Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
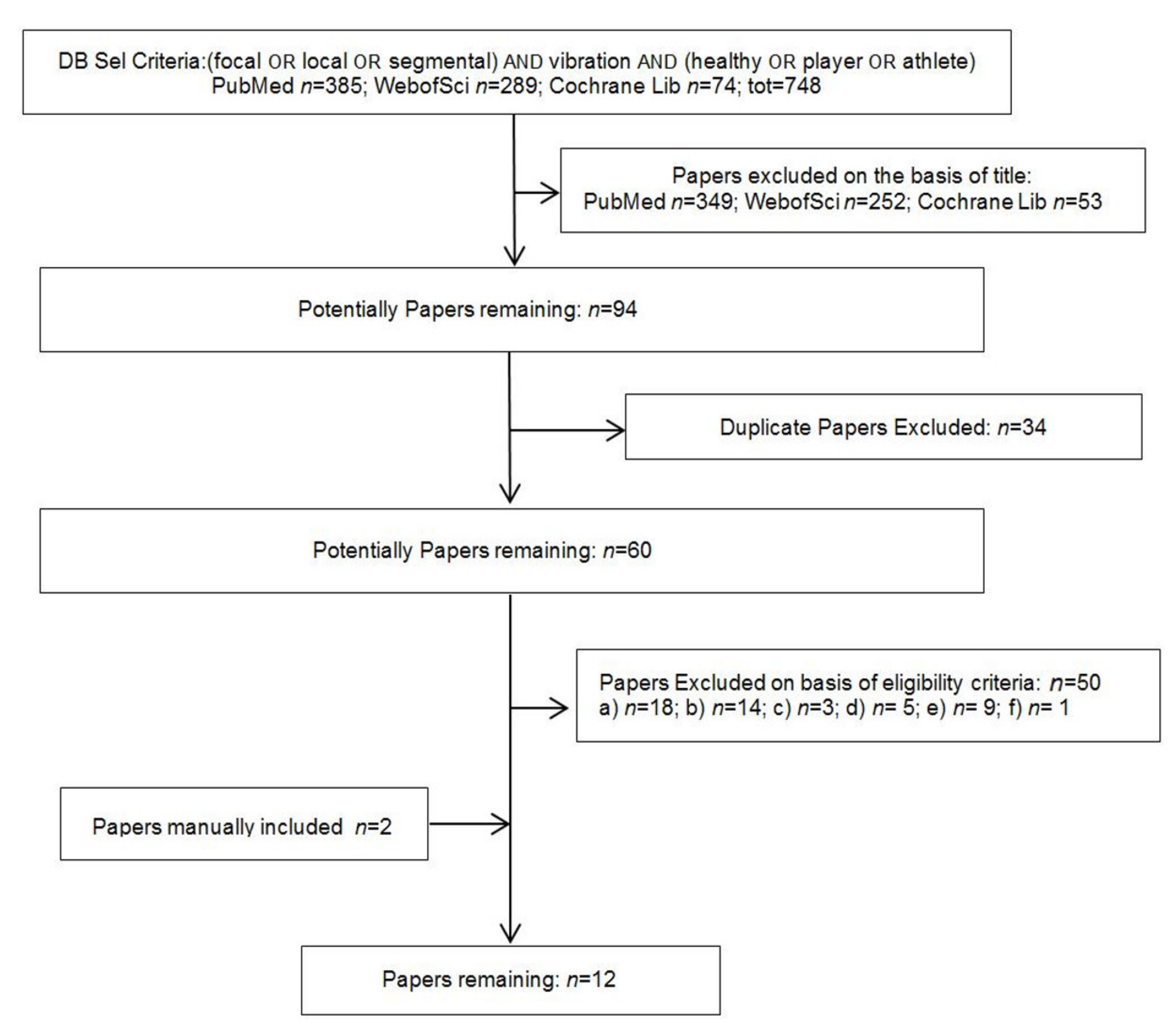
2.2. Selection Criteria
2.3. Study Eligibility
2.4. Assessment of Methodological Quality
3. Results
3.1. Assessment of Methodological Quality
3.2. Outcome Measures and After-Effects
3.3. Bias
4. Discussion
4.1. Outcomes
4.2. Focal Vibration and Fatigue
4.3. After-Effect Duration
4.4. FVT Versus Traditional Training Protocols
4.5. Protocol Parameters
4.5.1. Vibration Frequency and Amplitude
4.5.2. Dose Administration
4.5.3. Treated Muscles
4.5.4. State of the Muscle during the Treatment
4.6. Suggested Physiological Mechanisms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lum, D.; Barbosa, T.M. Brief Review: Effects of isometric strength training on strength and dynamic performance. Int. J. Sports Med. 2019, 40, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, J.S.; Rusko, H.K.; Nummela, A.T.; Paavolainen, L.M.; Häkkinen, K. Concurrent endurance and explosive type strength training increases activation and fast force production of leg extensor muscles in endurance athletes. J. Strength Cond. Res. 2007, 21, 613–620. [Google Scholar]
- Filipovic, A.; Kleinöder, H.; Dörmann, U.; Mester, J. Electromyostimulation—A systematic review of the effects of different electromyostimulation methods on selected strength parameters in trained and elite athletes. J. Strength Cond. Res. 2012, 26, 2600–2614. [Google Scholar] [CrossRef]
- Costantino, C.; Gimigliano, R.; Olvirri, S.; Gimigliano, F. Whole body vibration in sport: A critical review. J. Sports Med. Phys. Fit. 2014, 54, 757–764. [Google Scholar]
- Bianconi, R.; van der Meulen, J.J. The response to vibration of the end organs of mammalian muscle spindles. J. Neurophysiol. 1963, 26, 177–190. [Google Scholar] [CrossRef]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J. Physiol. 1976, 261, 673–693. [Google Scholar] [CrossRef]
- Burke, D.; Hagbarth, K.-E.; Löfstedt, L.; Wallin, B.G. The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. 1976, 261, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Roll, J.P.; Vedel, J.P.; Roll, R. Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Prog. Brain Res. 1989, 80, 113–123. [Google Scholar]
- Matthwes, P.B.C. Mammalian Muscle Receptors and Their Central Actions; Edward Arnold: London, UK, 1972. [Google Scholar]
- Thompson, A.K.; Wolpaw, J.R. Restoring walking after spinal cord injury: Operant conditioning of spinal reflexes can help. Neuroscientist 2015, 21, 203–215. [Google Scholar] [CrossRef]
- Bongiovanni, L.G.; Hagbarth, K.E. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J. Physiol. 1990, 423, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef]
- Cochrane, D.J. Vibration exercise: The potential benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef]
- Stania, M.; Juras, G.; Słomka, K.; Chmielewska, D.; Kŕol, P. The application of whole-body vibration in physiotherapy—A narrative review. Physiol. Int. 2016, 103, 133–145. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Lesinski, M.; Fernandez-del-Olmo, M. Small and inconsistent effects of whole body vibration on athletic performance: A systematic review and meta-analysis. Eur. J. Appl. Physiol. 2015, 115, 1605–1625. [Google Scholar] [CrossRef] [PubMed]
- Abercromby, A.F.J.; Amonette, W.E.; Layne, C.S.; McFarlin, B.K.; Hinman, M.R.; Paloski, W.H. Vibration Exposure and Biodynamic Responses during Whole-Body Vibration Training. Med. Sci. Sports Exerc. 2007, 39, 1794–1800. [Google Scholar] [CrossRef]
- Shinohara, M. Effects of Prolonged Vibration on Motor Unit Activity and Motor Performance. Med. Sci. Sports Exerc. 2005, 37, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, J.; Masani, K.; Kouzaki, M.; Hiroaki, K.; Fukunaga, T. Difference in after effects following prolonged Achilles tendon vibration on muscle activity during maximal voluntary contraction among plantar flexor synergists. J. Appl. Physiol. 2005, 98, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Clarke, M.; McNamara, B.; Moran, K. Influence of resistance load on neuromuscular response to vibration training. J. Strength Cond. Res. 2009, 23, 420. [Google Scholar] [CrossRef]
- Couto, B.P.; Silva, H.R.; Barbosa, M.P.; Szmuchrowski, L.A. Chronic Effects of Different Frequencies of Local Vibrations. Int. J. Sports Med. 2012, 32, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Folland, J.P. Prolonged infrapatellar tendon vibration does not influence quadriceps maximal or explosive isometric force production in man. Eur. J. Appl. Physiol. 2014, 114, 1757–1766. [Google Scholar] [CrossRef]
- Goebel, R.T.; Kleinöder, H.; Yue, Z.; Gosh, R.; Mester, J. Effect of Segment-Body Vibration on Strength Parameters. Sports Med. Open 2015, 1, 14. [Google Scholar] [CrossRef]
- Celletti, C.; Fara, M.A.; Filippi, G.M.; La Torre, G.; Tozzi, R.; Vanacore, N.; Camerota, F. Focal Muscle Vibration and Physical Exercise in Postmastectomy Recovery: An Explorative Study. Biomed. Res. Int. 2017, 2017, 7302892. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J. Effectiveness of using wearable vibration therapy to alleviate muscle soreness. Eur. J. Appl. Physiol. 2017, 117, 501–509. [Google Scholar] [CrossRef]
- Contemori, S.; Dieni, C.V.; Sullivan, J.A.; Ferraresi, A.; Occhigrossi, C.; Calabrese, F.; Pettorossi, V.E.; Biscarini, A.; Panichi, R. Sensory infow manipulation induces learning-like phenomena in motor behavior. Eur. J. Appl. Physiol. 2020, 120, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Germann, D.; El Bouse, A.; Shnier, J.; Abdelkader, N.; Kazemi, M. Effects of local vibration therapy on various performance parameters: A narrative literature review. J. Can. Chiropr. Assoc. 2018, 62, 170–181. [Google Scholar] [PubMed]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Ferraresi, A.; Rodio, A.; Azzena, G.B.; Filippi, G.M. Motor performance changes induced by muscle vibration. Eur. J. Appl. Physiol. 2006, 98, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Ring, H.; Rainoldi, A. High frequency vibration conditioning stimulation centrally reduces myoelectrical manifestation of fatigue in healthy subjects. J. Electromyogr. Kinesiol. 2009, 19, 998–1004. [Google Scholar] [CrossRef]
- Filippi, G.M.; Brunetti, O.; Botti, F.M.; Panichi, R.; Roscini, M.; Camerota, F.; Cesari, M.; Pettorossi, V.E. Improvement of stance control and muscle performance induced by focal muscle vibration in young-elderly women: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2009, 90, 2019–2025. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Mancinelli, R.; Toniolo, L.; Cancellara, L.; Paoli, A.; Puglielli, C.; Iodice, P.; Doria, C.; Bosco, G.; D’Amelio, L.; et al. Effects of local vibrations on skeletal muscle trophism in elderly people: Mechanical, cellular, and molecular events. Int. J. Mol. Med. 2009, 24, 503–512. [Google Scholar] [CrossRef]
- Lapole, T.; Pérot, C. Effects of repeated Achilles tendon vibration on triceps surae force production. J. Electromyogr. Kinesiol. 2010, 20, 648–654. [Google Scholar] [CrossRef]
- Iodice, P.; Bellomo, R.G.; Gialluca, G.; Fano, G.; Saggini, R. Acute and cumulative effects of focused high-frequency vibrations on the endocrine system and muscle strength. Eur. J. Appl. Physiol. 2011, 111, 897–904. [Google Scholar] [CrossRef]
- Brunetti, O.; Botti, F.M.; Roscini, M.; Brunetti, A.; Panichi, R.; Filippi, G.M.; Biscarini, A.; Pettorossi, V.E. Focal vibration of quadriceps muscle enhances leg power and decreases knee joint laxity in female volleyball players. J. Sports Med. Phys. Fit. 2012, 52, 595–596. [Google Scholar]
- Brunetti, O.; Botti, F.M.; Brunetti, A.; Biscarini, A.; Scarponi, A.M.; Filippi, G.M.; Pettorossi, V.E. Effects of focal vibration on bone mineral density and motor performance of postmenopausal osteoporotic women. J. Sports Med. Phys. Fit. 2015, 55, 118–127. [Google Scholar]
- Aprile, I.; di Sipio, E.; Germanotta, M.; Simbolotti, C.; Padua, L. Muscle focal vibration in healthy subjects: Evaluation of the effects on upper limb motor performance measured using a robotic device. Eur. J. Appl. Physiol. 2016, 116, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Souron, R.; Farabet, A.; Féasson, L.; Belli, A.; Millet, G.Y.; Lapole, T. Eight weeks of local vibration training increases dorsiflexor muscle cortical voluntary activation. J. Appl. Physiol. 2017, 122, 1504–1515. [Google Scholar] [CrossRef]
- Feltroni, L.; Monteleone, S.; Petrucci, L.; Carlisi, E.; Mazzacane, B.; Schieppati, M.; Dalla Toffola, E. Potentiation of muscle strength by focal vibratory stimulation on quadriceps femoris. G Ital. Med. Lav. Ergon. 2018, 40, 90–96. [Google Scholar]
- Filippi, G.M.; Fattorini, L.; Summa, A.; Zagaglia, A.; Rodio, A. Effects of focal vibration on power and work in multiple wingate tests. Biol. Sport 2020, 37, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Souron, R.; Besson, T.; Lapole, T.; Millet, G.Y. Neural adaptations in quadriceps muscle after 4 weeks of local vibration training in young versus older subjects. Appl. Physiol. Nutr. Metab. 2018, 43, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Celletti, C.; Fattorini, L.; Camerota, F.; Ricciardi, D.; La Torre, G.; Landi, F.; Filippi, G.M. Focal muscle vibration as a possible intervention to prevent falls in elderly women: A pragmatic randomized controlled trial. Aging Clin. Exp. Res. 2015, 27, 857–863. [Google Scholar] [CrossRef]
- Caserotti, P.; Aagaard, P.; Puggaard, L. Changes in power and force generation during coupled eccentric–concentric versus concentric muscle contraction with training and aging. Eur. J. Appl. Physiol. 2008, 103, 151–161. [Google Scholar] [CrossRef]
- Andersson, E.A.; Frank, P.; Pontén, M.; Ekblom, B.; Ekblom, M.; Moberg, M.; Sahlin, K. Improving strength, power, muscle aerobic capacity, and glucose tolerance through short-term progressive strength training among elderly people. J. Vis. Exp. 2017, 125, 55518. [Google Scholar] [CrossRef]
- Cuevas-Trisan, R. Balance problems and fall risks in the elderly. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 727–737. [Google Scholar] [CrossRef]
- Attanasio, G.; Camerota, F.; Ralli, M.; Galeoto, G.; La Torre, G.; Galli, M.; de Vincentiis, M.; Greco, A.; Celletti, C. Does focal mechanical stimulation of the lower limb muscles improve postural control and sit to stand movement in elderly? Aging Clin. Exp. Res. 2018, 30, 1161–1166. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P. Alteration of proprioceptive messages induced by tendon vibration in man: A microneurographic study. Exp. Brain Res. 1989, 76, 213–222. [Google Scholar] [CrossRef]
- Fallon, J.B.; Macefield, V.G. Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle Nerve 2007, 36, 21–29. [Google Scholar] [CrossRef]
- Fattorini, L.; Tirabasso, A.; Lunghi, A.; Sacco, F.; Marchetti, E. Muscular forearm activation in hand-grip tasks with superimposition of mechanical vibrations. J. Electromyogr. Kinesiol. 2016, 26, 143–145. [Google Scholar] [CrossRef]
- Marconi, B.; Filippi, G.M.; Koch, G.; Pecchioli, C.; Salerno, S.; Don, R.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J. Neurol. Sci. 2008, 275, 51–59. [Google Scholar] [CrossRef]
- Marconi, B.; Filippi, G.M.; Koch, G.; Giacobbe, V.; Pecchioli, C.; Versace, V.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil. Neural Repair 2011, 25, 48–60. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; van Baelen, M.; Swinnen, S.P. Corticospinal excitability changes following prolonged muscle tendon vibration. Neuroreport 2003, 14, 2001–2004. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J. Physiol. 2003, 551, 649–660. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J. Physiol. 2004, 561, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, K.; Williamon, A.; Butler, K.; Cordivari, C.; Lees, A.J.; Rothwell, J.C. Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain 2005, 128, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, R.; Rothwell, J.C. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur. J. Neurosci. 2006, 23, 822–829. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. Spatial attention affects sensorimotor reorganisation in human motor cortex. Exp. Brain Res. 2006, 170, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise mechanisms and recommendations for training practices. Sports Med. 2006, 36, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Mrówczynski, W.; Celichowski, J.; Raikova, R.; Krutki, P. Physiological consequences of doublet discharges on motoneuronal firing and motor unit force. Front. Cell Neurosci. 2015, 10, 81. [Google Scholar] [CrossRef]
- Mason, J.; Howatson, G.; Frazer, A.K.; Pearce, A.J.; Jaberzadeh, S.; Avela, J.; Kidgell, D.J. Modulation of intracortical inhibition and excitation in agonist and antagonist muscles following acute strength training. Eur. J. Appl. Physiol. 2019, 119, 2185–2214. [Google Scholar] [CrossRef]
- Dai, W.; Pi, Y.L.; Ni, Z.; Tan, X.Y.; Zhang, J.; Wu, Y. Maintenance of balance between motor cortical excitation and inhibition after long-term training. Neuroscience 2016, 336, 114–122. [Google Scholar] [CrossRef]
- Sato, D.; Yamazaki, Y.; Yamashiro, K.; Onishi, H.; Baba, Y.; Ikarashi, K.; Maruyama, A. Elite competitive swimmers exhibit higher motor cortical inhibition and superior sensorimotor skills in a water environment. Behav. Brain Res. 2020, 395, 112835. [Google Scholar] [CrossRef]
- Siddique, U.; Rahman, S.; Frazer, A.; Leung, M.; Pearce, A.J.; Kidgell, D.J. Task-dependent modulation of corticospinal excitability and inhibition following strength training. J. Electromyogr. Kinesiol. 2020, 52, 102411. [Google Scholar] [CrossRef]
- Lestienne, F. Effects of inertial load and velocity on the braking process of voluntary limb movements. Exp. Brain Res. 1979, 35, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D.; Obeso, J.A.; Rothwell, J.C. The function of the antagonist muscle during fast limb movements in man. J. Physiol. 1983, 335, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.M.; Wiegner, A.W.; Shahani, B.T. Role of agonist and antagonist muscles in fast arm movements in man. Exp. Brain Res. 1986, 63, 331–340. [Google Scholar] [CrossRef]
- Wierzbicka, M.M.; Wiegner, A.W. Accuracy of motor responses in subjects with and without control of antagonist muscle. J. Neurophysiol. 1996, 75, 2533–2541. [Google Scholar] [CrossRef]
- Karst, G.M.; Hasan, Z. Antagonist muscle activity during human forearm movements under varying kinematic and loading conditions. Exp. Brain Res. 1987, 67, 391–401. [Google Scholar] [CrossRef]
- Teulings, H.L.; Contreras-Vidal, J.L.; Stelmach, G.E.; Adler, C.H. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp. Neurol. 1997, 146, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, G.A.; Kaneko, M. Mechanical work and efficiency in level walking and running. J. Appl. Physiol. 1977, 18, 467–471. [Google Scholar] [CrossRef]
- Fattorini, L.; Tirabasso, A.; Lunghi, A.; Sacco, F.; Marchetti, E. Muscular synchronization and hand-arm fatigue. Int. J. Ind. Ergon. 2017, 62, 13–16. [Google Scholar] [CrossRef]
- Di Prampero, P.E.; Capelli, C.; Pagliaro, P.; Antonutto, G.; Girardis, M.; Zamparo, P.; Soule, R.G. Energetics of best performances in middle-distance running. J. Appl. Physiol. 1993, 74, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Kerkhoff, G. Modulation and rehabilitation of spatial neglect by sensory stimulation. Prog. Brain Res. 2003, 142, 257–314. [Google Scholar]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Biscarini, A.; Filippi, G.M.; Schieppati, M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin. Neurophysiol. 2015, 126, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
| Study | Tested Sbjts | Age (Years) | Main Protocol Features: FV Frequency; Application Duration; Num appl; Mechanical Signal Amplitude | Body Part Treated & Muscle Status during Treatment | Follow-up | Outcomes at the Follow-Up End (POST vs. PRE) |
|---|---|---|---|---|---|---|
| Fattorini L. et al., 2006 [30] | 21 (6 F; 15 M) | 31.0 ± 2.4 | 100 Hz, 3 applications/day, lasting 10 min each one, repeated for 3 consecutive days; p-p ≈ 0.05–0.15 mm | Quadriceps (one leg); Contracted/relaxed | 2 weeks | RFD ≈+ 27% *; Fatigue resistance (isotonic conditions) ≈+40% ** (vibrated side) ≈+15% (untreated side) MVC Unchanged; No changes with muscle relaxed during treatment |
| Casale et al., 2009 [31] | 10 (M) | 25–50 | 300 Hz, 1 application/day, lasting 30 min, repeated for 5 consecutive days; p-p ≈ 2 mm | Biceps brachii (one arm); Relaxed | 48 h | Fatigue index1 ≈ −20% * MVC Unchanged |
| Filippi GM et al., 2009 [32] | 60 (F) | 60–72 | 100 Hz, 3 applications/day, lasting 10 min each one, repeated for 3 consecutive days; p-p ≈ 0.2–0.5 mm | Quadriceps; Contracted/relaxed | 90 days | Power ≈ +35% * Squat Jump height ≈ +55% * Postural Sway Area2 ≈ –35% * Velocity of Sway3 ≈ –35% *; No changes with muscle relaxed during treatment |
| Pietrangelo T et al., 2009 [33] | 9 (5F; 4M) | 71.0 ± 5.7 (F); 75.3 ± 6.9 (M) | 300 Hz, for 15 min, 1 application/week for 8 weeks and 3 applications/weeks during the subsequent 4 weeks; Amplitude unknown | Quadriceps; Contracted | 16 weeks | MVC ≈ +40% * (M) ≈ +50% * (F); Muscle fiber cross-sectional Area: Unchanged Fast Myosin isoforms: +12% * |
| Lapole et al. 2010 [34] | 29 (Gender unknown) | 21.7 ± 1.7 College students | 50 Hz, 1 application/day, lasting 1 h, repeated for 14 consecutive days; p-p ≈ 0.2 mm | Triceps surae; Relaxed | 24 h | MVC ≈ +6.9% * EMG ≈ +9.3% * Twitch properties: Unchanged Muscle mass: Unchanged |
| Iodice et al., 2011 [35] | 36 (Gender unknown) | 21.5 College students | 300 Hz, 1 application/day, lasting 30 min, repeated 3 time/week, for 4 consecutive weeks: p-p ≈ 2 mm | Quadriceps Gluteus maximus Biceps femoris, GastrocnemiusTibialis anterior; Relaxed | 2 months | MVC ≈ +32.5% * Torque ≈ +33% * |
| Brunetti et al., 2012 [36] | 18 (F) | 22,7 Volleyball players | 100 Hz; 3 applications/day, lasting 10 min each one, repeated for 3 consecutive days: p-p ≈ 0.3–0.5 mm | Quadriceps; Contracted/relaxed | 240 days | Squat Jump height ≈ +26% ** Counter Movement Jump height = +13% **Knee Laxity ≈ −18% **; No changes with muscle relaxed during treatment |
| Brunetti O et al., 2015 [37] | 40 (F) | 65.2 ± 3.0 Postmenopausal women | 100 Hz, 3 applications/day, lasting 10 min each one, repeated for 3 consecutive days; p-p ≈ 0.3–0.5 mm | Quadriceps; Contracted | 360 days | Power ≈ +40% ** Squat Jump height ≈ +40% ** Postural Sway Area2 ≈ -35%, ** |
| Aprile et al., 2016 [38] | 48 (36F; 12M) | 25–50 | 100 Hz, 1 application/day, lasting 30 min, for 3 consecutive days; 200 Hz, 1 application/day, lasting 30 min, for 3 consecutive days; Pressure 150 mBar | Deltoids, Biceps Pectoralis; Muscle status unknown | 10 days | Fatigue resistance: ≈ +30%* (@200 Hz) Unchanged (@100 Hz) Movement Smoothness: ≈ +27% * (@200 Hz) Unchanged (@100 Hz) |
| Souron et al., 2017 [39] | 44 (24 F; 20 M) | 20 ± 1.0 | 100 Hz, 1 application/day, lasting1 h, repeated for 3 days/week, for 8 consecutive weeks; p-p ≈ 1 mm | Tibialis anterior (one leg); Relaxed | 2 weeks | MVC ≈ +12% ** (vibrated side) MVC ≈ +10% * (untreated side) |
| Feltroni L, et al., 2018 [40] | 27 (12 F; 15 M) | 22.2 ± 2.7 | 80/300 Hz, 1 application/day, lasting 30 min, repeated for 5 consecutive days. Pressure 240 mBar | Quadriceps; Relaxed | 4 weeks | Peak torque ≈ +29% * (both at 80 and 300 Hz) |
| Filippi GM et al., 2020 [41] | 28 (M) | 24 ± 3.0 University & phd students | 100 Hz, 3 applications/day, lasting 10 min each one, repeated for 3 consecutive days. p-p ≈ 0.2–0.5 mm | Quadriceps; Contracted | 2 weeks | Peak Power ≈ +10% * Average Peak ≈ +7% * Total Work ≈ +8%* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, L.; Rodio, A.; Pettorossi, V.E.; Filippi, G.M. Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review. J. Funct. Morphol. Kinesiol. 2021, 6, 39. https://doi.org/10.3390/jfmk6020039
Fattorini L, Rodio A, Pettorossi VE, Filippi GM. Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review. Journal of Functional Morphology and Kinesiology. 2021; 6(2):39. https://doi.org/10.3390/jfmk6020039
Chicago/Turabian StyleFattorini, Luigi, Angelo Rodio, Vito E. Pettorossi, and Guido M. Filippi. 2021. "Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review" Journal of Functional Morphology and Kinesiology 6, no. 2: 39. https://doi.org/10.3390/jfmk6020039
APA StyleFattorini, L., Rodio, A., Pettorossi, V. E., & Filippi, G. M. (2021). Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review. Journal of Functional Morphology and Kinesiology, 6(2), 39. https://doi.org/10.3390/jfmk6020039









