Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Methods
2.5. Study Selection
2.6. Data Collection Process and Items
2.7. Risk of Bias
2.8. Summary Measures
2.9. Data Analysis and Synthesis
3. Results
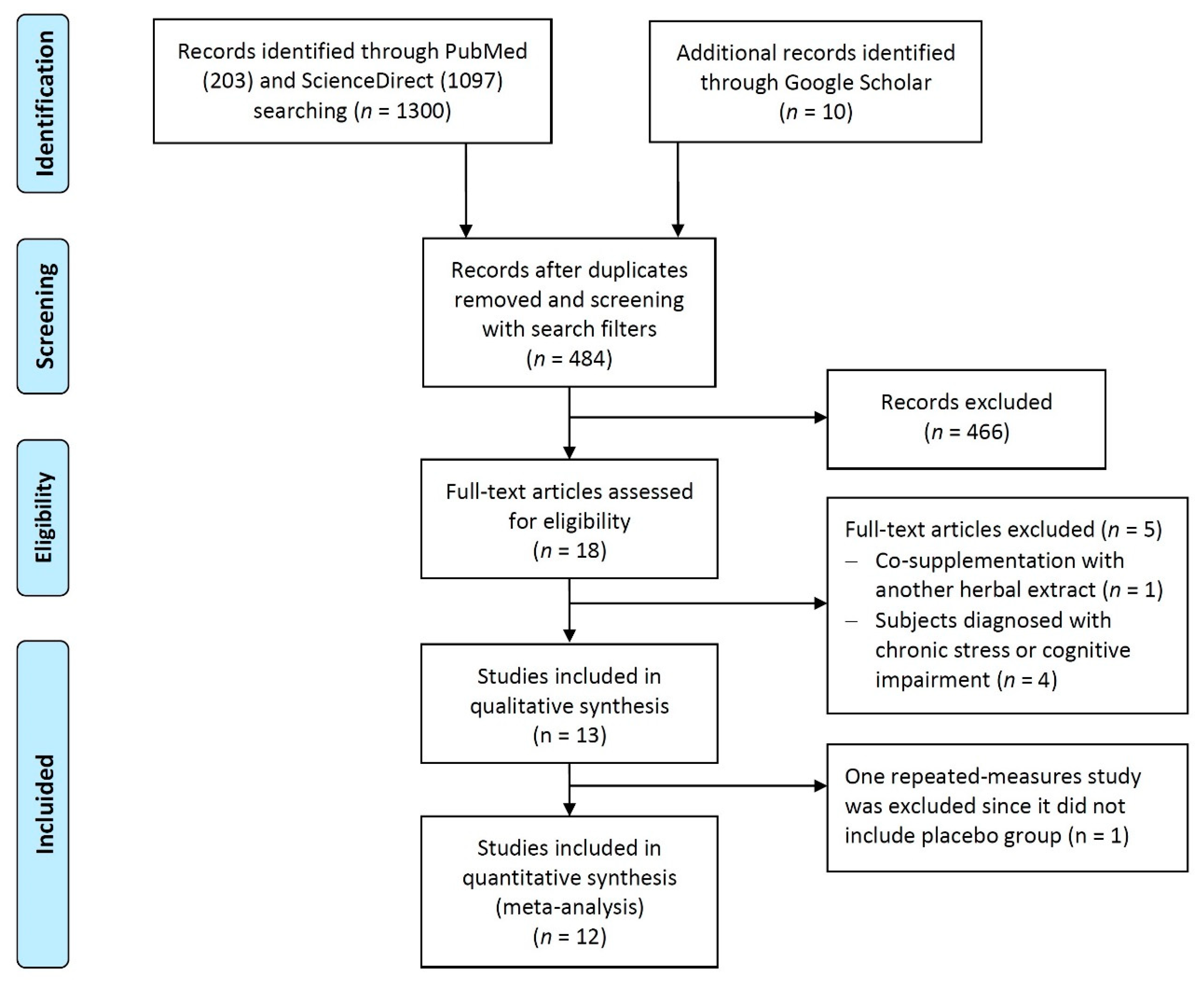
3.1. Study Selection
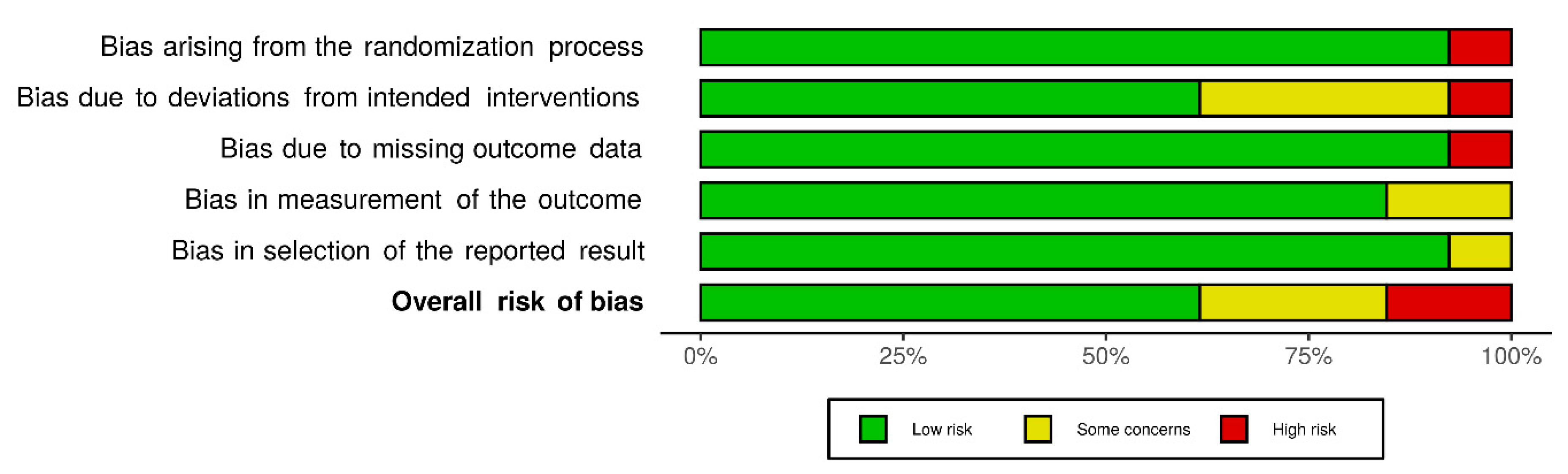
3.2. Risk of Bias within Studies
3.3. Results of Individual Studies
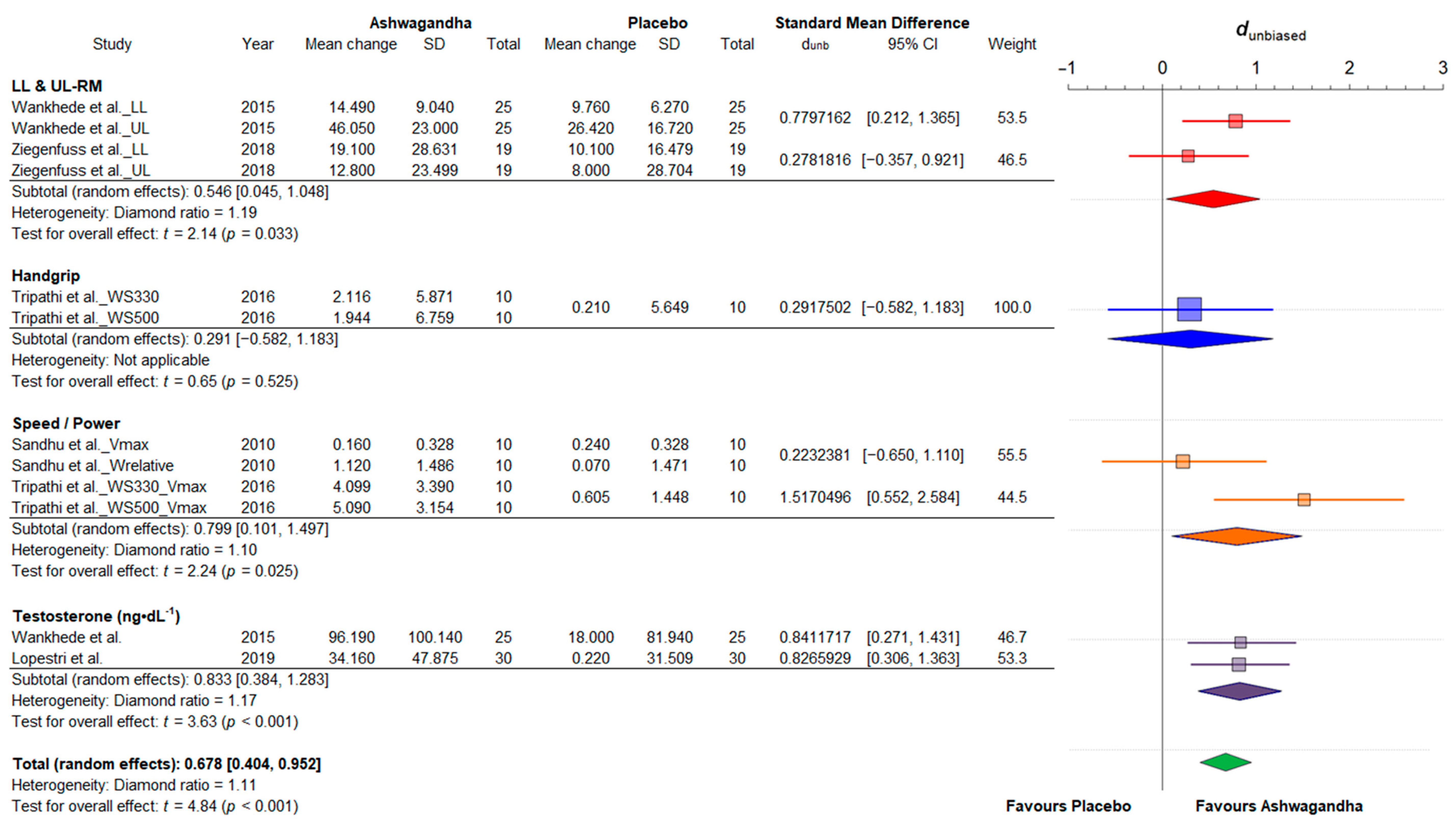
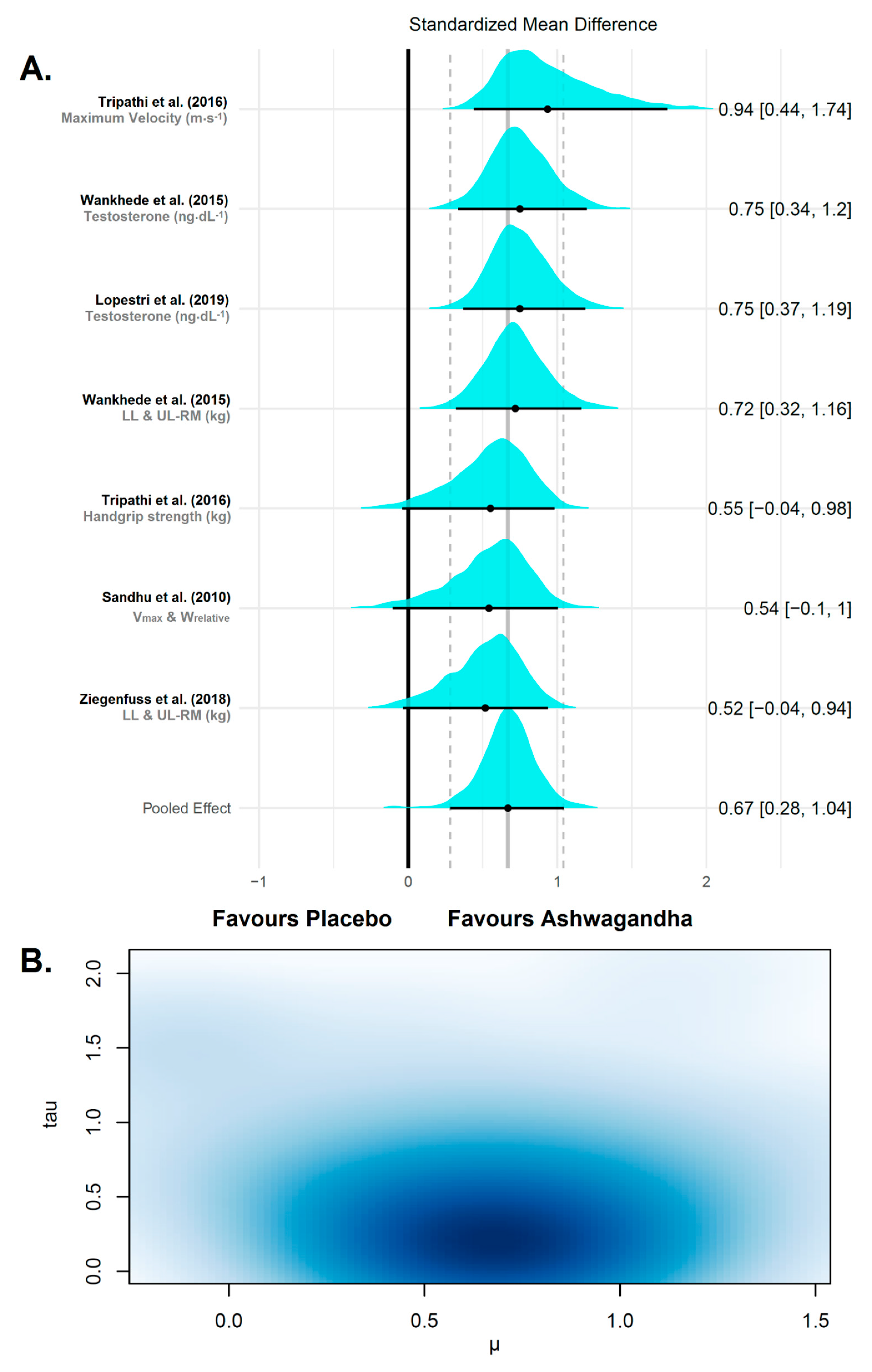
3.4. Synthesis of Results
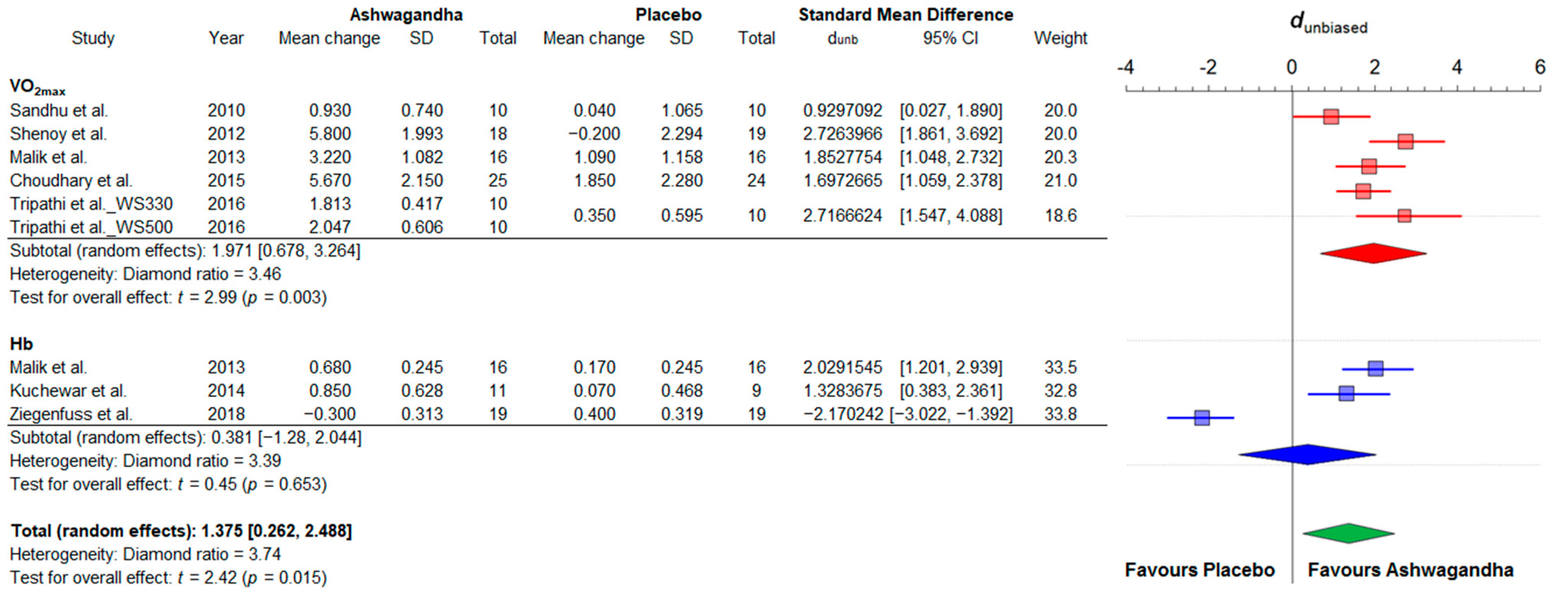
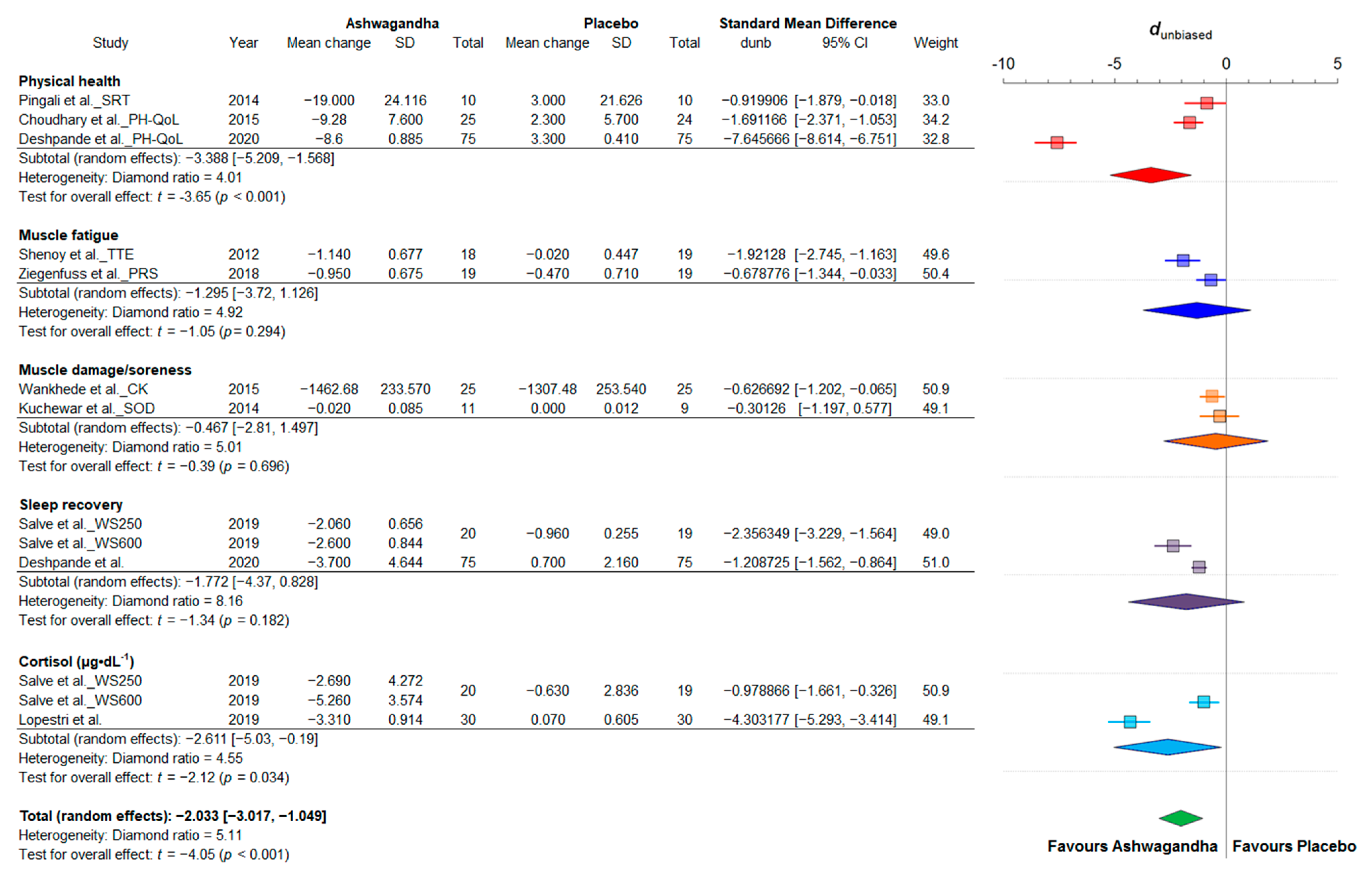
3.4.1. Strength and Power
3.4.2. Cardiorespiratory Fitness
3.4.3. Fatigue and Recovery
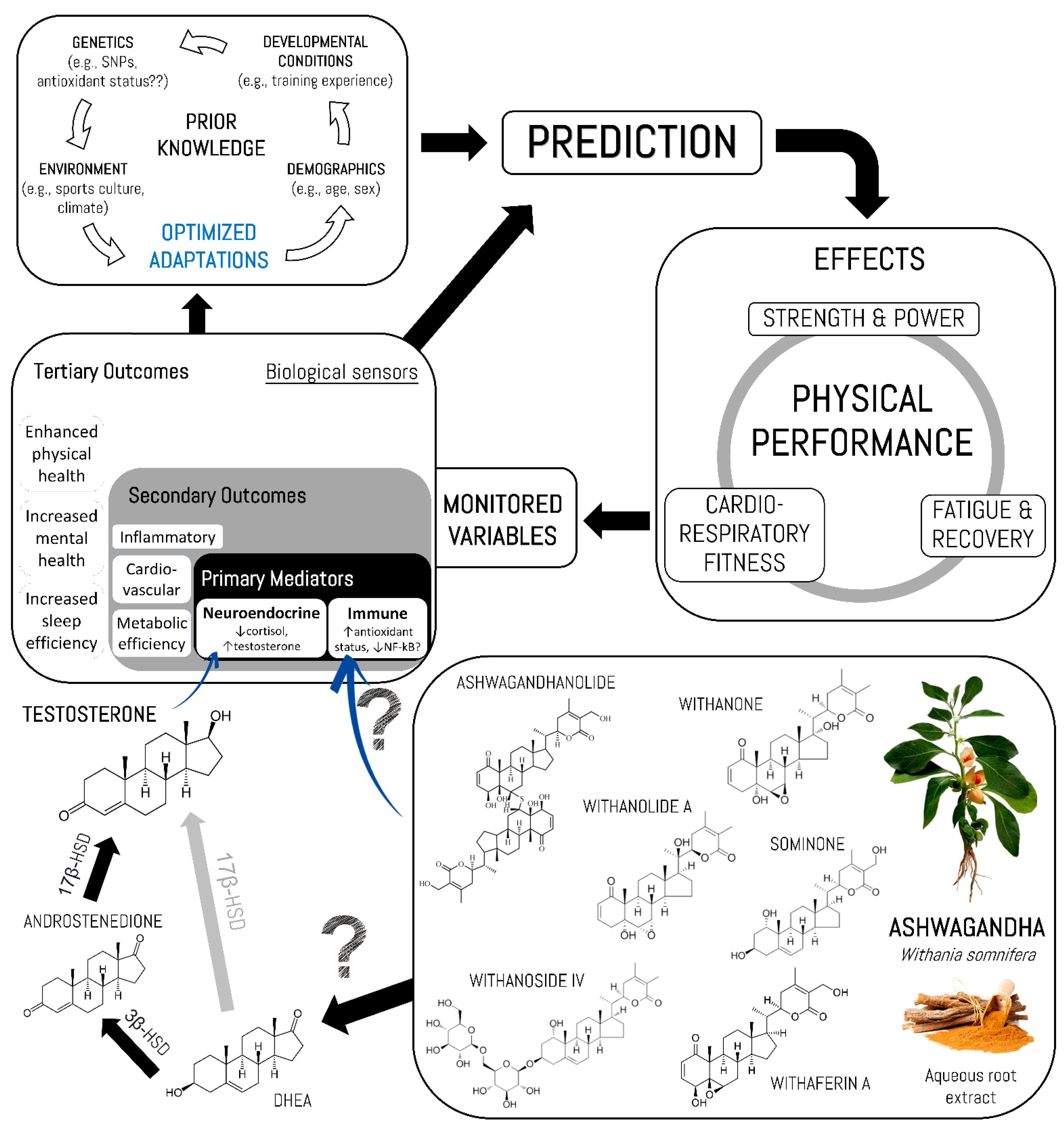
4. Discussion
4.1. Summary of Evidence
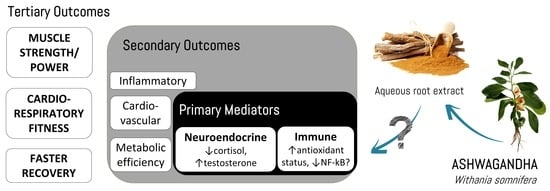
4.2. Potential Mechanisms of Action
4.3. Practical Applications
4.4. Future Directions
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Keßler, C.; Gupta, H. Rasayanas: Ayurvedische Behandlung von altersbedingten Erkrankungen. Z. Komplementärmedizin 2015, 7, 22–25. [Google Scholar] [CrossRef]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar] [PubMed]
- Brekhman, I.I.; Dardymov, I.V. New Substances of Plant Origin which Increase Nonspecific Resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, M.; De Jager, P.; Gilca, M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- Tripathi, S.; Sangwan, R.S.; Mishra, B.; Jadaun, J.S.; Sangwan, N.S. Berry transcriptome: Insights into a novel resource to understand development dependent secondary metabolism in Withania somnifera (Ashwagandha). Physiol. Plant. 2019, 168, 148–173. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Gansukh, E.; Baskar, V.; Kai, G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L) and their biological activities. Food Chem. Toxicol. 2019, 132, 110659. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Jana, S. Metabolite Profiling in Withania somnifera Roots Hydroalcoholic Extract Using LC/MS, GC/MS and NMR Spectroscopy. Chem. Biodivers. 2017, 14, e1600280. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kaileh, M.; Vanden Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharmacol. 2005, 144, 961–971. [Google Scholar] [CrossRef]
- Pratte, M.A.; Nanavati, K.B.; Young, V.; Morley, C.P. An Alternative Treatment for Anxiety: A Systematic Review of Human Trial Results Reported for the Ayurvedic Herb Ashwagandha (Withania somnifera). J. Altern. Complement. Med. 2014, 20, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Shivaram, S.B.; Bavage, S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine 2018, 50, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Nasimi Doost Azgomi, R.; Zomorrodi, A.; Nazemyieh, H.; Fazljou, S.M.B.; Sadeghi Bazargani, H.; Nejatbakhsh, F.; Moini Jazani, A.; Ahmadi AsrBadr, Y. Effects of Withania somnifera on Reproductive System: A Systematic Review of the Available Evidence. Biomed. Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Bavage, S.; Shivaram, S.B. Withania somnifera (Indian ginseng) in diabetes mellitus: A systematic review and meta-analysis of scientific evidence from experimental research to clinical application. Phytother. Res. 2020, 34, 1041–1059. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2019, 34, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Dhadde, S.B.; Vandal, R.; Shivakumar, B.S.; Charan, C.S. Withania somnifera(Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. J. Pharm. Pharmacol. 2015, 67, 879–899. [Google Scholar] [CrossRef]
- Pérez-Gómez, J.; Villafaina, S.; Adsuar, J.C.; Merellano-Navarro, E.; Collado-Mateo, D. Effects of Ashwagandha (Withania somnifera) on VO2max: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1119. [Google Scholar] [CrossRef]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 43. [Google Scholar] [CrossRef]
- Ziegenfuss, T.; Kedia, A.; Sandrock, J.; Raub, B.; Kerksick, C.; Lopez, H. Effects of an Aqueous Extract of Withania somnifera on Strength Training Adaptations and Recovery: The STAR Trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef]
- Estarli, M.; Aguilar Barrera, E.S.; Martínez-Rodríguez, R.; Baladia, E.; Duran Agüero, S.; Camacho, S.; Buhring, K.; Herrero-López, A.; Gil-González, D.M. Ítems de referencia para publicar Protocolos de Revisiones Sistemáticas y Metaanálisis: Declaración PRISMA-P 2015. Rev. Esp. Nutr. Hum. Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Barbui, C.; Cipriani, A.; Brambilla, P.; Watanabe, N. Imputing missing standard deviations in meta-analyses can provide accurate results. J. Clin. Epidemiol. 2006, 59, 7–10. [Google Scholar] [CrossRef]
- Goulet-Pelletier, J.-C.; Cousineau, D. A review of effect sizes and their confidence intervals, Part I: The Cohen’s d family. Quant. Methods Psychol. 2018, 14, 242–265. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, West Sussex, UK, 2011. [Google Scholar]
- Cumming, G. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-Analysis; Routledge: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Bürkner, P.-C. Advanced Bayesian Multilevel Modeling with the R Package brms. R J. 2018, 10, 395. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software]. Available online: https://www.R-project.org/ (accessed on 20 November 2020).
- Sandhu, J.; Shah, B.; Shenoy, S.; Padhi, M.M.; Chauhan, S.; Lavekar, G.S. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Rege, N.; Shirolkar, S.; Pandey, S.; Tadvi, F.; Solanki, P.; Vaidya, R.; Vaidya, A.; Kene, K. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.; Chaskar, U.; Sandhu, J.; Paadhi, M. Effects of eight-week supplementation of Ashwagandha on cardiorespiratory endurance in elite Indian cyclists. J. Ayurveda Integr. Med. 2012, 3, 209. [Google Scholar] [CrossRef]
- Malik, A.; Mehta, V.; Dahiya, V. Effect of Ashwagandha (Withania somnifera) root powder supplementation on the VO2 max. and hemoglobin in hockey players. Int. J. Behav. Soc. Mov. Sci. 2013, 2, 91–99. [Google Scholar]
- Kuchewar, V.; Borkar, M.; Nisargandha, M. Evaluation of antioxidant potential of Rasayana drugs in healthy human volunteers. AYU (Int. Q. J. Res. Ayurveda) 2014, 35, 46. [Google Scholar] [CrossRef]
- Pingali, U.; Pilli, R.; Fatima, N. Effect of standardized aqueous extract of Withania somniferaon tests of cognitive and psychomotor performance in healthy human participants. Pharmacogn. Res. 2014, 6, 12. [Google Scholar] [CrossRef]
- Choudhary, B.; Shetty, A.; Langade, D.G. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in improving cardiorespiratory endurance in healthy athletic adults. AYU (Int. Q. J. Res. Ayurveda) 2015, 36, 63. [Google Scholar] [CrossRef]
- Tripathi, R.K.; Salve, B.A.; Petare, A.U.; Raut, A.A.; Rege, N.N. Effect of Withania somnifera on physical and cardiovascular performance induced by physical stress in healthy human volunteers. Int. J. Basic Clin. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kruschke, J.K.; Liddell, T.M. The Bayesian New Statistics: Hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon. Bull. Rev. 2017, 25, 178–206. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Reid, M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl Physiol. 2008, 104, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.A.; Pérez-Idárraga, A.; Odriozola-Martínez, A.; Kreider, R.B. The 4R’s Framework of Nutritional Strategies for Post-Exercise Recovery: A Review with Emphasis on New Generation of Carbohydrates. Int. J. Environ. Res. Public Health 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Medved, I.; Goodman, C.A.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J. Physiol 2006, 576, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; McKenna, M.J. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J. Appl Physiol. 2004, 96, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X.; McKenna, M.J. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl Physiol. 2004, 97, 1477–1485. [Google Scholar] [CrossRef]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philos. Ethicsand Humanit. Med. 2009, 4. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, R.; Gan, L.; Lau, W.B.; Cao, X.; Zhao, J.; Ma, X.; Christopher, T.A.; Lopez, B.L.; Wang, Y. Withaferin A inhibits apoptosis via activated Akt-mediated inhibition of oxidative stress. Life Sci. 2018, 211, 91–101. [Google Scholar] [CrossRef]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.; McCord, J.M. Phytochemical Combination PB125 Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Antioxidants 2019, 8, 119. [Google Scholar] [CrossRef]
- Velmurugan, K.; Alam, J.; McCord, J.M.; Pugazhenthi, S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic. Biol. Med. 2009, 46, 430–440. [Google Scholar] [CrossRef]
- Bakkar, N.; Guttridge, D.C. NF-kappaB signaling: A tale of two pathways in skeletal myogenesis. Physiol. Rev. 2010, 90, 495–511. [Google Scholar] [CrossRef]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol. Cancer 2006, 5, 1434–1445. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Ravindranath, P.P.; Mohan, R. Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: Potential application to choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4138–4145. [Google Scholar] [CrossRef]
- Schumacher, Y.O.; Jankovits, R.; Bultermann, D.; Schmid, A.; Berg, A. Hematological indices in elite cyclists. Scand. J. Med. Sci. Sports 2002, 12, 301–308. [Google Scholar] [CrossRef]
- Borges, G.F.; Rama, L.M.; Pedreiro, S.; Rosado, F.; Alves, F.; Santos, A.M.; Paiva, A.; Teixeira, A.M. Haematological changes in elite kayakers during a training season. Appl. Physiol. Nutr. Metab. 2012, 37, 1140–1146. [Google Scholar] [CrossRef]
- Rietjens, G.J.; Kuipers, H.; Hartgens, F.; Keizer, H.A. Red blood cell profile of elite olympic distance triathletes. A three-year follow-up. Int. J. Sports Med. 2002, 23, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.U.; Garvican-Lewis, L.A.; Schmidt, W.F.; Gore, C.J. Relationship between changes in haemoglobin mass and maximal oxygen uptake after hypoxic exposure. Br. J. Sports Med. 2013, 47, i26–i30. [Google Scholar] [CrossRef] [PubMed]
- Otto, J.M.; Montgomery, H.E.; Richards, T. Haemoglobin concentration and mass as determinants of exercise performance and of surgical outcome. Extrem. Physiol. Med. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Lundby, C.; Koskolou, M.; Boushel, R. Importance of hemoglobin concentration to exercise: Acute manipulations. Respir. Physiol. Neurobiol. 2006, 151, 132–140. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D.; Smith, S.J. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study Examining the Hormonal and Vitality Effects of Ashwagandha (Withania somnifera) in Aging, Overweight Males. Am. J. Men’s Health 2019, 13, 155798831983598. [Google Scholar] [CrossRef]
- Gabrielsen, J.S. Iron and Testosterone: Interplay and Clinical Implications. Curr. Sex. Health Rep. 2017, 9, 5–11. [Google Scholar] [CrossRef]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11. [Google Scholar] [CrossRef]
- Ramakanth, G.S.H.; Uday-Kumar, C.; Kishan, P.V.; Usharani, P. A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J. Ayurveda Integr. Med. 2016, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, R.S.; Chaurasiya, N.D.; Misra, L.N.; Lal, P.; Uniyal, G.C.; Sharma, R.; Sangwan, N.S.; Suri, K.A.; Qazi, G.N.; Tuli, R. Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha). Curr. Sci. 2004, 86, 461–465. [Google Scholar]
- Tomar, V.; Beuerle, T.; Sircar, D. A validated HPTLC method for the simultaneous quantifications of three phenolic acids and three withanolides from Withania somnifera plants and its herbal products. J. Chromatogr. B 2019, 1124, 154–160. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract. J. Evid. Based Complement. Altern. Med. 2016, 22, 96–106. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Kelgane, S.B.; Salve, J.; Sampara, P.; Debnath, K. Efficacy and Tolerability of Ashwagandha Root Extract in the Elderly for Improvement of General Well-being and Sleep: A Prospective, Randomized, Double-blind, Placebo-controlled Study. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
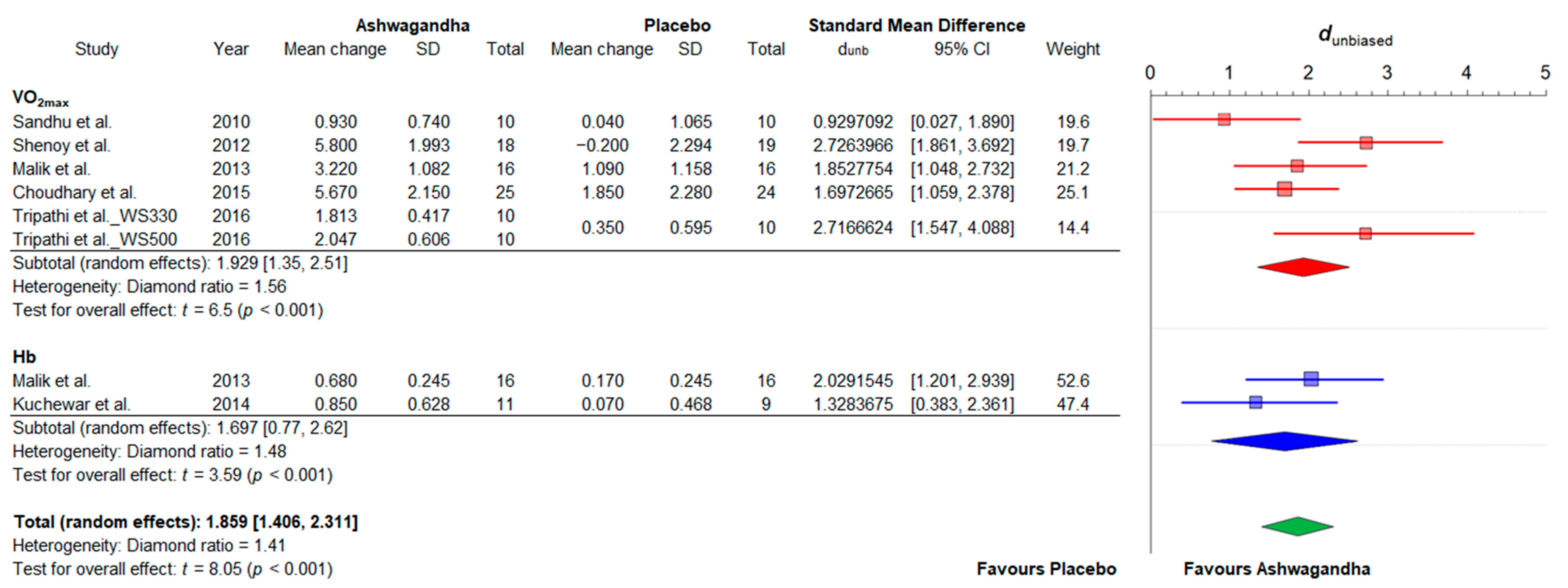
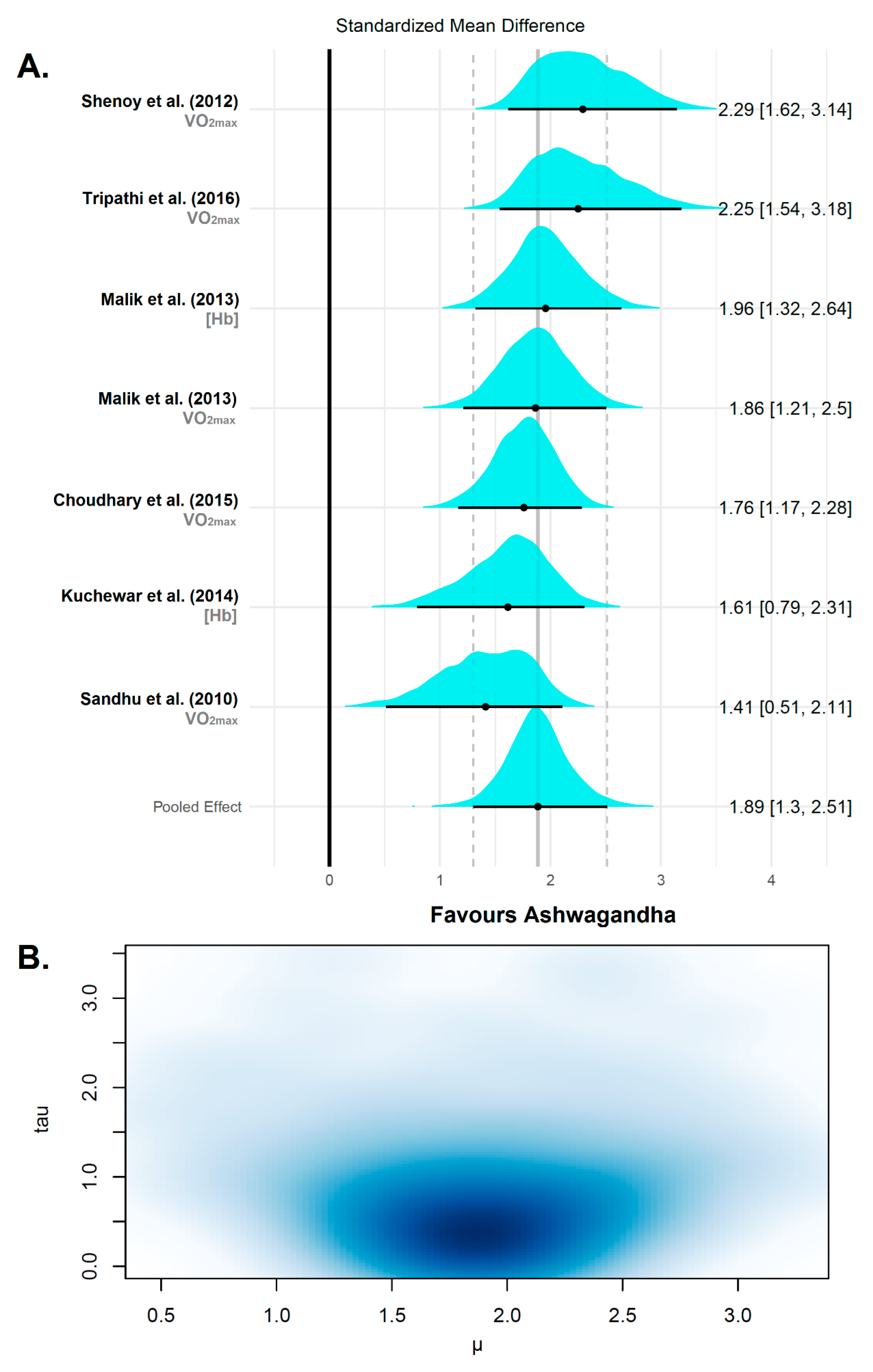
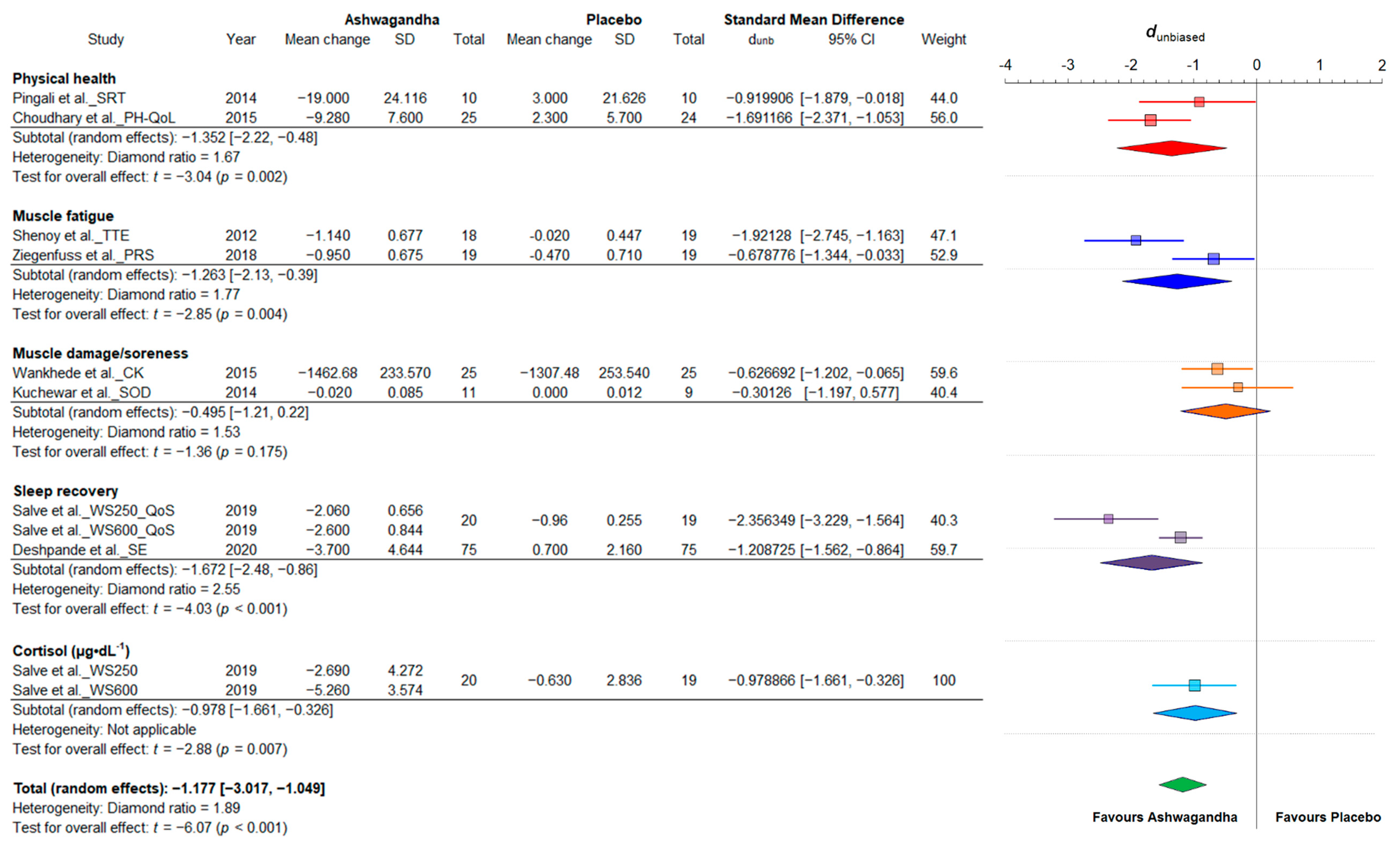
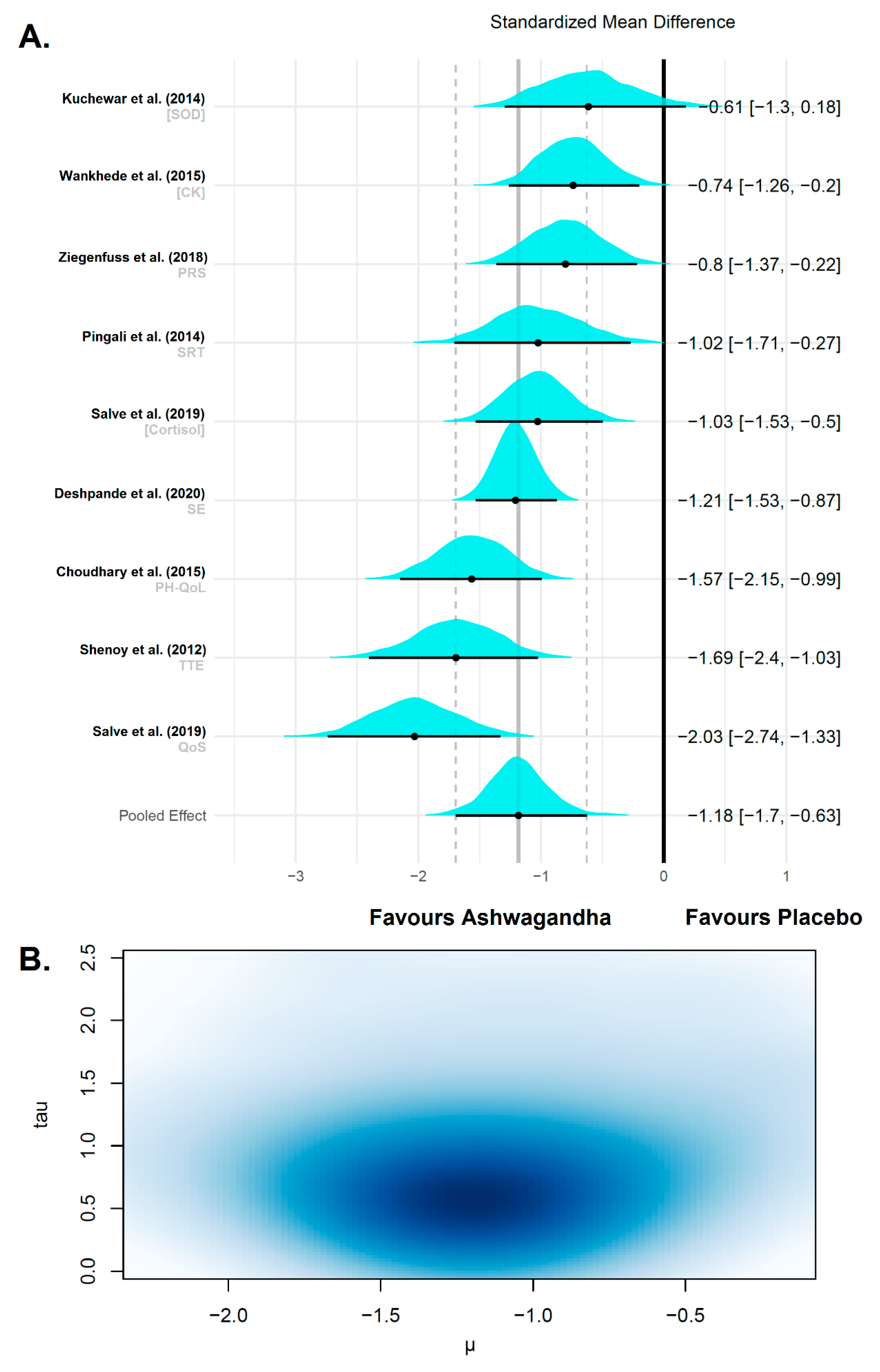
| Reference | Sample (F:M) | Age/ BMI | Ashwagandha (WS) Supplementation | Analyzed Variables | Change | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Design/ Length | Dosage (mg) | (Withanolides) | ||||||
| Sandhu et al., 2010 [31] | 40 (18:22) | 20.6 (2.5) 21.9 (2.2) | WS (n = 10) TA (n = 10) WS+TA (n = 10) Placebo (flour, n = 10) | RCT-SB/ 8 weeks | 500 (o.d. qAM) | ARE (ND) |
| +2.9% †* +8.8% †* +10.1% †* +6.8% †* | WS may be useful against physical exhaustion and to improve speed, lower limb muscle strength and neuromuscular coordination. |
| Raut et al. 2012 [32] | 18 (6:12) | 24.3 (2.1) 24.2 (2.7) | WS (n = 18) | CT-O/ 30 days | 750 per day × 10 days, 1000 per day × 10 days and 1250 per day × 10 days (b.i.d.) | ARE 8:1 (ND) | Muscle strength (kg):
| +8.0% NS +21.5% † +15.4% † −26.9% NS | WS supplementation is not only safe but has positive effects on muscle strength. |
| Shenoy et al., 2012 [33] | 40 (20:17) | 19.6 (1.4) 19.6 (1.9) | WS (n = 18) Placebo (starch, n = 19) | RCT/ 8 weeks | 1000 (500 b.i.d.) | ARE (ND) |
| +13% †* +7.2% †* +1.8% NS | First study to report significant improvements in the sports performance of elite athletes. |
| Malik et al., 2013 [34] | 32 (0:32) | 17.4 (1.7) 20.9 (2.9) | WS (n = 16) Placebo (sugar, n = 16) | RCT-SB/ 8 weeks | 500 (q.h.s.) | ARE (ND) |
| +6.67% †* +5.14% †* | WS supplementation for eight weeks improves VO2max and [Hb] in hockey players. |
| Kuchewar et al., 2014 [35] | 30 (20:10) | 18 to 45 years old | WS (n = 11) Guduchi (n = 10) Placebo (CaCO3, n = 9) | RCT-DB/ 6 meses | 1000 (500 b.i.d.) | ARE (ND) |
| +6.29% † +8.12% † +4.45% † +8.15% † −18.2% † +18.1% † | WS contributes to the increase of [Hb] and hematological markers while preventing oxidative stress. |
| Pingali et al., 2014 [36] | 20 (0:20) | 24.9 (4.18) 22.38 (1.1) | WS (n = 10) Placebo (carbohydrates mixture, n = 10) | RCT-DB Crossver/14 days (14 days wash-out) | 1000 (500 b.i.d.) | ARLE Sensoril® ≥10% withanolides and ≤0.5% withaferin-A | Reaction time:
| −6.2% †* −3.39% †* −8.14% †* −3.16% †* | Fourteen days of WS supplementation decreases reaction time compared to placebo, indicating a positive effect on cognitive and psychomotor function. |
| Choudhary et al., 2015 [37] | 49 (ND) | 20 to 45 years old/ 18.5 to 24.9 kg·m−2 | WS (n = 25) Placebo (saccharose, n = 24) | RCT-DB/ 3 months | 600 (300 b.i.d.) | ARE KSM‑66® 5% withanolides |
| +6.2% †* +14.7% †* +19.6% †* | WS root extract increases cardiorespiratory endurance and improves quality of life in healthy athletic adults. |
| Wankhede et al., 2015 [19] | 50 (0:50) | 28.0 (8.0) /NA | WS (n = 25) Placebo (starch, n = 25) | RCT-DB/ 8 weeks | 600 (300 b.i.d.) | ARE KSM‑66® 5% withanolides |
| +138% †* +51.9% †* +17.1% †* +3.32% †* +8.0% † +16.0% †* +15.2% †* −98.9% †* | WS supplementation during a resistance training program increases muscle strength and size while accelerating post-exercise recovery in untrained subjects. |
| Tripathi et al., 2016 [38] | 30 (0:30) | 27.3 (2.48) 22.9 (1.44) | WS330 (n = 10) WS500 (n = 10) Placebo (starch, n = 10) | RCT-O/ 28 days | 330 and 500 per group (o.d. qAM) | ARE (ND) |
| WS330: +15.7% †* +15.9 †* +9.3% NS +4.95% † +5.40% † WS500 +15.9% †* +16.2% †* +12.0% † +4.93% † +6.09% † | WS supplementation for 28 days increases muscle strength and cardiorespiratory capacity in healthy men. |
| Ziegenfuss et al., 2018 [20] | 38 (0:38) | 24.4 (4.2) 26.2 (3.4) | WS (n = 19) Placebo (flour, n = 19) | RCT-DB/ 3 months | 500 (o.d. qAM) | ARLE Sensoril® ≥10% withanolides and ≤0.5% withaferin-A |
| +18.0% †* +8.45% †* +4.64% † +22.8% † +13.6% † +11.3% †* +11.6% †* +28.1% † −21.2% † +14.4% † −1.93% † | WS supplementation (500 mg·day−1) for twelve weeks of a resistance training program improves upper and lower limb muscle strength and power and perceived recovery in recreationally active subjects. |
| Salve et al., 2019 [39] | 58 (ND) | 31.1 (7.5) /NA | WS250 (n = 19) WS600 (n = 20) Placebo (starch, n = 19) | RCT-DB/ 8 weeks | 250 and 600 per group (o.d. qAM) | ARE KSM‑66® 5% withanolides |
| WS250: −33.7% †* −16.5 †* −13.0% NS −41.0% †* WS600 −38.3% †*⁑ −32.6% †*⁑ −16.3% †* −46.0% †*⁑ | Eight weeks of supplementation of aqueous WS root extract was associated with a significant reduction of stress levels and improved the overall quality of life. |
| Lopestri et al., 2019 [40] | 60 (23:37) | 42.2 (2.44) 24.6 (0.60) | WS (n = 30) Placebo (toasted rice, n = 30) | RCT-DB/ 60 days | 240 (o.d. qPM) | ARLE Shoden® ≥35% withanolide glycosides |
| −40.8% †* −30.0 † −23.3% †* −8.15% †* +11.4 † | WS reduces anxiety levels, [cortisol] and [DHEA-S], with a non-significant tendency to increase [testosterone] in men. |
| Deshpande et al., 2020 [41] | 150 (78:72) | 36.8 (10.9) 25.0 (3.9) | WS (n = 75) Placebo (rice powder, n = 75) | RCT-DB/ 6 weeks | 120 (o.d. qPM) | ARLE Shoden® ≥35% withanolide glycosides (42 mg HPLC) |
| +72% †* −27.2% †* −14.7% †* +4.8% †* +4.6% †* +13.1 † +11.8 † | 120 mg of WS extract increases the overall quality of sleep (time and efficiency) and increases the quality of life at the physical and psychological levels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, D.A.; Moreno, Y.; Gho, C.; Petro, J.L.; Odriozola-Martínez, A.; Kreider, R.B. Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis. J. Funct. Morphol. Kinesiol. 2021, 6, 20. https://doi.org/10.3390/jfmk6010020
Bonilla DA, Moreno Y, Gho C, Petro JL, Odriozola-Martínez A, Kreider RB. Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2021; 6(1):20. https://doi.org/10.3390/jfmk6010020
Chicago/Turabian StyleBonilla, Diego A., Yurany Moreno, Camila Gho, Jorge L. Petro, Adrián Odriozola-Martínez, and Richard B. Kreider. 2021. "Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis" Journal of Functional Morphology and Kinesiology 6, no. 1: 20. https://doi.org/10.3390/jfmk6010020
APA StyleBonilla, D. A., Moreno, Y., Gho, C., Petro, J. L., Odriozola-Martínez, A., & Kreider, R. B. (2021). Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis. Journal of Functional Morphology and Kinesiology, 6(1), 20. https://doi.org/10.3390/jfmk6010020