ** | −325.05 ± 110.97 ** | ||
| ρ | 0.64 | ||||
| R2y,y1 | 0.03 | 0.07 | |||
| R2e | 0.37 | ||||
| R20 | 0.04 | ||||
| R21 | 0.10 | ||||
| AIC | 10,478 | 10,100 | 10,069 | ||
| BIC | 10,482 | 10,115 | 10,077 |
| Model A Unconditional Means Model | Model B Unconditional Growth Model | Model C Personal Level Covariate | |||
|---|---|---|---|---|---|
| Initial status | Intercept | γ00 | 310.27 ± 9.39 *** | 292.06 ± 9.64 *** | 279.85 ± 13.97 *** |
| Intervention | γ01 | 23.21 ± 19.20 | |||
| Rate of change | Intercept (time) | γ10-1 | 34.70 ± 4.58 *** | 20.05 ± 6.51 ** | |
| γ10-2 | Reference | Reference | |||
| γ10-3 | 13.72 ± 4.61 ** | 20.63 ± 6.58 ** | |||
| γ10-4 | 31.81 ± 6.10 *** | 45.81 ± 8.61 *** | |||
| Interaction | Time * intervention | ||||
| γ11-1 | 27.76 ± 8.95 ** | ||||
| γ11-2 | Reference | ||||
| γ11-3 | −13.11 ± 9.04 | ||||
| γ11-4 | −26.57 ± 11.83 * | ||||
| Level 1 | Within-person | δ2e | 2528.76 ± 121.45 *** | 1740.58 ± 86.28 *** | 1736.73 ± 86.20 *** |
| Level 2 | In initial status | δ20 | 4355.68 ± 899.26 *** | 6965.27 ± 1460.77 *** | 6331.01 ± 1345.51 *** |
| In rate of change | δ21 | 19.84 ± 4.67 *** | 16.82 ± 4.08 *** | ||
| Covariance | δ01 | −235.36 ± 69.32 *** | −188.39 ± 60.84 ** | ||
| ρ | 0.63 | ||||
| R2y,y1 | 0.03 | 0.07 | |||
| R2e | 0.28 | ||||
| R20 | 0.11 | ||||
| R21 | 0.17 | ||||
| AIC | 9971 | 9727 | 9688 | ||
| BIC | 9975 | 9735 | 9696 |
| Model A Unconditional Means Model | Model B Unconditional Growth Model | Model C Personal Level Covariate | |||
|---|---|---|---|---|---|
| Initial status | Intercept | γ00 | 53.09 ± 5.98 *** | 47.99 ± 5.36 *** | 55.43 ± 7.84 *** |
| Intervention | γ01 | −14.28 ± 10.77 | |||
| Rate of change | Intercept (time) | γ10-1 | 14.74 ± 2.38 *** | 8.99 ± 3.45 * | |
| γ10-2 | Reference | Reference | |||
| γ10-3 | 9.62 ± 2.33 *** | 11.55 ± 3.38 *** | |||
| γ10-4 | 20.50 ± 3.31 *** | 23.29 ± 4.80 *** | |||
| Interaction | Time * intervention | ||||
| γ11-1 | 10.91 ± 4.74 * | ||||
| γ11-2 | Reference | ||||
| γ11-3 | −3.71 ± 4.65 | ||||
| γ11-4 | −5.39 ± 6.59 | ||||
| Level 1 | Within-person | δ2e | 716.61 ± 34.42 *** | 345.29 ± 17.14 *** | 344.68 ± 17.13 *** |
| Level 2 | In initial status | δ20 | 1781.34 ± 364.24 *** | 1200.33 ± 254.36 *** | 1223.10 ± 261.47 *** |
| In rate of change | δ21 | 11.36 ± 2.43 *** | 11.09 ± 2.38 *** | ||
| Covariance | δ01 | −12.05 ± 17.93 | −13.31 ± 17.99 | ||
| Ρ | 0.71 | ||||
| R2y,y1 | 0.03 | 0.04 | |||
| R2e | 0.48 | ||||
| R20 | 0.03 | ||||
| R21 | 0.01 | ||||
| AIC | 8833 | 8316 | 8289 | ||
| BIC | 8837 | 8323 | 8297 |
| Model A Unconditional Means Model | Model B Unconditional Growth Model | Model C Personal Level Covariate | |||
|---|---|---|---|---|---|
| Initial status | Intercept | γ00 | 7.96 ± 1.34 *** | 5.80 ± 1.40 *** | 7.36 ± 1.98 *** |
| Intervention | γ01 | −2.79 ± 2.73 | |||
| Rate of change | Intercept (time) | γ10-1 | 4.00 ± 0.90 *** | 6.98 ± 1.30 * | |
| γ10-2 | Reference | Reference | |||
| γ10-3 | 1.97 ± 0.91 * | 1.87 ± 1.31 | |||
| γ10-4 | 3.53 ± 1.20 ** | 5.37 ± 1.73 ** | |||
| Interaction | Time * intervention | ||||
| γ11-1 | 5.62 ± 1.79 ** | ||||
| γ11-2 | Reference | ||||
| γ11-3 | 0.17 ± 1.80 | ||||
| γ11-4 | −3.50 ± 2.38 | ||||
| Level 1 | Within-person | δ2e | 93.03 ± 4.46 *** | 69.34 ± 3.44 *** | 68.21 ± 3.39 *** |
| Level 2 | In initial status | δ20 | 86.49 ± 18.33 *** | 142.82 ± 31.53 *** | 128.28 ± 28.90 ** |
| In rate of change | δ21 | 0.73 ± 0.17 *** | 0.71 ± 0.17 *** | ||
| Covariance | δ01 | −6.36 ± 1.99 ** | −5.65 ± 1.87 | ||
| ρ | 0.48 | ||||
| R2y,y1 | 0.02 | 0.06 | |||
| R2e | 0.24 | ||||
| R20 | 0.11 | ||||
| R21 | 0.03 | ||||
| AIC | 6913 | 6747 | 6713 | ||
| BIC | 6917 | 6755 | 6721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Blasio, A.; Morano, T.; Lancia, F.; Viscioni, G.; Di Iorio, A.; Grossi, S.; Cianchetti, E.; Cugusi, L.; Gobbo, S.; Bergamin, M.; et al. Effects of Activity Tracker-Based Counselling and Live-Web Exercise on Breast Cancer Survivors during Italy COVID-19 Lockdown. J. Funct. Morphol. Kinesiol. 2021, 6, 50. https://doi.org/10.3390/jfmk6020050
Di Blasio A, Morano T, Lancia F, Viscioni G, Di Iorio A, Grossi S, Cianchetti E, Cugusi L, Gobbo S, Bergamin M, et al. Effects of Activity Tracker-Based Counselling and Live-Web Exercise on Breast Cancer Survivors during Italy COVID-19 Lockdown. Journal of Functional Morphology and Kinesiology. 2021; 6(2):50. https://doi.org/10.3390/jfmk6020050
Chicago/Turabian StyleDi Blasio, Andrea, Teresa Morano, Federica Lancia, Gianluca Viscioni, Angelo Di Iorio, Simona Grossi, Ettore Cianchetti, Lucia Cugusi, Stefano Gobbo, Marco Bergamin, and et al. 2021. "Effects of Activity Tracker-Based Counselling and Live-Web Exercise on Breast Cancer Survivors during Italy COVID-19 Lockdown" Journal of Functional Morphology and Kinesiology 6, no. 2: 50. https://doi.org/10.3390/jfmk6020050
APA StyleDi Blasio, A., Morano, T., Lancia, F., Viscioni, G., Di Iorio, A., Grossi, S., Cianchetti, E., Cugusi, L., Gobbo, S., Bergamin, M., D’Eugenio, A., Masini, L., Rinaldi, M., Scognamiglio, M. T., Vamvakis, A., & Napolitano, G. (2021). Effects of Activity Tracker-Based Counselling and Live-Web Exercise on Breast Cancer Survivors during Italy COVID-19 Lockdown. Journal of Functional Morphology and Kinesiology, 6(2), 50. https://doi.org/10.3390/jfmk6020050







