Nordic Walking and Free Walking Improve the Quality of Life, Cognitive Function, and Depressive Symptoms in Individuals with Parkinson’s Disease: A Randomized Clinical Trial
Abstract
1. Introduction
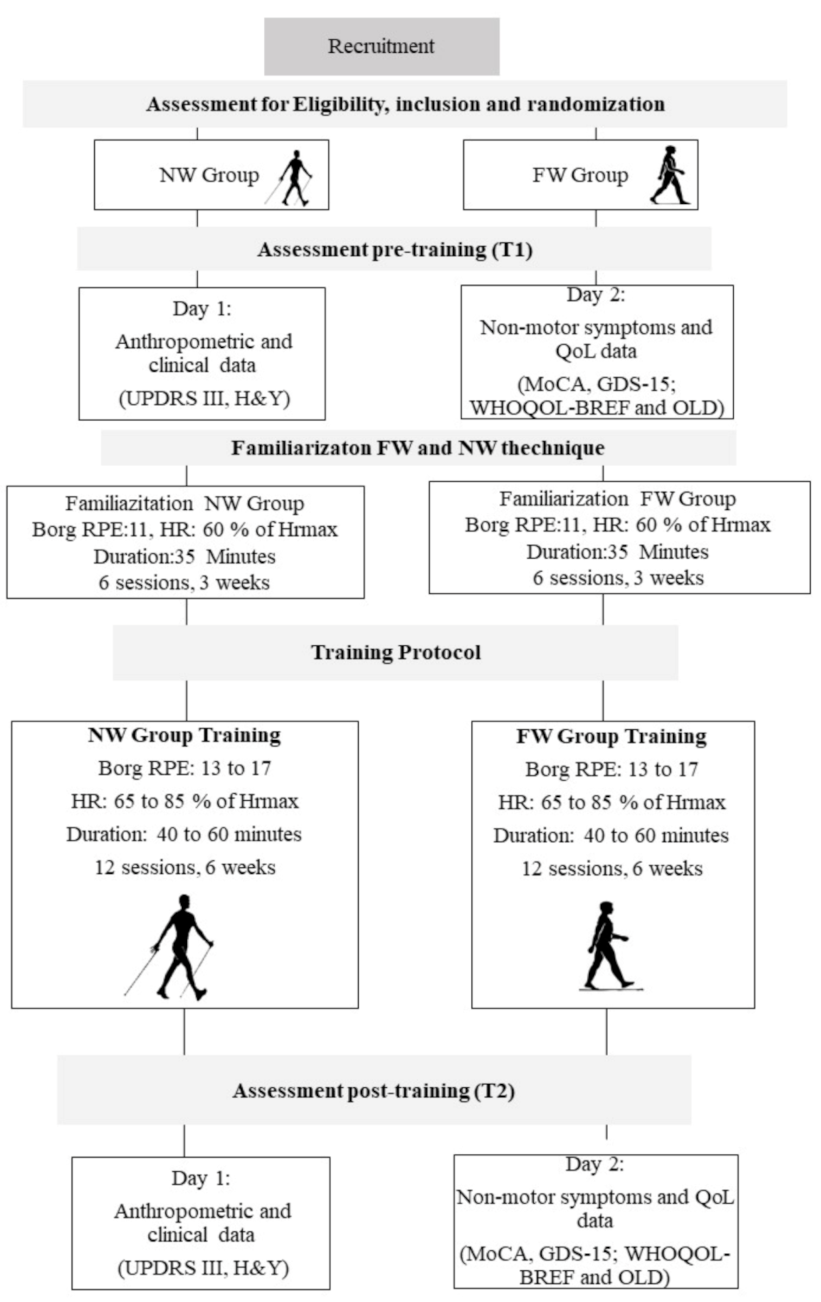
2. Materials and Methods
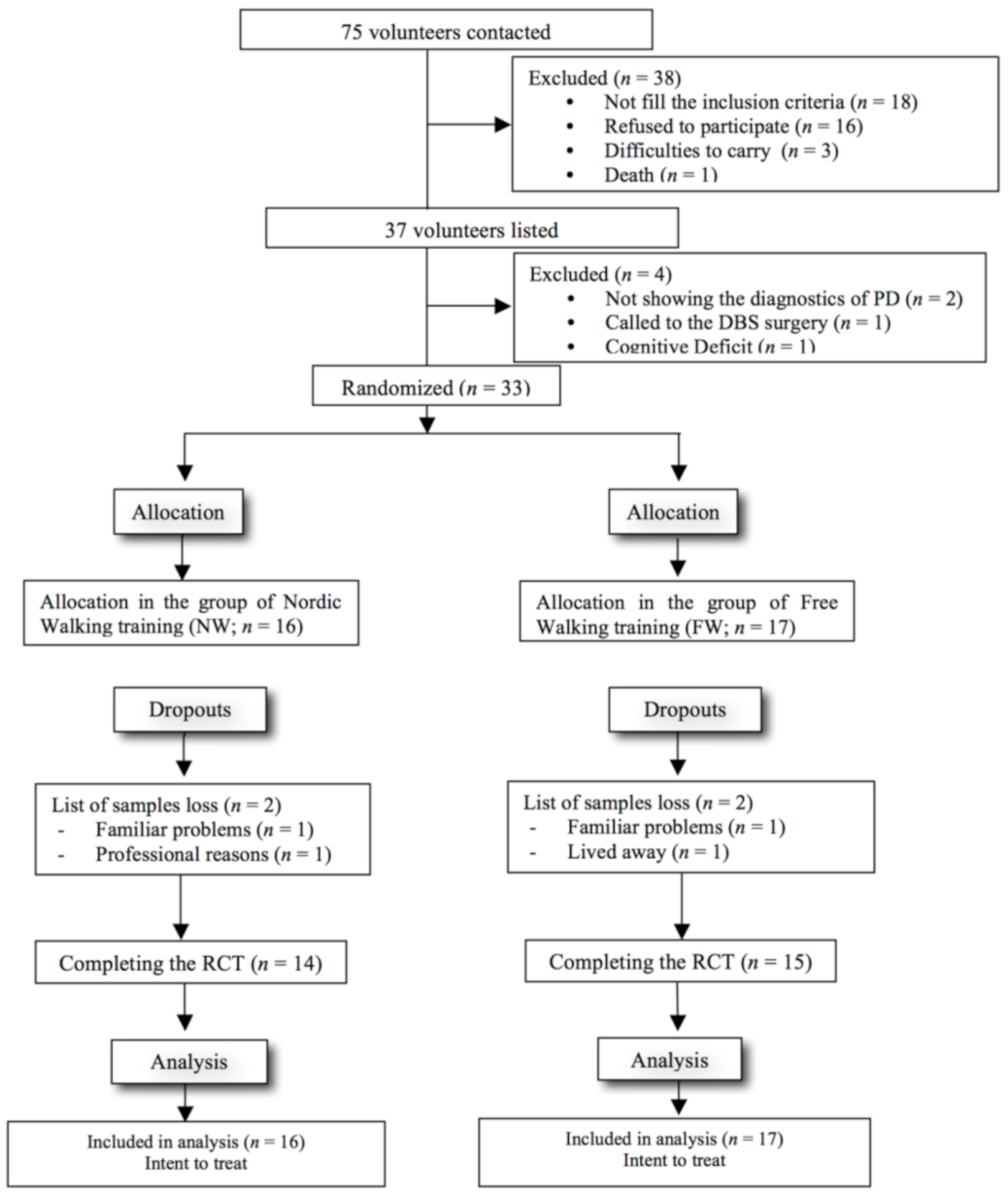
2.1. Sample and Randomization
2.2. Outcomes
2.3. Assessments
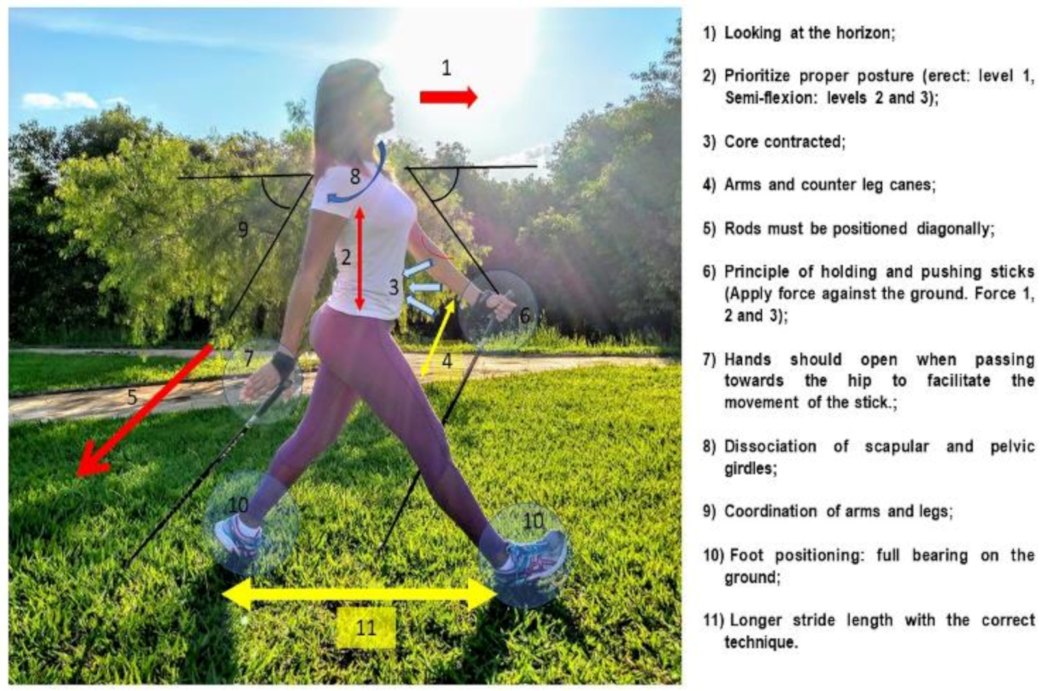
2.4. Exercise Training
2.5. Statistical Analysis
3. Results
3.1. Demographic, Anthropometric, and Clinical Characteristics of the Sample
3.2. Adherence
3.3. Quality of Life
3.4. Non-Motor Symptoms
3.5. Motor Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frazzitta, G.; Maestri, R.; Ghilardi, M.F.; Riboldazzi, G.; Perini, M.; Bertotti, G.; Boveri, N.; Buttini, S.; Lombino, F.L.; Uccellini, D.; et al. Intensive rehabilitation increases BDNF serum levels in Parkinsonian patients: A randomized study. Neurorehabilit. Neural Repair 2014, 28, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Liang, D.; Deng, K.; Wu, J.; Cai, L.; Yan, J.H. Broad Impairment of Executive Functions in Patients with Parkinson’s disease: A Meta-Analysis. Rev. Neuropsychiatry 2018, 10, 625–643. [Google Scholar] [CrossRef]
- Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; O’Brien, J.T.; Coleman, S.Y.; Brooks, D.J.; Barker, R.A.; Burn, D.J. Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms. Mov. Disord. 2014, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Olchik, M.R.; Ayres, A.; Ghisi, M.; Schuh, A.F.; Rieder, C.R. Impacto da performance cognitiva na qualidade de vida de indivíduos com doença de Parkinson. Dement. Neuropsychol. 2016, 10, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Steffens, R.D.; Vilarino, G.T.; Sieczkowska, S.M.; Coimbra, D.R. Does volume of physical exercise have an effect on depression in patients with fibromyalgia? J. Affect. Disord. 2017, 208, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Rosenbaum, S.; Richards, J.; Ward, P.B.; Veronese, N.; Solmi, M.; Cadore, E.L.; Stubbs, B. Exercise for depression in older adults: A meta-analysis of randomized controlled trials adjusting for publication bias. Braz. J. Psychiatry 2016, 38, 247–254. [Google Scholar] [CrossRef]
- Marinho, M.S.; Chaves, P.D.; Tarabal, T.D. Dupla-tarefa na doença de Parkinson: Uma revisão sistemática de ensaios clínicos aleatorizados. Rev. Bras. Geriatr. Gerontol. 2014, 17, 191–199. [Google Scholar] [CrossRef][Green Version]
- Tuon, T.; Valvassori, S.S.; Dal Pont, G.C.; Paganini, C.S.; Pozzi, B.G.; Luciano, T.F.; Souza, P.S.; Quevedo, J.; Souza, C.T.; Pinho, R.A. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef]
- dos Santos Delabary, M.; Komeroski, I.G.; Monteiro, E.P.; Costa, R.R.; Haas, A.N. Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: A systematic review with meta-analysis. Aging Clin. Exp. Res. 2018, 30, 727–735. [Google Scholar] [CrossRef]
- Reuter, I.; Mehnert, S.; Leone, P.; Kaps, M.; Oechsner, M.; Engelhardt, M. Effects of a flexibility and relaxation programme, walking, and nordic walking on parkinson’s disease. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef]
- Fritz, B.; Rombach, S.; Godau, J.; Berg, D.; Horstmann, T.; Grau, S. The influence of Nordic Walking training on sit-to-stand transfer in Parkinson patients. Gait Posture 2011, 34, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.P.; Franzoni, L.T.; Cubillos, D.M.; de Oliveira Fagundes, A.; Carvalho, A.R.; Oliveira, H.B.; Pantoja, P.D.; Schuch, F.B.; Rieder, C.R.; Martinez, F.G.; et al. Effects of Nordic walking training on functional parameters in Parkinson’s disease: A randomized controlled clinical trial. Scand. J. Med. Sci. Sport 2017, 27. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Zoppirolli, C.; Bortolan, L.; Schena, F.; Pellegrini, B. Shared and task-specific muscle synergies of Nordic walking and conventional walking. Scand. J. Med. Sci. Sport. 2018, 28, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Memon, A.A. Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, L.T.; Monteiro, E.P.; Oliveira, H.B.; da Rosa, R.G.; Costa, R.R.; Rieder, C.; Martinez, F.G.; Peyré-Tartaruga, L.A. A 9-Week Nordic and Free Walking Improve Postural Balance in Parkinson’s Disease. Sports Med. Int. Open 2018, 2, E28–E34. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A.; et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 2015, 37, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.; Giladi, N.; Gruendlinger, L.; Hausdorff, J.M. Six Weeks of Intensive Treadmill Training Improves Gait and Quality of Life in Patients with Parkinson’s Disease: A Pilot Study. Arch. Phys. Med. Rehabil. 2007, 88, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Den Oudsten, B.L.; Van Heck, G.L.; De Vries, J. Quality of life and related concepts in Parkinson’s disease: A systematic review. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1528–1537. [Google Scholar] [CrossRef]
- Veiga, B.A.; Borges, V.; Silva, S.M.; Goulart, F.D.; Cendoroglo, M.S.; Ferraz, H.B. Depression in Parkinson’s disease: Clinical-epidemiological correlates and comparison with a controlled group of non-parkinsonian geriatric patients. Braz. J. Psychiatry 2009, 31, 39–42. [Google Scholar] [CrossRef][Green Version]
- Fujita, E.; Yakushi, K.; Takeda, M.; Islam, M.M.; Nakagaichi, M.; Taaffe, D.R.; Takeshima, N. Proficiency in pole handling during Nordic walking influences exercise effectiveness in middle-aged and older adults. PLoS ONE 2018, 13, e0208070. [Google Scholar] [CrossRef]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Rizzo, M.; Newman, S.R.; Mehta, S.; Grabowski, T.J.; et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 2014, 83, 413 LP–425 LP. [Google Scholar] [CrossRef] [PubMed]
- Nagano-Saito, A.; Martinu, K.; Monchi, O. Function of basal ganglia in bridging cognitive and motor modules to perform an action. Front. Neurosci. 2014, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wild, L.B.; de Lima, D.B.; Balardin, J.B.; Rizzi, L.; Giacobbo, B.L.; Oliveira, H.B.; de Lima Argimon, I.I.; Peyré-Tartaruga, L.A.; Rieder, C.R.; Bromberg, E. Characterization of cognitive and motor performance during dual-tasking in healthy older adults and patients with Parkinson’s disease. J. Neurol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, B.; Peyré-Tartaruga, L.A.; Zoppirolli, C.; Bortolan, L.; Bacchi, E.; Figard-Fabre, H.; Schena, F. Exploring muscle activation during nordic walking: A comparison between conventional and uphill walking. PLoS ONE 2015, 10, e0138906. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, B.; Peyré-Tartaruga, L.A.; Zoppirolli, C.; Bortolan, L.; Savoldelli, A.; Minetti, A.E.; Schena, F. Mechanical energy patterns in nordic walking: Comparisons with conventional walking. Gait Posture 2017, 51, 234–238. [Google Scholar] [CrossRef]
- Gomeñuka, N.A.; Oliveira, H.B.; Silva, E.S.; Costa, R.R.; Kanitz, A.C.; Liedtke, G.V.; Schuch, F.B.; Peyré-Tartaruga, L.A. Effects of Nordic walking training on quality of life, balance and functional mobility in elderly: A randomized clinical trial. PLoS ONE 2019, 14, e0211472. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Zaman, A.; Stegemöller, E.L. Sedentary Behavior and Quality of Life in Individuals with Parkinson’s Disease. Neurorehabilit. Neural Repair 2019, 33, 595–601. [Google Scholar] [CrossRef]
- Casamali, F.F.; Schuch, F.B.; Scortegagna, S.A.; Legnani, E.; De Marchi, A.C. Accordance and reproducibility of the electronic version of the WHOQOL-BREF and WHOQOL-OLD questionnaires. Exp. Gerontol. 2019, 125, 110683. [Google Scholar] [CrossRef]
- Dos Santos Delabary, M.; Monteiro, E.P.; Donida, R.G.; Wolffenbuttel, M.; Peyré-Tartaruga, L.A.; Haas, A.N. Can Samba and Forró Brazilian rhythmic dance be more effective than walking in improving functional mobility and spatiotemporal gait parameters in patients with Parkinson’s disease? BMC Neurol. 2020, 20, 305. [Google Scholar] [CrossRef]
| Variable | NW Group (n = 16) | FW Group (n = 17) | p Value |
|---|---|---|---|
| Age (years) | 64.9 ± 10.2 | 70.5 ± 5.8 | 0.062 |
| Body mass (kg) | 79.0 ± 15.1 | 68.9 ± 11.9 | 0.041 * |
| Women, n (%) | 3 (18) | 10 (58) | 0.757 |
| Stature (m) | 1.67 ± 0.08 | 1.59 ± 0.14 | 0.049 * |
| Body Mass Index (kg/m2) | 28.5 ± 4.2 | 27.4 ± 5.8 | 0.556 |
| Fat percentage (%) | 21.42 ± 5.7 | 24.13 ± 9.3 | 0.367 |
| Length of lower limb (m) | 0.88 ± 0.02 | 0.85 ± 0.03 | 0.087 |
| Time of clinical diagnostics of PD (years) | 5.5 ± 3.3 | 5.1 ± 4.1 | 0.757 |
| UPDRS III | 15.00 ± 3.2 | 23.19 ± 3.9 | 0.128 |
| Hoehn & Yahr (scale of 1 a 4) | 1.5 ± 0.5 | 2.0 ± 1.0 | 0.123 |
| MoCA (score de 0 a 30) | 16.67 ± 1.4 | 21.96 ± 1.1 | 0.004 * |
| Berg Balance Scale | 51.19 ± 1.2 | 47.44 ± 2.5 | 0.988 |
| Clinical Symptoms | R = 7; L = 9 | R = 9; L = 7 | NA |
| Affected leg | 9 | 6 | NA |
| Instability (change of balance) | 7 | 7 | NA |
| Tremor | 6 | 8 | NA |
| Postural changes | 14 | 6 | NA |
| Rigidity | 4 | 11 | NA |
| Bradykinesia | 4 | 0 | NA |
| Dyskinesia | 3 | 2 | NA |
| Freezing | 5 | 5 | NA |
| Historic of falls | |||
| Drugs (Dosages) | 250 ± 0.0 | 181.89 ± 46.81 | NA |
| Levodopa + Carbidopa | 161 ± 53.21 | 250 ± 108.40 | NA |
| Prolopa | 150 ± 93.24 | 200 ± 0.0 | NA |
| Sifrol | - | 400 ± 0.0 | NA |
| Biperideno | - | 175 ± 75.00 | NA |
| Benserazida | - | 150. ± 00 | NA |
| Selegina |
| PRE | POST | p value | ||||
|---|---|---|---|---|---|---|
| Domains of QoL | Intervention | Mean (CI 95%) | Mean (CI 95%) | Group | Time | Time × Group |
| WHOQOL OLD | ||||||
| QoL general | FW | 53.97 | 55.76 | <0.001 * | 0.233 | 0.989 |
| NW | (41.88; 57.89) 67.89 | (47.04; 60.56) 69.71 | ||||
| Sensory ability | FW | (59.34; 73.11) 56.36 (48.39; 64.32) | (63.34; 78.84) 59.16 (52.68; 65.63) | 0.001 * | 0.982 | 0.536 |
| NW | 69.25 (62.26; 76.23) | 66.64 (58.97; 74.32) | ||||
| Autonomy | FW | 51.96 (47.33; 56.58) | 50.17 (40.86; 59.41) | <0.001 * | 0.131 | 0.033 * |
| NW | 64.38 (58.32; 70.44) | 75.32 (68.76; 81.88) | ||||
| Past activities/present/future | FW | 69.46 (46.37; 74.32) | 63.41 (49.69; 75.87) | 0.025 * | 0.091 | 0.045 * |
| NW | 71.18 (68.39; 73.79) | 72.74 (71.48; 82.50) | ||||
| Social participation | FW | 50.53 (44.26; 56.80) | 57.38 (53.06; 61.70) | <0.001 * | 0.007 * | 0.934 |
| NW | 61.63 (56.35; 66.92) | 68.92 (63.52; 74.33) | ||||
| Death and dying | FW | 47.34 (40.48; 54.21) | 52.00 (43.78; 60.22) | 0.039 * | 0.325 | 0.047 * |
| NW | 65.94 (57.15; 74.55) | 52.41 (41.02; 63.08) | ||||
| Intimacy | FW | 48.57 (42.71; 54.43) | 52.61 (45.65; 59.58) | <0.001 * | 0.033 * | 0.544 |
| NW | 76.20 (69.50; 82.90) | 83.50 (75.81; 91.18) | ||||
| WHOQOL BREF | ||||||
| QoL general | FW | 50.06 (43.16; 56.97) | 63.70 (55.73; 71.67) | 0.002 * | <0.001 * | 0.629 |
| NW | 66.30 (59.81; 72.79) | 76.88 (71.25; 82.50) | ||||
| Physical | FW | 51.63 (46.69; 56.57) | 54.80 (49.04; 60.56) | 0.003 * | 0.037 * | 0.453 |
| NW | 60.36 (54.43; 66.28) | 67.23 (61.34; 73.11) | ||||
| Psychological | FW | 57.53 (51.88; 63.19) | 58.65 (54.22; 63.09) | 0.006 * | 0.019 * | 0.093 |
| NW | 67.65 (61.16; 74;14) | 75.02 (66.58; 83.47) | ||||
| Environmental | FW | 60.26 (54.71; 65.80) | 61.81 (57.74; 65.89) | 0.025 * | 0.091 | 0.313 |
| NW | 65.71 (60.32; 69.96) | 71.39 (65.76; 77.03) | ||||
| Social relationship | FW | 58.28 (52.75; 63.82) | 59.72 (55.44; 63.99) | <0.001 * | 0.216 | 0.433 |
| NW | 65.63 (61.11; 70.16) | 72.05 (66.90; 77.19) | ||||
| PRE | POST | |||||
|---|---|---|---|---|---|---|
| Variable | Intervention | Means ± SE | Means ± SE | Group | Time | Group × Time |
| Cognitive function (MoCA) | FW | 16.06 ± 1.1 | 17.29 ± 1.7 | 0.004 * | 0.046 * | 0.784 |
| NW | 21.50 ± 1.1 | 22.43 ± 1.2 | ||||
| Depressive symptoms (GDS—15) | FW | 4.6 ± 0.4 | 3.7 ± 0.4 | 0.014 * | <0.001 * | 0.583 |
| NW | 2.8 ± 0.3 | 1.2 ± 0.3 | ||||
| Motor Symptoms (UPDRS III) | FW | 23.19 ± 3.9 | 17.43 ± 3.8 | 0.128 | <0.001 * | 0.352 |
| NW | 15.10± 3.2 | 11.64 ± 2.1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passos-Monteiro, E.; B. Schuch, F.; T. Franzoni, L.; R. Carvalho, A.; A. Gomeñuka, N.; Becker, M.; Rieder, C.R.M.; Andrade, A.; G. Martinez, F.; S. Pagnussat, A.; et al. Nordic Walking and Free Walking Improve the Quality of Life, Cognitive Function, and Depressive Symptoms in Individuals with Parkinson’s Disease: A Randomized Clinical Trial. J. Funct. Morphol. Kinesiol. 2020, 5, 82. https://doi.org/10.3390/jfmk5040082
Passos-Monteiro E, B. Schuch F, T. Franzoni L, R. Carvalho A, A. Gomeñuka N, Becker M, Rieder CRM, Andrade A, G. Martinez F, S. Pagnussat A, et al. Nordic Walking and Free Walking Improve the Quality of Life, Cognitive Function, and Depressive Symptoms in Individuals with Parkinson’s Disease: A Randomized Clinical Trial. Journal of Functional Morphology and Kinesiology. 2020; 5(4):82. https://doi.org/10.3390/jfmk5040082
Chicago/Turabian StylePassos-Monteiro, Elren, Felipe B. Schuch, Leandro T. Franzoni, Alberito R. Carvalho, Natalia A. Gomeñuka, Marindia Becker, Carlos R. M. Rieder, Alexandro Andrade, Flávia G. Martinez, Aline S. Pagnussat, and et al. 2020. "Nordic Walking and Free Walking Improve the Quality of Life, Cognitive Function, and Depressive Symptoms in Individuals with Parkinson’s Disease: A Randomized Clinical Trial" Journal of Functional Morphology and Kinesiology 5, no. 4: 82. https://doi.org/10.3390/jfmk5040082
APA StylePassos-Monteiro, E., B. Schuch, F., T. Franzoni, L., R. Carvalho, A., A. Gomeñuka, N., Becker, M., Rieder, C. R. M., Andrade, A., G. Martinez, F., S. Pagnussat, A., & A. Peyré-Tartaruga, L. (2020). Nordic Walking and Free Walking Improve the Quality of Life, Cognitive Function, and Depressive Symptoms in Individuals with Parkinson’s Disease: A Randomized Clinical Trial. Journal of Functional Morphology and Kinesiology, 5(4), 82. https://doi.org/10.3390/jfmk5040082






