Reliability and Validity of Near-Infrared Spectroscopy Mitochondrial Capacity Measurement in Skeletal Muscle
Abstract
:1. Introduction
- Measuring reliability of the forearm, calf and thigh in a within day consistency study.
- Measuring reliability in the forearm across days.
- Testing if the NIRS measurement parameter is influenced by measurement location compared in the forearm, calf and thigh.
- Analysis of the influence of hot and cold interventions on the mitochondrial capacity measurement.
- Comparing sensitivity analysis to proof the power for clinical interventions using hot and cold interventions for validity.
2. Materials and Methods
2.1. Ethical Approval
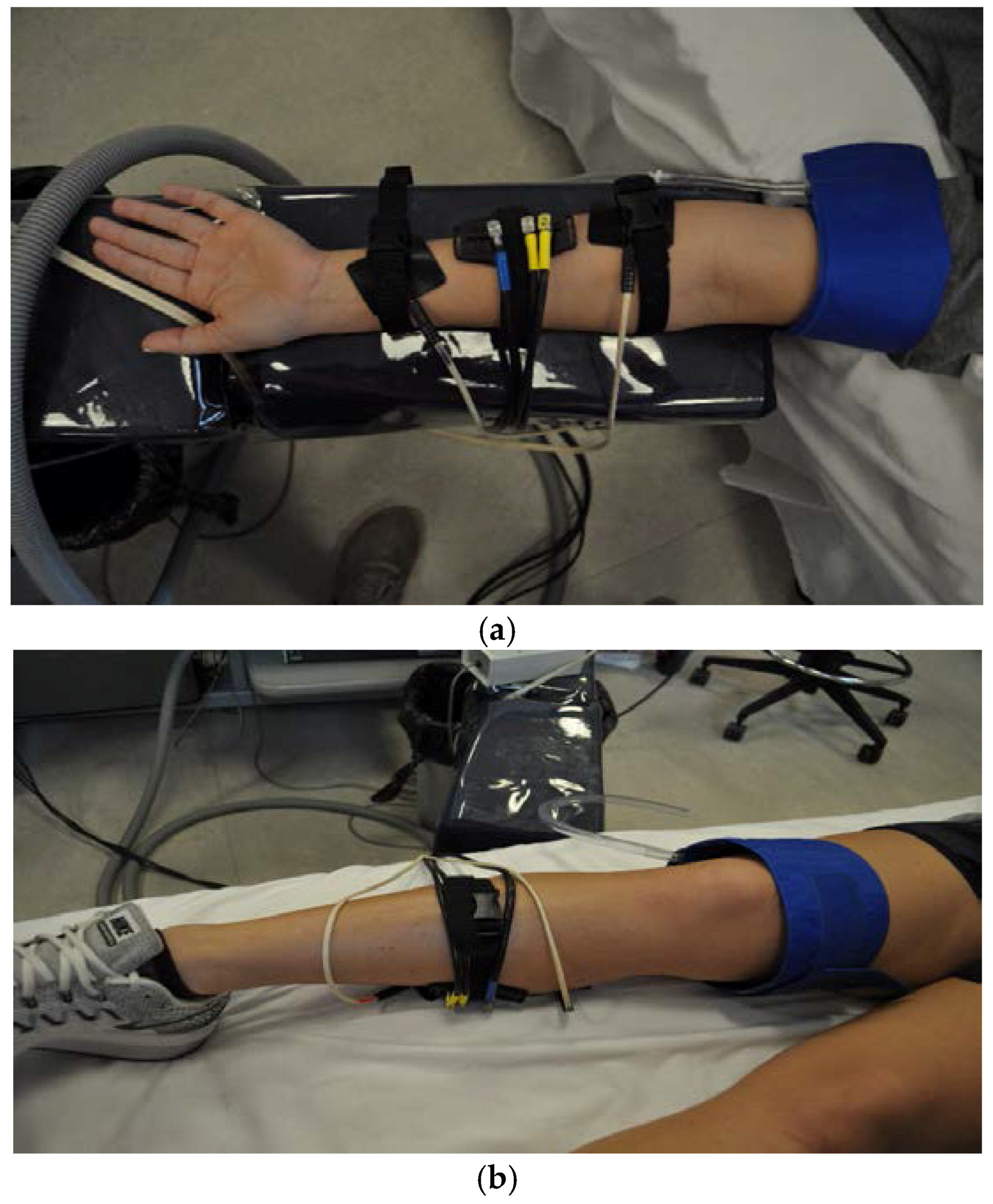
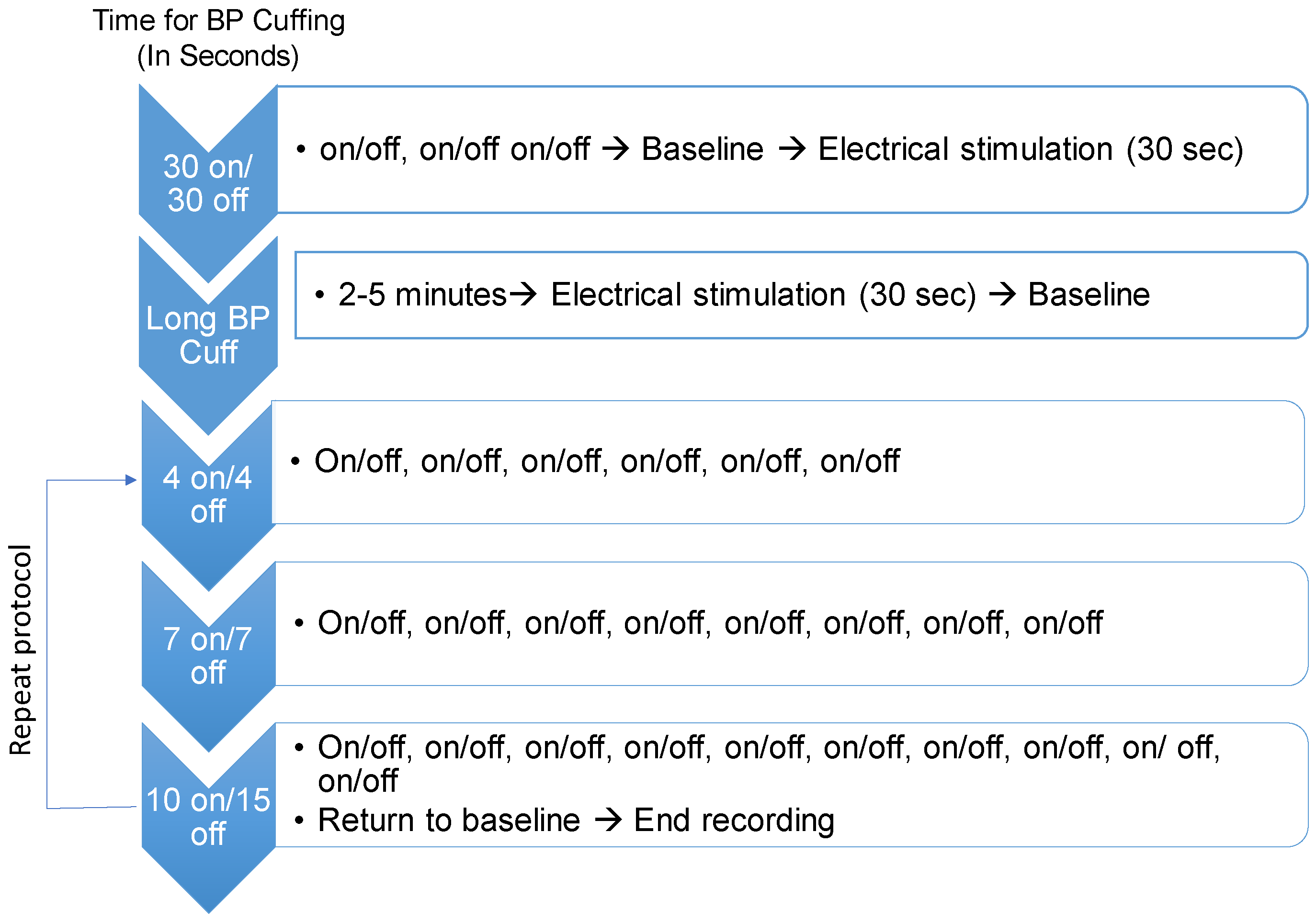
2.2. Participants and Procedures
2.2.1. Visit 1
2.2.2. Visit 2
2.2.3. Visit 3–4
2.3. Statistics
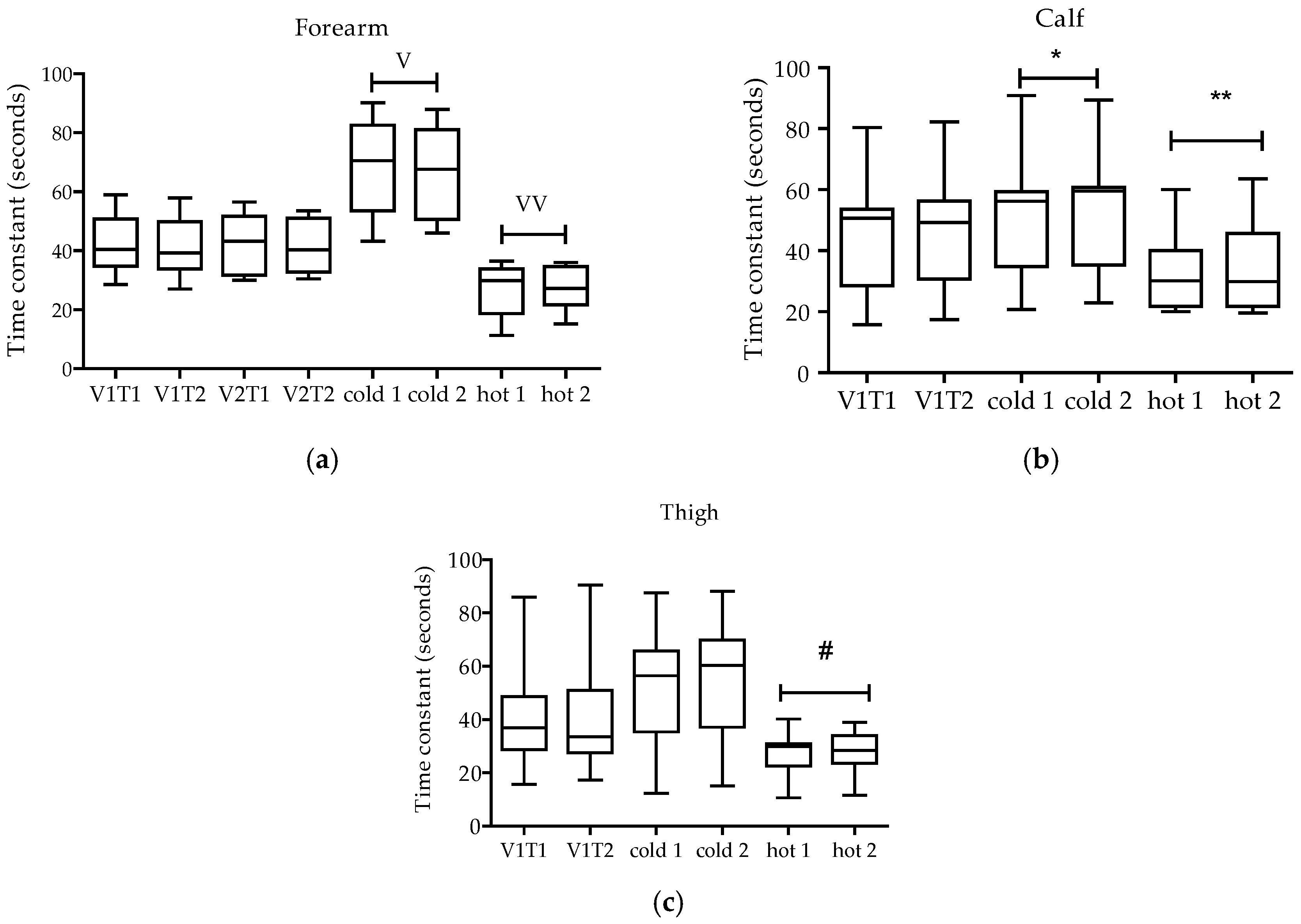
3. Results
4. Discussion
4.1. Reliability
4.2. Validity
4.3. Limitations
Author Contributions
Conflicts of Interest
References
- Ferrari, M.; Binzoni, T.; Quaresima, V. Oxidative metabolism in muscle. Philos. Trans. R. Soc. B 1997, 352, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. R. Soc. A 2011, 369, 4591–4604. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Hamaoka, T. Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle? Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar] [PubMed]
- Southern, W.M.; Reynolds, M.A.; McCully, K.K. Reproducibility of skeletal muscle oxidative function and postexercise kinetics using near-infrared spectroscopy. Med. Sci. Sport Exerc. 2013, 45, 8. [Google Scholar]
- Southern, W.M.; Ryan, T.E.; Kepple, K.; Murrow, J.R.; Nilsson, K.R.; McCully, K.K. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Ryan, T.E.; Young, H.-J.; McCully, K.K. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur. J. Appl. Physiol. 2013, 113, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Allen, C.; Avila, P.; Bina, J.; Konrade, J.; Munns, S. Soft tissue thermodynamics before, during, and after cold pack therapy. Med. Sci. Sport Exerc. 2002, 34, 45–50. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, J. Comparison of muscle temperature increases produced by moist hot pack and thermostim probe. J. Sport Rehabilit. 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.S. The effects of hot packs and exercise on local blood flow. Phys. Ther. 1972, 52, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Karunakara, R.G.; Lephart, S.M.; Pincivero, D.M. Changes in forearm blood flow during single and intermittent cold application. J. Orthop. Sports Phys. Ther. 1999, 29, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Tomchuk, D.; Rubley, M.D.; Holcomb, W.R.; Guadagnoli, M.; Tarno, J.M. The magnitude of tissue cooling during cryotherapy with varied types of compression. J. Athl. Train. 2010, 45, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zemke, J.E.; Andersen, J.; Guion, W.K.; McMillan, J.; Joyner, A.B. Intramuscular temperature responses in the human leg to two forms of cryotherapy: Ice massage and ice bag. J. Orthop. Sports Phys. Ther. 1998, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Myrer, J.W.; Draper, D.O.; Durrant, E. Contrast therapy and intramuscular temperature in the human leg. J. Athl. Train. 1994, 29, 318–322. [Google Scholar] [PubMed]
- Belitsky, R.B.; Odam, S.J.; Hubley-Kozey, C. Evaluation of the effectiveness of wet ice, dry ice, and cryogenic packs in reducing skin temperature. Phys. Ther. 1987, 67, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, W.A.; Robey, K.W. The sampling distribution of the coefficient of variation. Ann. Math. Stat. 1936, 7, 129–144. [Google Scholar] [CrossRef]
- Plichta, M.M.; Herrmann, M.J.; Baehne, C.G.; Ehlis, A.C.; Richter, M.M.; Pauli, P.; Fallgatter, A.J. Event-related functional near-infrared spectroscopy (fnirs): Are the measurements reliable? Neuroimage 2006, 31, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Mantia, A.M.; Neidert, L.E.; Kluess, H.A. Reliability and Validity of Near-Infrared Spectroscopy Mitochondrial Capacity Measurement in Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2018, 3, 19. https://doi.org/10.3390/jfmk3020019
La Mantia AM, Neidert LE, Kluess HA. Reliability and Validity of Near-Infrared Spectroscopy Mitochondrial Capacity Measurement in Skeletal Muscle. Journal of Functional Morphology and Kinesiology. 2018; 3(2):19. https://doi.org/10.3390/jfmk3020019
Chicago/Turabian StyleLa Mantia, Anna M., Leslie E. Neidert, and Heidi A. Kluess. 2018. "Reliability and Validity of Near-Infrared Spectroscopy Mitochondrial Capacity Measurement in Skeletal Muscle" Journal of Functional Morphology and Kinesiology 3, no. 2: 19. https://doi.org/10.3390/jfmk3020019
APA StyleLa Mantia, A. M., Neidert, L. E., & Kluess, H. A. (2018). Reliability and Validity of Near-Infrared Spectroscopy Mitochondrial Capacity Measurement in Skeletal Muscle. Journal of Functional Morphology and Kinesiology, 3(2), 19. https://doi.org/10.3390/jfmk3020019



