Abstract
Establish reliability of near-infrared spectroscopy (NIRS) mitochondrial capacity measurement and assess validity using heating and cooling interventions. We recruited 13 participants for four visits. Two visits consisted of a series of blood pressure occlusions proximal to the NIRS probe and electrical stimulation to the thigh, calf and forearm for reliability. Visits 3 and 4 required heating or cooling of the three muscles, using the protocol above. The between day reliability coefficient of variation (CV) in the forearm was 3% and time constant (TC) average was 41.9 ± 9.2 seconds (s). The within day CV in the forearm = 4.5%, the calf = 5.5% and the thigh = 4.6% (n = 13). Within day reliability for the cold condition in the forearm = 5.9%, calf = 4.3% and thigh = 4.4% and within day for the hot condition in the forearm = 6.4%, calf = 5.3% and thigh = 4.6%. The cold condition increased TC in the forearm, but decreased TC with heat (p < 0.05). The thigh decreased mitochondrial capacity with heat (p < 0.05), but cold was no different from control. These results suggest NIRS mitochondrial capacity has good within and between day reliability and temperature changes the mitochondrial capacity time constant.
1. Introduction
Near-infrared spectroscopy (NIRS) is a noninvasive device used to measure changes in oxygenated and deoxygenated hemoglobin/myoglobin and total hemoglobin, blood volume or flow in localized muscles during rest and exercise [1]. A more recent application of NIRS is for the assessment of muscle oxygen consumption and uses muscle field stimulation to increase muscle metabolic activity and arterial occlusions to create recovery slopes for each occlusion [2]. The arterial occlusion slopes are fit to an exponential curve and a time constant is calculated as a measure of mitochondrial capacity [3]. Depending on the mitochondrial time constant, higher mitochondria capacity correlates to a lower time constant, while lower mitochondrial capacity relates to a higher time constant.
The NIRS device uses multiple, continuous wavelengths in the 700–900 nm range to penetrate into biological tissue. The light emitted from the device follows a “banana shaped” curve into the tissue at a depth equal to half the distance between the light source and the detector [4,5]. A common tissue depth used for mitochondrial capacity testing is 1.5 cm, which is the depth used in the current study.
The mitochondrial capacity recovery protocol approach was validated in young, healthy individuals following exercise training [6] and individuals with spinal cord injury compared to healthy controls [7]. However, there is a lack of research looking at NIRS mitochondrial capacity in a variety of muscles in the same person and the validity and reliability of these measurements using clinically relevant manipulations. Surface cooling and heating of the muscle is a known way to increase and decrease muscle metabolism, respectively, and is easily applied in clinical settings [8,9]. Heating and cooling therapies applied to the skin directly, are ways to heat or cool the skin surface and deeper tissues. Moist heat packs are a form of superficial heating modality that penetrates 1–2 cm deep [10]. Blood flow increases when the body temperature is raised or through heating the muscle. When the skin surface is raised to above 40 °C, an increase in blood flow is attained and the metabolic demand increases [11] resulting in increased metabolic activity. Cold as therapeutic modality, decreases blood flow through vasoconstriction and permeability of the local vessels in order to reduce swelling [12] and reduces metabolic demand, which should reduce mitochondrial capacity. The aim of this study was to establish validity and reliability of skeletal muscle NIRS mitochondrial capacity measurements in young, healthy individuals. We did this through:
- Measuring reliability of the forearm, calf and thigh in a within day consistency study.
- Measuring reliability in the forearm across days.
- Testing if the NIRS measurement parameter is influenced by measurement location compared in the forearm, calf and thigh.
- Analysis of the influence of hot and cold interventions on the mitochondrial capacity measurement.
- Comparing sensitivity analysis to proof the power for clinical interventions using hot and cold interventions for validity.
We predicted that the NIRS mitochondrial capacity recovery protocol will be effective in proof of power for clinical interventions. Cooling the muscle will increase the time constant and thus, decrease mitochondria capacity, but heating the muscle will decrease the time constant and increase mitochondrial capacity demonstrating validity. We will have overall good across day reliability in the forearm and within day reliability in all three muscles.
2. Materials and Methods
2.1. Ethical Approval
All parts of the following study were reviewed and approved by the Institutional Review Board at Auburn University prior to beginning the study (IRB protocol #: 17-050 EP1702, approval date 14 February 2017) and conformed to the standards set by the latest revision of the Declaration of Helsinki. Written informed consent was obtained from all subjects before participation.
2.2. Participants and Procedures
For this study, 13 men and women (men = 8, women = 5), between the ages of 19–35 years, were recruited locally from the Auburn University campus and a signed informed consent was obtained from each participant prior to beginning any data collection. Participants were required to complete a health questionnaire regarding their current health status. Questions included if they were acutely ill, pregnant, or previously diagnosed with hypertension or diabetes. Participant’s weight and height were recorded for body mass index (BMI) measurements to ensure that the amount of fat mass did not interfere with the NIRS signal. Anyone with a BMI of 25 kg/m2 or greater were excluded from the study. We asked these questions to ensure they were apparently healthy to participate in the study.
2.2.1. Visit 1
After collection of the informed consent, anthropometric measurements (height and weight for BMI measurements) and health questionnaire, participants were asked to lay in the supine position with their left arm extended and level to their heart in a quiet room with a constant temperature of about 20 °C. The participants were then set up with a small blood pressure cuff (SC10D, D.E. Hokanson Inc., Bellevue, WA, USA) placed proximal to the forearm connected to a rapid cuff inflator (D.E. Hokanson Inc.).
The NIRS optode (Artinis Medical System, Oxymon MKIII, Elst, The Netherlands) was secured with Velcro straps and placed at the level of the largest circumference of the forearm (see Figure 1 for a photo of the set-up). The NIRS signals were obtained using a continuous-wave NIRS device, which consisted of two channels (2 equivalent pulsed light sources, 2 avalanche photodiode detectors, shielding from ambient light), and used intensity-modulated light at a frequency of 1 Hz, and laser diodes at two wavelengths (762 and 846 mm) corresponding to the absorption wavelengths of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb), with an autosensing power supply (~40 W at 110–240 V). The probe was set for two source-detector separation distances of 30–40 mm apart to collect the data using a “banana shaped” curve signal into the skin [4]. This distance was set to fit the healthy population used and is subject to change with other populations depending on the amount of adipose tissue under the skin. The NIRS data were collected at 10 Hz with optode 2 × 1 setting. Field stimulation (4 Hz, square wave, duration of 1.1 milliseconds; Grass Stimulator model S48F, Grass Medical Instruments Quincy, MA, USA), and a Stimulation Isolation Unit (model SIU8TB; Grass Medical Instruments Quincy, MA, USA) of the muscles was elicited by placing the electrical stimulation pads on either end of the optode on the skin to stimulate the forearm muscle where the optode could monitor the level of electrical-evoked muscle contraction. The intensity of the stimulation was slowly increased (using the stimulation isolation unit) until the desired movement was reached or until the participant experienced discomfort.
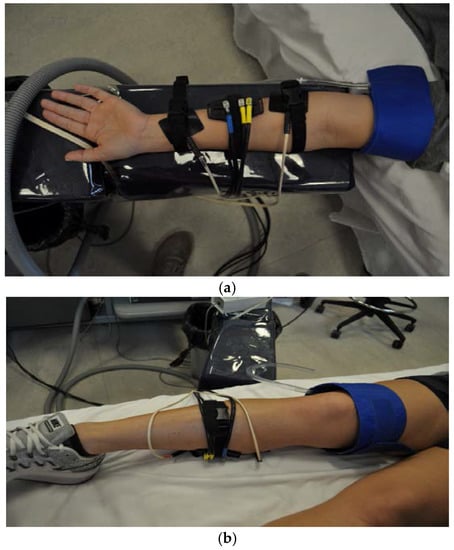
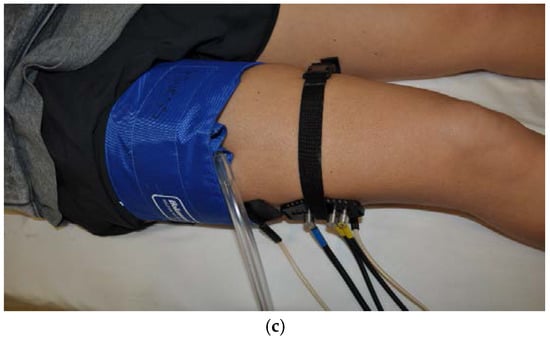
Figure 1.
The set up for the near-infrared spectroscopy (NIRS), arterial blood pressure cuff and electrical stimulation for each muscle. (a) The forearm; (b) is the calf; (c) is the thigh.
The same arrangement and settings of the NIRS optode, blood pressure cuff and electrical stimulation was used on the vastus lateralis (thigh) and gastrocnemius (calf) while they participant lay in the supine position with their leg extended out on the bed. The thigh blood pressure cuff was placed as high up on the participant’s leg as possible and a medium blood pressure cuff (SC12D cuff, D.E. Hokanson Inc.) was used. The NIRS optode was placed on the muscle belly of the vastus lateralis and on either end of the optode, the electrical stimulus pads were placed.
For the calf, the medium blood pressure cuff (SC12D cuff, D.E. Hokanson Inc.) was placed above the knee, slightly above the patella. The NIRS optode was placed on the muscle belly of the gastrocnemius and the electrical stimulation pads were set up on either end of the optode on the skin. The NIRS mitochondrial capacity protocol was used to test the forearm, calf and thigh.
The NIRS mitochondrial capacity protocol consisted of thirty-second blood pressure cuff occlusions at 230 mmHg to establish resting values. Next, the muscle was electrical stimulated for 30 seconds and immediately after, the blood pressure cuff was inflated for 5 min to 230 mmHg. Another electrical stimulation of 30 s occurred and the recovery assessment of the protocol commenced. The recovery consisted of 24 short blood pressure cuff occlusions also at 230 mmHg and was repeated twice [3] (see Figure 2 for a diagram of the protocol).
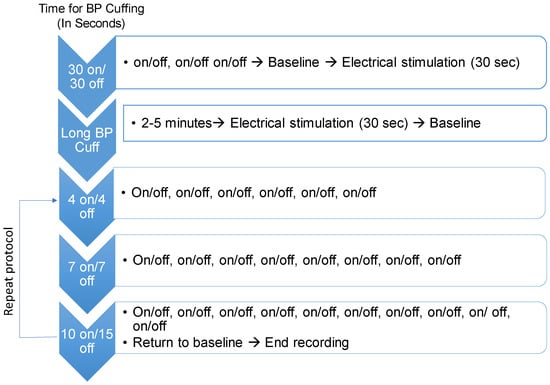
Figure 2.
NIRS mitochondrial capacity protocol that was used on the forearm, calf and thigh. Heating and cooling of the muscle occurred prior and during the start of this protocol.
2.2.2. Visit 2
The next visit occurred 24 h later and consisted of testing the forearm only for NIRS mitochondrial capacity protocol to establish day to day reliability.
2.2.3. Visit 3–4
Participants returned 24 h after visit 2 to participate in one of two treatments, heating (increasing the skin temperature to 40 °C) or cooling (decreasing the skin temperature to 15.6 °C) of the thigh, calf and forearm to increase the temperature of the muscle superficially. The order of the visits was counterbalanced. The skin surface was cooled to 15.6 °C using four commercially available cold packs for 20 min on the forearm and calf, and 25 min on the thigh [13]. The forearm and calf skin surface was heated to 40 °C using a commercially available electric heat pack for 20 min, while the thigh was heated for 25 min. The skin temperatures were measured with a basal digital thermometer (CVS Health- Non-Touch Thermometer, CVS Health, Woondsocket, RI, USA) and checked every 2 min to maintain a consistent temperature.
Treatment areas with greater subcutaneous tissue thickness require longer periods of exposure to both heating and cooling [14]. These temperatures were chosen to penetrate deep into the skin to extend their effects into the muscle [15].The tissues are able to maintain these temperatures for up to an hour as long as the heat or cold pack was properly touching the skin surface [16]. After 24 h, the participants returned to complete the second treatment.
2.3. Statistics
The data were exported as an excel file and analyzed in MATLAB (The MathWorks, Inc., Natick, MA, USA) and a Portamon Analysis System, a proprietary system created by Kevin McCully to assess the mitochondrial capacity recovery protocol (McCully and Ryan. “Systems and Methods for Measuring Mitochondrial Capacity”. US Patent #9,706,959 (2017)). The mitochondrial capacity time constant was found using the slopes from the arterial pressure occlusions in the recovery protocol to create a curve from the averaged oxygenated and deoxygenated blood signal. Statistical comparisons were made using a one-way repeated measures ANOVA. Tukey’s post-hoc analysis was performed if significance was found in the ANOVA. Normality of the data was assessed using a Shapiro-Wilk Normality test. For reliability, a coefficient of variation (CV) was calculated as the ratio of the standard deviation over the mean and was expressed using a percentage. CV is used to represent biometry and is sufficient extensive in some knowledge of sampling distribution. An instance of this is when measuring reliability or reproducibility of repeated trials on the same individual [17,18]. A Bland-Altman analysis (Difference vs average) was also used to assess reliability and the bias and standard deviation of the bias were reported. α was set at 0.05.
3. Results
Data are presented as mean±standard deviation. The average age of the 13 participants (8 male and 5 female), was 26 ± 2 years and the average BMI was 22 ± 2 kg/m2. The within day CV in the forearm was 4.5% (Bland Altman for V1 bias = 0.5 ± 2.5 s; for V2 = 2.0 ± 2.5 s), the calf was 5.5% (Bland Altman bias = −0.2 ± 3.5 s) and the thigh was 4.6% (Bland Altman bias=−0.1 ± 3.2 s; n = 13). The between day reliability for the forearm was 3% (Bland Altman for T1 bias = −0.5 ± 3.2 s; for T2 = 1.1 ± 3.8 s). For the cold condition, the within day reliability for the forearm was 5.9% (Bland Altman bias = 0.3 ± 4.0s), the calf was 4.3% (Bland Altman bias = −1.8 ± 2.9 s) and the thigh was 4.4% (Bland Altman bias = −1.7 ± 2.7 s). For the hot condition the coefficient of variation in the forearm was 6.4% (Bland Altman bias = −0.7 ± 2.5 s), the calf was 5.3% (Bland Altman bias = −1.0 ± 3.0 s) and the thigh was 4.6% (Bland Altman bias = 0.01 ± 2.0 s).
For the forearm, the time constant (TC) average for visits 1 and 2 was 41.9 ± 9.2 seconds (s). The cold condition resulted in an increase in the time constant (averaged time constant: 67.4 ± 15.1 s; p < 0.05) and the hot condition resulted in a decrease in the time constant (average time constant: 27.4 ± 7.9 s; p < 0.05) (Figure 3a).
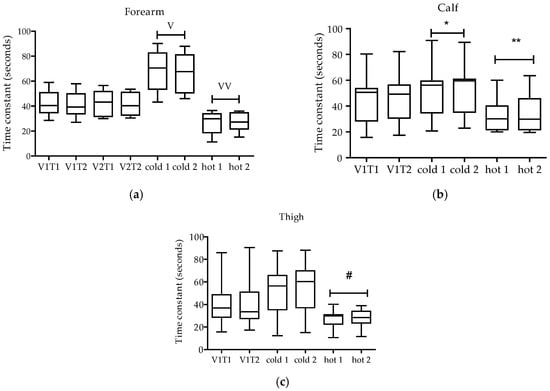
Figure 3.
Time constants in seconds, for the forearm, calf and thigh for each visit (V). V1T1 represents the first test run in visit one. V1T2 represents the second test in visit one. V2T1 represents the first test run in visit two. V2T2 represents second test in visit two. In the forearm (a) and the calf (b), the cold condition resulted in an increase in the time constant and the hot condition resulted in a decrease in the time constant. In the thigh muscle (c), only the hot condition resulted in a decrease in the time constant. V p < 0.05 different from all visits and hot all. VV p < 0.05 different from all visits and cold all. * p < 0.05 different from all V1 all and hot all. ** p < 0.05 different from all V1 all and cold all. # p < 0.05 different from cold all. The box plot shows the median (line), interquartile range (box) and the 95th percentile range (whiskers).
In the calf, the average time constant for the normal temperature condition (V1) was 44.1 ± 18.1 s (Figure 3b). The cold condition increased the time constant (average time constant: 51.6 ± 19.1 s; p < 0.05) and heat decreased the time constant (32.7 ± 12.8 s; p < 0.05). In the thigh (average time constant 41.5 ± 19.3 s), heat decreased the time constant (averaged time constant: 27.2 ± 8.5 s) compared to cold (53.3 ± 22.9 s; p < 0.05), but hot and cold were not different from control (Figure 3c).
4. Discussion
The primary findings in this study were that NIRS mitochondrial capacity time constants are increased by cold and decreased by heat in the forearm and calf, which suggests good construct validity. The time constant in the thigh decreased with heat, but did not change with cold. NIRS mitochondrial capacity has excellent between day consistency in the forearm and within day reliability in the forearm, calf and thigh during three temperature conditions in healthy people. These data suggest that the NIRS mitochondrial capacity measurement is sensitive enough to change with commonly applied clinical interventions and highly reliable within a day and across days.
4.1. Reliability
We saw very good within day reliability (coefficient of variation less than 6.5%) [19] for all muscle groups with all conditions (control, cold and hot). The forearm had excellent between day reliability (coefficient of variation of 3%). A previous study showed that the mitochondrial capacity measurement using NIRS achieved good between day reproducibility (12–17% coefficient of variation) in the calf muscle using various types of voluntary exercise [5]. It appears that the best reliability is achieved with electrical stimulation in all muscle groups.
4.2. Validity
Unique to the present study, the forearm, calf and thigh were tested in healthy individuals to establish NIRS mitochondrial capacity validity by testing the influence of hot and cold interventions on the mitochondrial capacity time constant measurement. We found that cooling the muscle resulted in a longer time constant in the forearm and calf, but not in the thigh. This is likely due to difficulty of cooling a large muscle area. Enwemeka et al. [8] cooled the quadriceps femoris with cold packs and measured the depth of muscle cooling. They found that cold packs cool well up to a 2 cm depth, but not deeper. The NIRS detection depth for this study was 1.5 cm, but it is likely that the muscle stimulation created heat, which blunted the cooling effects on the mitochondrial capacity measurement.
Heating resulted in decreases in the time constant indicating increased mitochondrial capacity in the forearm, calf and the thigh. This is consistent with findings by Little et al. [9] that took muscle biopsies immediately after an intense exercise bout where the muscle temperature was elevated and found increased citrate synthase and cytochrome C oxidase activity. Southern et al. [6] also tested validity of the NIRS mitochondrial capacity measurement by performing wrist-flexor exercise training in healthy people and people with heart failure. They found that the training resulted in improved mitochondrial capacity in the dominant and non-dominant arm of the healthy group, but saw no changes in the heart failure group after training. Further, Erikson et al. [7] showed that the NIRS mitochondrial capacity measurement was sensitive enough to detect differences in the vastus lateralis of healthy people vs people with spinal cord injuries. Overall, the current study found that the most robust response to heating and cooling were in the forearm and calf muscles. Hot and cold interventions allowed for a comparison of sensitivity to proof the power for clinical interventions. There are some challenges to measuring the NIRS mitochondrial capacity measurement in the thigh. The challenges are discussed in the limitations section below.
In conclusion, this study demonstrates well within day NIRS reliability in the forearm, calf and thigh and between day reliability in the forearm in young healthy participants. Validity of the NIRS mitochondrial capacity measurement with hot and cold interventions was good in that it changed the mitochondrial capacity time constant by increasing with cold and decreasing with heat. These data support NIRS mitochondrial capacity measurements as a sensitive and valuable tool for assessing muscle metabolism. Clinically, this protocol could be used to assess muscle metabolic changes in people with limited mobility to monitor muscle health and possibly to better understand the properties of the muscle’s mitochondrial capacity non-invasively.
4.3. Limitations
There are some challenges with the thigh measurement. The occluding cuff is placed at the proximal end of the thigh, therefore, people with larger thigh circumferences are more difficult to occlude and the cuff is more likely to move with inflation of the cuff. This does not appear to affect reliability, but does create noise in the measurements. In addition, thigh length is an issue because of the number of instruments that have to be placed on specific locations on the thigh. This measurement is optimal when the participant has a small thigh circumference and has a long femoral length making the thigh a sub-optimal location for most clinical populations where obesity is common. We did not eliminate participants based on thigh circumference or length, but people with sub-optimal thighs required more labor-intensive analysis than participants that met the optimal conditions for thigh measurements.
Author Contributions
Anna M. La Mantia, Leslie E. Neidert and Heidi A. Kluess conceived, designed and performed the experiment, analyzed data, contributed to the materials, and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferrari, M.; Binzoni, T.; Quaresima, V. Oxidative metabolism in muscle. Philos. Trans. R. Soc. B 1997, 352, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. R. Soc. A 2011, 369, 4591–4604. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Hamaoka, T. Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle? Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar] [PubMed]
- Southern, W.M.; Reynolds, M.A.; McCully, K.K. Reproducibility of skeletal muscle oxidative function and postexercise kinetics using near-infrared spectroscopy. Med. Sci. Sport Exerc. 2013, 45, 8. [Google Scholar]
- Southern, W.M.; Ryan, T.E.; Kepple, K.; Murrow, J.R.; Nilsson, K.R.; McCully, K.K. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Ryan, T.E.; Young, H.-J.; McCully, K.K. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur. J. Appl. Physiol. 2013, 113, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Allen, C.; Avila, P.; Bina, J.; Konrade, J.; Munns, S. Soft tissue thermodynamics before, during, and after cold pack therapy. Med. Sci. Sport Exerc. 2002, 34, 45–50. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, J. Comparison of muscle temperature increases produced by moist hot pack and thermostim probe. J. Sport Rehabilit. 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.S. The effects of hot packs and exercise on local blood flow. Phys. Ther. 1972, 52, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Karunakara, R.G.; Lephart, S.M.; Pincivero, D.M. Changes in forearm blood flow during single and intermittent cold application. J. Orthop. Sports Phys. Ther. 1999, 29, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Tomchuk, D.; Rubley, M.D.; Holcomb, W.R.; Guadagnoli, M.; Tarno, J.M. The magnitude of tissue cooling during cryotherapy with varied types of compression. J. Athl. Train. 2010, 45, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zemke, J.E.; Andersen, J.; Guion, W.K.; McMillan, J.; Joyner, A.B. Intramuscular temperature responses in the human leg to two forms of cryotherapy: Ice massage and ice bag. J. Orthop. Sports Phys. Ther. 1998, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Myrer, J.W.; Draper, D.O.; Durrant, E. Contrast therapy and intramuscular temperature in the human leg. J. Athl. Train. 1994, 29, 318–322. [Google Scholar] [PubMed]
- Belitsky, R.B.; Odam, S.J.; Hubley-Kozey, C. Evaluation of the effectiveness of wet ice, dry ice, and cryogenic packs in reducing skin temperature. Phys. Ther. 1987, 67, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, W.A.; Robey, K.W. The sampling distribution of the coefficient of variation. Ann. Math. Stat. 1936, 7, 129–144. [Google Scholar] [CrossRef]
- Plichta, M.M.; Herrmann, M.J.; Baehne, C.G.; Ehlis, A.C.; Richter, M.M.; Pauli, P.; Fallgatter, A.J. Event-related functional near-infrared spectroscopy (fnirs): Are the measurements reliable? Neuroimage 2006, 31, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).