Effect of Overnight Fasted Exercise on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
3. Evaluation of Articles
4. Statistical Analysis
5. Results
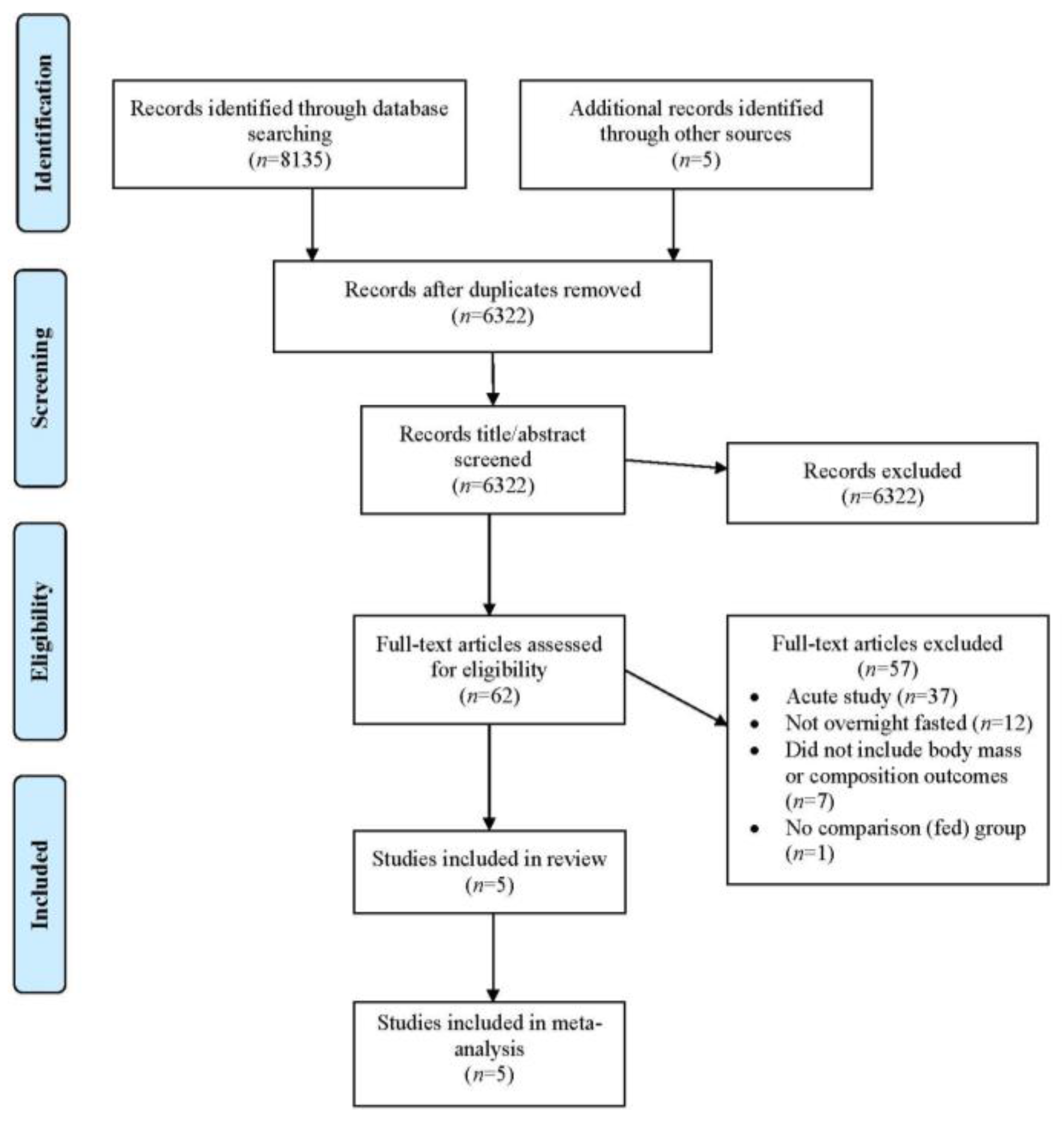
5.1. Description of Studies
5.2. Effect on Body Mass and Composition
5.3. Risk of Bias
6. Discussion
7. Practical Applications
Author Contributions
Conflicts of Interest
References
- Clark, J.E. Diet, exercise or diet with exercise: Comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J. Diabetes Metab. Disord. 2015, 14, 1–28. [Google Scholar]
- Forbes, G.B. Lean body mass-body fat interrelationships in humans. Nutr. Rev. 1987, 45, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. Body fat and fat-free mass inter-relationships: Forbes’s theory revisited. Br. J. Nutr. 2007, 97, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; D’Orso, M. Body for Life: 12 weeks to mental and physical strength; Harper Collins: New York, NY, USA, 1999. [Google Scholar]
- Goldhamer, A.; Lisle, D.; Parpia, B.; Anderson, S.V.; Campbell, T.C. Medically supervised water-only fasting in the treatment of hypertension. J. Manip. Physiol. Ther. 2001, 24, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, N.; Schnitzler, A.; Hunter, G.; Davis, B. Body composition and energy metabolism in resting and exercising muslims during ramadan fast. J. Sports Med. Phys. Fit. 1992, 32, 156–163. [Google Scholar]
- Maughan, R.J.; Fallah, J.; Coyle, E.F. The effects of fasting on metabolism and performance. Br. J. Sports Med. 2010, 44, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.; Coconcelli, L.; Kruel, L.F. Effects of aerobic exercise performed in fasted v. Fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism; Cengage Learning: Belmont, CA, USA, 2012. [Google Scholar]
- Jeukendrup, A.E. Regulation of fat metabolism in skeletal muscle. Ann. N. Y. Acad. Sci. 2002, 967, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2011; pp. 8.1–8.43. [Google Scholar]
- Ugille, M.; Moeyaert, M.; Beretvas, S.N.; Ferron, J.M.; van den Noortgate, W. Bias corrections for standardized effect size estimates used with single-subject experimental designs. J. Exp. Educ. 2014, 82, 358–374. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Elsevier Science: Burlington, MA, USA, 2013; pp. 25–27. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Derave, W.; Eijnde, B.O.; Hesselink, M.K.; Koninckx, E.; Rose, A.J.; Schrauwen, P.; Bonen, A.; Richter, E.A.; Hespel, P. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J. Appl. Physiol. 2008, 104, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Percival, M.E.; Ludzki, A.; Tarnopolsky, M.A.; Gibala, M.J. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 2013, 21, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A.; Wilborn, C.D.; Krieger, J.W.; Sonmez, G.T. Body composition changes associated with fasted versus non-fasted aerobic exercise. J. Int. Soc. Sports Nutr. 2014, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Pelgrim, K.; Deldicque, L.; Hesselink, M.; Van Veldhoven, P.P.; Hespel, P. Training in the fasted state improves glucose tolerance during fat-rich diet. J. Physiol. 2010, 588, 4289–4302. [Google Scholar] [CrossRef] [PubMed]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Wycherley, T.; Luscombe-Marsh, N.; Taylor, P.; Brinkworth, G.; Riley, M. Dairy intake enhances body weight and composition changes during energy restriction in 18–50-year-old adults-a meta-analysis of randomized controlled trials. Nutrients 2016, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Marcolin, G.; Zonin, F.; Neri, M.; Sivieri, A.; Pacelli, Q.F. Exercising fasting or fed to enhance fat loss? Influence of food intake on respiratory ratio and excess postexercise oxygen consumption after a bout of endurance training. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yamamoto, Y.; Iwayama, K.; Nakamura, K.; Yamaguchi, S.; Hibi, M.; Nabekura, Y.; Tokuyama, K. Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism 2013, 62, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Alderman, B.L. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J. Appl. Physiol. 2014, 116, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L.; MacLean, P.S.; Hill, J.O. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc. Sport Sci. Rev. 2009, 37, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [PubMed]
- Skelly, L.E.; Andrews, P.C.; Gillen, J.B.; Martin, B.J.; Percival, M.E.; Gibala, M.J. High-intensity interval exercise induces 24-h energy expenditure similar to traditional endurance exercise despite reduced time commitment. Appl. Physiol. Nutr. Metab. 2014, 39, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Johnson, N.A.; Mielke, G.I.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; Berg, R.; Ward, R.; Keech, A. The effects of high-intensity interval training vs. Moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Truesdale, K.P.; McClain, J.E.; Cai, J. The definition of weight maintenance. Int. J. Obes. 2006, 30, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American college of sports medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Sadri, S.; Sargent, R.G.; Ward, D. Weight control and calorie expenditure: Thermogenic effects of pre-prandial and post-prandial exercise. Addict. Behav. 1989, 14, 347–351. [Google Scholar] [CrossRef]
- Goben, K.W.; Sforzo, G.A.; Frye, P.A. Exercise intensity and the thermic effect of food. Int. J. Sport Nutr. 1992, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Ha, M.S.; Lee, Y.J. The effects of various intensities and durations of exercise with and without glucose in milk ingestion on postexercise oxygen consumption. J. Sports Med. Phys. Fit. 1999, 39, 341–347. [Google Scholar]
- Lemon, P.W.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 624–629. [Google Scholar] [PubMed]
- Carraro, F.; Stuart, C.A.; Hartl, W.H.; Rosenblatt, J.; Wolfe, R.R. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am. J. Physiol. Endocrinol. Metab. 1990, 259, E470–E476. [Google Scholar]
- Sheffield-Moore, M.; Yeckel, C.; Volpi, E.; Wolf, S.; Morio, B.; Chinkes, D.; Paddon-Jones, D.; Wolfe, R. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E513–E522. [Google Scholar] [CrossRef] [PubMed]
- Klempel, M.C.; Kroeger, C.M.; Varady, K.A. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism 2013, 62, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Veldhuis, J.D.; Johnson, M.L.; Furlanetto, R.; Evans, W.S.; Alberti, K.G.; Thorner, M.O. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Investig. 1988, 81, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brooks, G.A. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 1999, 86, 479–487. [Google Scholar] [PubMed]
- Hansen, D.; De Strijcker, D.; Calders, P. Impact of endurance exercise training in the fasted state on muscle biochemistry and metabolism in healthy subjects: Can these effects be of particular clinical benefit to type 2 diabetes mellitus and insulin-resistant patients? Sports Med. 2017, 47, 415–428. [Google Scholar] [CrossRef] [PubMed]
| Study | Group | Sex: M (%) | Age (Year) | Training Status | Exercise Prescription | Duration (wk) | Frequency (d/wk) |
|---|---|---|---|---|---|---|---|
| De Bock et al. [18] | Fasted (n = 10) | 100 | 21.2 ± 0.4 | Trained | Cycling: 60–120 min @ 75% 70-VO2 peak (supervised) | 6 | 3 |
| Fed (n = 10) | 100 | 21.2 ± 0.4 | Trained | ||||
| Gillen et al. [19] | Fasted (n = 8) | 0 | 27.0 ± 9.0 | Untrained | Cycling: 10 × 60 s efforts @ 90% HRmax with 60 s recovery (supervised) | 6 | 3 |
| Fed (n = 8) | 0 | 27.0 ± 7.0 | Untrained | ||||
| Schoenfeld et al. [20] | Fasted (n = 10) | 0 | 23.8 ± 3.0 | Trained | Treadmill: 60 min @ 70% MHR (supervised) | 4 | 3 |
| Fed (n = 10) | 0 | 21.0 ± 1.7 | Trained | ||||
| Van Proeyen et al. [21] | Fasted (n = 10) | 100 | 21.2 ± 1.0 | Trained | Cycling: 2/wk = 60 min & 2/wk = 90 min. Cycling performed @ 70–75% VO2 max and running @ 85% VO2 max (supervised) | 6 | 4 |
| Fed (n = 10) | 100 | 21.2 ± 1.0 | Trained | ||||
| Van Proeyen et al. [22] | Fasted (n = 10) | 100 | 23.0 ± 1.1 | Trained | Cycling: 2/wk = 60 min & 2/wk = 90 min performed @ ~70-VO2 max (supervised) | 6 | 4 |
| Fed (n = 10) | 100 | 22.1 ± 0.9 | Trained |
| Study | Fasted Exercise | Fed Exercise | Between Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre-Training | Post-Training | Hedges’ g | 95% CI | n | Pre-Training | Post-Training | Hedges’ g | 95% CI | Hedges’ g | 95% CI | p | |
| Body Mass (Males) | |||||||||||||
| De Bock et al. [18] | 10 | 74.3 ± 2.8 | 74.2 ± 3.0 | −0.03 (0.29) | −0.60 to 0.54 | 10 | 75.3 ± 3.0 | 75.5 ± 2.9 | 0.06 (0.29) | −0.51 to 0.63 | 0.10 (0.43) | −0.74 to 0.94 | 0.82 |
| Van Proeyen et al. [21] | 10 | 73.3 ± 9.8 | 74.1 ± 8.8 | 0.08 (0.29) | −0.49 to 0.65 | 10 | 70.2 ± 11.4 | 71.6 ± 10.7 | 0.12 (0.29) | −0.45 to 0.69 | 0.06 (0.43) | −0.78 to 0.90 | 0.89 |
| Van Proeyen et al. [22] | 10 | 76.0 ± 4.6 | 75.8 ± 4.3 | −0.04 (0.33) | −0.61 to 0.53 | 10 | 77.6 ± 3.7 | 76.9 ± 3.4 | −0.18 (0.29) | −0.75 to 0.39 | −0.12 (0.43) | −0.96 to 0.72 | 0.77 |
| Mean Effect | - | - | - | 0.01 (0.17) | −0.33 to 0.33 | - | - | 0.01 (0.17) | −0.33 to 0.33 | 0.02 (0.20) | −0.36 to 0.41 | 0.90 | |
| Body Mass (Females) | |||||||||||||
| Gillen et al. [19] | 8 | 79.0 ± 15.0 | 79.0 ± 15.0 | 0.01 (0.31) | −0.62 to 0.62 | 8 | 77.0 ± 12.0 | 77.0 ± 13.0 | 0.01 (0.31) | −0.62 to 0.62 | 0 (0.47) | −0.93 to 0.93 | 1.00 |
| Schoenfeld et al. [20] | 10 | 62.4 ± 7.8 | 60.8 ± 7.8 | −0.19 (0.29) | −0.76 to 0.39 | 10 | 62.0 ± 5.5 | 61.0 ± 5.7 | −0.16 (0.30) | −0.73 to 0.41 | 0.08 (0.43) | −0.76 to 0.92 | 0.84 |
| Mean Effect | - | - | - | −0.10 (0.21) | −0.52 to 0.32 | - | - | −0.09 (0.21) | −0.51 to 0.33 | 0.05 (0.32) | −0.58 to 0.67 | 0.88 | |
| Body Mass (Combined) | |||||||||||||
| Mean Effect | - | - | - | −0.04 (0.13) | −0.30 to 0.22 | - | - | −0.03 (0.13) | −0.29 to 0.23 | 0.02 (0.20) | −0.36 to 0.41 | 0.90 | |
| % Body Fat (Females) | |||||||||||||
| Gillen et al. [19] | 8 | 42.3 ± 8.1 | 41.6 ± 7.8 | −0.08 (0.32) | −0.70 to 0.54 | 8 | 40.9 ± 5.8 | 40.1 ± 5.4 | −0.13 (0.32) | −0.75 to 0.49 | 0.01 (0.47) | −0.91 to 0.94 | 0.98 |
| Schoenfeld et al. [20] | 10 | 26.3 ± 7.9 | 25.0 ± 7.7 | −0.15 (0.29) | −0.72 to 0.42 | 10 | 24.8 ± 8.4 | 24.1 ± 8.5 | −0.08 (0.29) | −0.64 to 0.49 | 0.07 (0.43) | −0.77 to 0.91 | 0.87 |
| Mean Effect | - | - | - | −0.12 (0.21) | −0.54 to 0.30 | - | - | −0.10 (0.21) | −0.52 to 0.32 | 0.05 (0.32) | −0.58 to 0.67 | 0.89 | |
| Lean Mass (Females) | |||||||||||||
| Gillen et al. [19] | 8 | 42.8 ± 5.5 | 43.3 ± 5.5 | 0.08 (0.32) | −0.54 to 0.70 | 8 | 43.5 ± 8.2 | 44.1 ± 7.8 | 0.07 (0.32) | −0.55 to 0.68 | 0.01 (0.47) | −0.91 to 0.94 | 0.98 |
| Schoenfeld et al. [20] | 10 | 45.9 ± 6.7 | 45.4 ± 6.1 | −0.07 (0.29) | −0.64 to 0.50 | 10 | 46.3 ± 3.8 | 46.1 ± 4.3 | −0.05 (0.29) | −0.61 to 0.52 | 0.05 (0.43) | −0.79 to 0.89 | 0.90 |
| Mean Effect | - | - | - | 0.01 (0.21) | −0.42 to 0.42 | - | - | - | 0.01 (0.21) | −0.41 to 0.42 | 0.04 (0.32) | −0.59 to 0.66 | 0.91 |
| Study | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) |
|---|---|---|---|---|---|---|
| De Bock et al. [18] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| Gillen et al. [19] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| Schoenfeld et al. [20] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| Van Proeyen et al. [21] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| Van Proeyen et al. [22] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackett, D.; Hagstrom, A.D. Effect of Overnight Fasted Exercise on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis. J. Funct. Morphol. Kinesiol. 2017, 2, 43. https://doi.org/10.3390/jfmk2040043
Hackett D, Hagstrom AD. Effect of Overnight Fasted Exercise on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2017; 2(4):43. https://doi.org/10.3390/jfmk2040043
Chicago/Turabian StyleHackett, Daniel, and Amanda D. Hagstrom. 2017. "Effect of Overnight Fasted Exercise on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis" Journal of Functional Morphology and Kinesiology 2, no. 4: 43. https://doi.org/10.3390/jfmk2040043
APA StyleHackett, D., & Hagstrom, A. D. (2017). Effect of Overnight Fasted Exercise on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology, 2(4), 43. https://doi.org/10.3390/jfmk2040043





