Physical Therapy Considerations for Chronic Kidney Disease and Secondary Sarcopenia
Abstract
:1. Introduction
2. Chronic Kidney Disease and Bone Mineral Density
3. Chronic Kidney Disease and Lean Body Mass
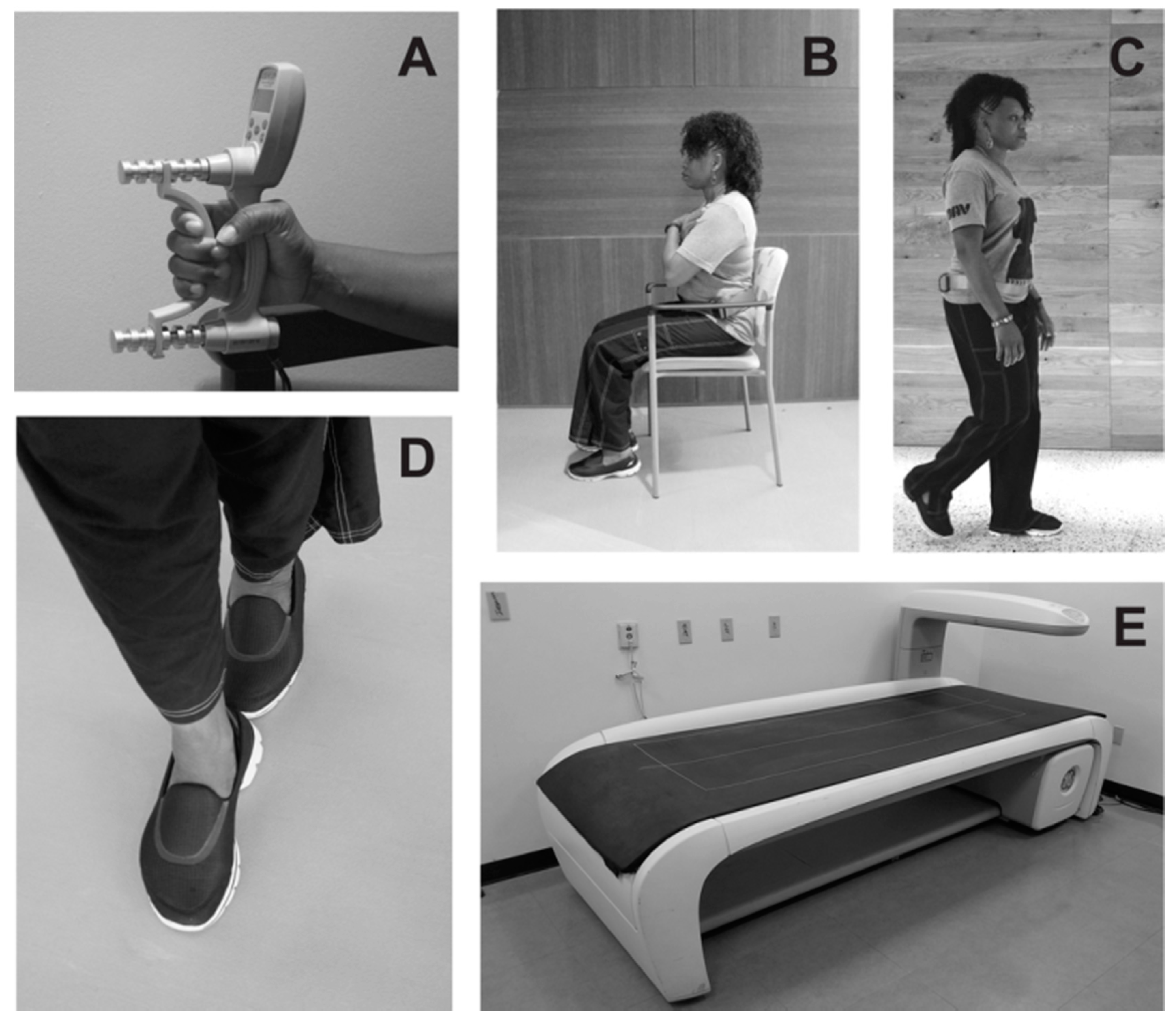
4. The Clinical Management of Secondary Sarcopenia Associated with Chronic Kidney Disease
5. Discussion
- ▪
- Sarcopenia screening with confirmatory assessments of objective muscle strength, functional status, and body composition, when appropriate
- ▪
- The detection of elevated fall risk along with an assessment of fall avoidance behavior
- ▪
- A formal exercise prescription designed to increase muscle strength, promote bone health, and improve balance.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, N.; Golzy, M.; Nainani, N.; Nader, N.D.; Carter, R.L.; Lohr, J.W.; Arora, P. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren. Fail. 2016, 38, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2014; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Koufaki, P.; Kouidi, E. Current best evidence recommendations on measurement and interpretation of physical function in patients with chronic kidney disease. Sports Med. 2010, 40, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Avin, K.G.; Moorthi, R.N. Bone is not alone: The effects of skeletal muscle dysfunction in chronic kidney disease. Curr. Osteoporos. Rep. 2015, 13, 173–179. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Lok, C.E.; Langsetmo, L.; Cheung, A.M.; Szabo, E.; Pearce, D.; Fusaro, M.; Wald, R.; Weinstein, J.; Jamal, S.A. Bone mineral density predicts fractures in chronic kidney disease. J. Bone Miner. Res. 2015, 30, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Yen, J.-F.; Lang, C.-L.; Yan, M.-T.; Lu, K.-C. Bisphophonates in CKD patients with low bone mineral density. Sci. World J. 2013, 2013, 837573. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B. Vitamin D and human skeletal muscle. Scand. J. Med. Sci. Sports 2010, 20, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos. Int. 2002, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Darain, H.; Sah, S. Effect of progressive resistive exercise training in improving mobility and functional ability of middle adulthood patients with chronic kidney disease. Saudi J. Kidney Dis. Transplant. 2015, 26, 912. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Adams, B.; Hernandez, H.J.; DiPietro, L.; Blackman, M.R. Disparities in the consequences of sarcopenia: Implications for African American Veterans. Front. Physiol. 2014, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Kim, T.H.; Yoon, S.Y.; Chung, J.H.; Hwang, H.-J. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS ONE 2015, 10, e0130740. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Garland, S.J.; House, A.A.; Overend, T.J. Morphological, electrophysiological, and metabolic characteristics of skeletal muscle in people with end-stage renal disease: A critical review. Physiother. Can. 2011, 63, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Liu, L.-K.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K. ILAS Research Group Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: Results from the I-Lan longitudinal aging study. J. Am. Med. Dir. Assoc. 2013, 14, 528.e1–528.e7. [Google Scholar] [CrossRef] [PubMed]
- Rubbieri, G.; Mossello, E.; Di Bari, M. Techniques for the diagnosis of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-Y.; Huang, T.-Y.; Wu, Y.-T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J. Am. Geriatr. Soc. 2008, 56, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Fielding, R.; Visser, M.; van Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.; Avelar, I.S.; Andrade, S.R.S.; Vieira, M.F.; Silva, M.S. Body composition of chronic renal patients: Anthropometry and bioimpedance vector analysis. Rev. Lat. Am. Enferm. 2013, 21, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Lowrie, E.G.; Wilmore, D.W.; Gonzalez, J.; Lew, N.L.; Ling, J.; Leboff, M.S.; Gottlieb, M.N.; Huang, W.; Zebrowski, B. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. JASN 1995, 6, 75–81. [Google Scholar] [PubMed]
- Mialich, M.S.; Sicchieri, J.M.F.; Junior, A.A.J. Analysis of body composition: A critical review of the use of bioelectrical impedance analysis. Int. J. Clin. Nutr. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Young, K.C.; Thiebaud, R.S.; Nahar, V.K.; Hollaway, K.M.; Stover, C.D.; Ford, M.A.; Bass, M.A.; Loftin, M. Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med. Biol. 2015, 41, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ismail, C.; Zabal, J.; Hernandez, H.J.; Woletz, P.; Manning, H.; Teixeira, C.; DiPietro, L.; Blackman, M.R.; Harris-Love, M. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front. Physiol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, A.Y.; Meskers, C.G.M.; van den Eshof, N.; Westendorp, R.G.; Sipilä, S.; Stenroth, L.; Sillanpää, E.; McPhee, J.S.; Jones, D.A.; Narici, M.V.; et al. Diagnostic criteria for sarcopenia and physical performance. Age 2014. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Bohannon Association of grip and knee extension strength with walking speed of older women receiving home-care physical therapy. J. Frailty Aging 2015, 4, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M. Assessment of physical performance and disability in older persons. Muscle Nerve 1997, 5, S14–S16. [Google Scholar] [CrossRef]
- Beaudart, C.; Biver, E.; Reginster, J.-Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Van Beveren, J.; et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: The SarQoL. Age Ageing 2015, 44, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes: SARC-F. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar] [PubMed]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Dam, T.-T.L.; Barber, V.; Judge, J.O.; Studenski, S.A.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; et al. Strength and function response to clinical interventions of older women categorized by weakness and low lean mass using classifications from the Foundation for the National Institute of Health sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Meredith, C.N.; O’Reilly, K.P.; Knuttgen, H.G.; Evans, W.J. Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. J. Appl. Physiol. 1988, 64, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Daly, R.M.; Sanders, K.M.; Ebeling, P.R. Fall and fracture risk in sarcopenia and dynapenia with and without obesity: The role of lifestyle interventions. Curr. Osteoporos. Rep. 2015, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Heiwe, S.; Jacobson, S.H. Exercise training for adults with chronic kidney disease. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Watson, E.L.; Greening, N.J.; Viana, J.L.; Aulakh, J.; Bodicoat, D.H.; Barratt, J.; Feehally, J.; Smith, A.C. Progressive resistance exercise training in CKD: A feasibility study. Am. J. Kidney Dis. 2015, 66, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.S.; Frémont, P.; Khan, K.; Poirier, P.; Fowles, J.; Wells, G.D.; Frankovich, R.J. Physical activity prescription: A critical opportunity to address a modifiable risk factor for the prevention and management of chronic disease: A position statement by the Canadian Academy of Sport and Exercise Medicine. Br. J. Sports Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-1-4698-2666-0. [Google Scholar]
- Johansen, K.L.; Painter, P. Exercise in Individuals with CKD. Am. J. Kidney Dis. 2012, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
| Stage | GFR Level (mL/min/1.73 m2) | Description |
|---|---|---|
| 1 | 90 or above | Kidney damage that includes normal or high GFR |
| 2 | 60–89 | Kidney damage that includes slightly decreased GFR |
| 3A | 45–59 | Moderate CKD with mild-moderate decrease in GFR |
| 3B | 30–44 | Moderate CKD with moderate-severe decrease in GFR |
| 4 | 15–29 | Severe CKD with severe decrease in GFR |
| 5 | <15 | End stage renal disease/kidney failure where dialysis in required |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, H.J.; Obamwonyi, G.; Harris-Love, M.O. Physical Therapy Considerations for Chronic Kidney Disease and Secondary Sarcopenia. J. Funct. Morphol. Kinesiol. 2018, 3, 5. https://doi.org/10.3390/jfmk3010005
Hernandez HJ, Obamwonyi G, Harris-Love MO. Physical Therapy Considerations for Chronic Kidney Disease and Secondary Sarcopenia. Journal of Functional Morphology and Kinesiology. 2018; 3(1):5. https://doi.org/10.3390/jfmk3010005
Chicago/Turabian StyleHernandez, Haniel J., Gideon Obamwonyi, and Michael O. Harris-Love. 2018. "Physical Therapy Considerations for Chronic Kidney Disease and Secondary Sarcopenia" Journal of Functional Morphology and Kinesiology 3, no. 1: 5. https://doi.org/10.3390/jfmk3010005
APA StyleHernandez, H. J., Obamwonyi, G., & Harris-Love, M. O. (2018). Physical Therapy Considerations for Chronic Kidney Disease and Secondary Sarcopenia. Journal of Functional Morphology and Kinesiology, 3(1), 5. https://doi.org/10.3390/jfmk3010005






