Sarcopenia and Exercise “The State of the Art”
Abstract
1. Introduction
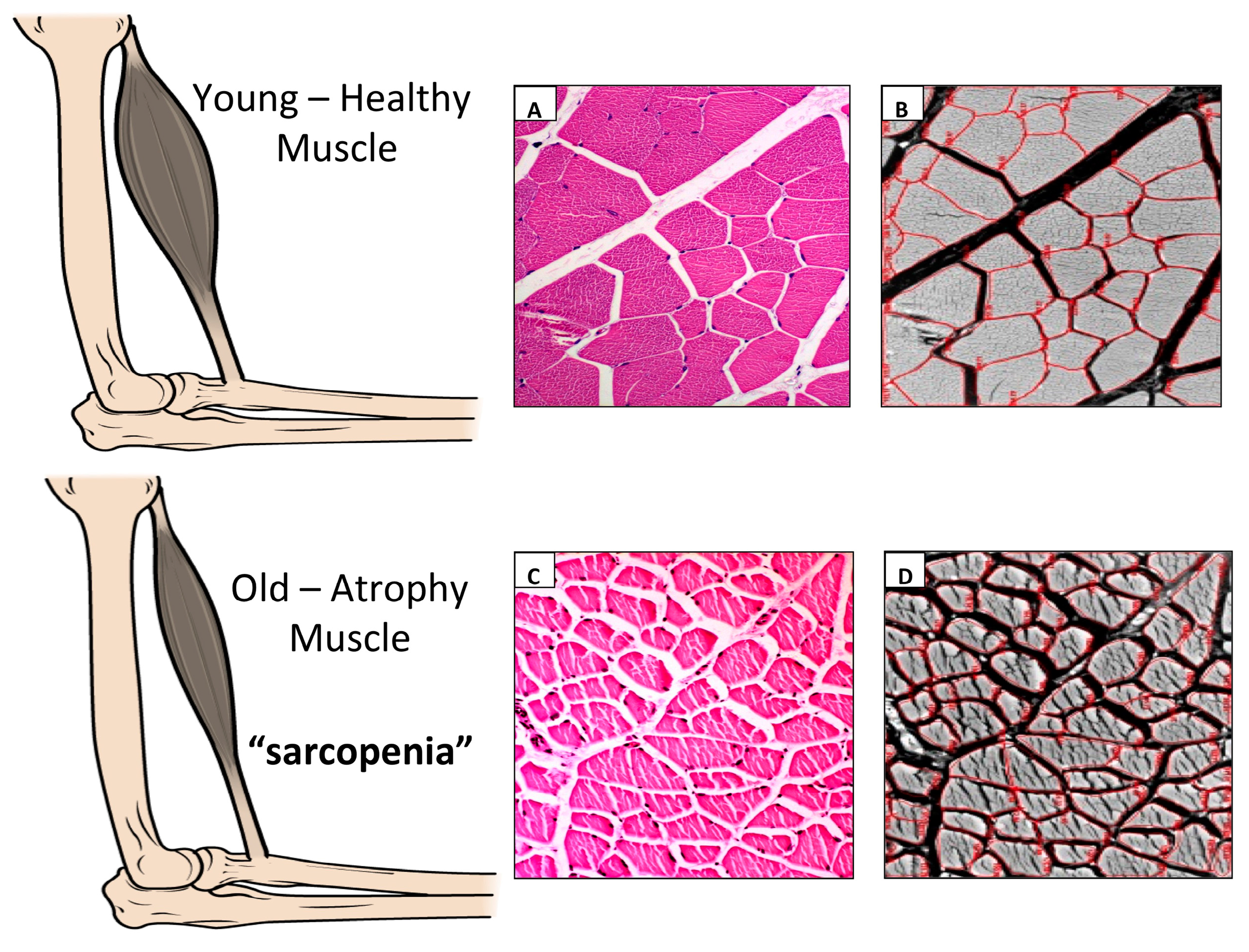
2. Sarcopenia
3. Exercise
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Musumeci, G.; Imbesi, R.; Szychlinska, M.A.; Castrogiovanni, P. Apoptosis and Skeletal Muscle in Aging. Open J. Apoptosis 2015, 4, 41–46. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Fried, L.P. Frailty and the older man. Med. Clin. N. Am. 1999, 83, 1173–1194. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Zmuda, J.M.; Cauley, J.A. Patterns and determinants of muscle strength change with aging in older men. Aging Male 2005, 8, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Chaves, P.; Koenig, K.; Walston, J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J. Am. Geriatr. Soc. 2002, 50, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Coleman, R.; Szychlinska, M.A.; Salvatorelli, L.; Parenti, R.; Magro, G.; Imbesi, R. Somitogenesis: From somite to skeletal muscle. Acta Histochem. 2015, 117, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, R.; D’Agata, V.; Musumeci, G.; Castrogiovanni, P. Skeletal muscle: From development to function. Clin. Ther. 2014, 165, 47–56. [Google Scholar]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.M.; Imbesi, R.; Conway, N.; Castrogiovanni, P. Morphological and Functional Aspects of Human Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2016, 1, 289–302. [Google Scholar] [CrossRef]
- Lynch, G.S. Overview of sarcopenia. In Sarcopenia—Age-Related Muscle Wasting and Weakness; Lynch, G.S., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–7. [Google Scholar]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Ageing and human muscle: Observations from Sweden. Can. J. Appl. Physiol. 1993, 18, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Downham, D.Y. The occurrence of fibre-type grouping in healthy human muscle: A quantitative study of crosssections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol. 1991, 81, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.V.; Faulkner, J.A. Skeletal muscle weakness in old age: Underlying mechanisms. Med. Sci. Sports Exerc. 1994, 26, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, X.; Fan, M. Signaling mechanisms involved in disuse muscle atrophy. Med. Hypotheses 2007, 69, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Ohira, Y.; Jiang, B.; Roy, R.R.; Oganov, V.; Ilyina-Kakueva, E.; Marini, J.F.; Edgerton, V.R. Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol. 1992, 73, 51S–57S. [Google Scholar] [PubMed]
- Anker, S.D.; Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 2002, 85, 51–66. [Google Scholar] [CrossRef]
- Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.D.; Selig, S.; Hare, D.L.; Hayes, A.; Krum, H.; Patterson, J.; Geerling, R.H.; Toia, D.; Carey, M.F. Reduced exercise tolerance in CHF may be related to factors other than impaired skeletal muscle oxidative capacity. J. Card. Fail. 2004, 10, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Massie, B.M.; Simonini, A.; Sahgal, P.; Wells, L.; Dudley, G.A. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J. Am. Coll. Cardiol. 1996, 27, 140–145. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- Delbono, O. Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle. Biogerontology 2005, 3, 265–270. [Google Scholar] [CrossRef]
- Lowe, D.A.; Husom, A.D.; Ferrington, D.A.; Thompson, L.V. Myofibrillar myosin ATPase activity in hindlimb muscles from young and aged rats. Mech. Ageing Dev. 2004, 125, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Lord, C.; Goulet, E.D.; Khalil, A.; Dionne, I.J. Effect of sarcopenia on cardiovascular disease risk factors in obese postmenopausal women. Obesity (Silver Spring) 2006, 14, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Omlin, A.; Baracos, V.E.; Solheim, T.S.; Tan, B.H.; Stone, P.; Kaasa, S.; Fearon, K.; Strasser, F. Cancer cachexia: A systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit. Rev. Oncol. Hematol. 2011, 80, 114–144. [Google Scholar] [CrossRef] [PubMed]
- Buehring, B.; Kirchner, E.; Sun, Z.; Calabrese, L. The frequency of low muscle mass and its overlap with low bone mineral density and lipodystrophy in individuals with HIV-a pilot study using DXA total body composition analysis. J. Clin. Densitom. 2012, 15, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.; Powers, S.K.; Goncalves, D.; Santos, A.; Mota, M.P.; Duarte, J.A. Physical inactivity is a major contributor to ovariectomy-induced sarcopenia. Int. J. Sports Med. 2012, 33, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Hepple, R.T. Viewpoint: Muscle atrophy is not always sarcopenia. J. Appl. Physiol. 2012, 113, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.H.; Rakowicz, W.; Gardner, R.; Pestronk, A. Frequent atrophic groups with mixed-type myofibers is distinctive to motor neuron syndromes. Muscle Nerve 2007, 36, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. 2001, 56, B209–B217. [Google Scholar] [CrossRef]
- Mosoni, L.; Mirand, P.P.; Houlier, M.L.; Arnal, M. Age-related changes in protein synthesis measured in vivo in rat liver and gastrocnemius muscle. Mech. Ageing Dev. 1993, 68, 209–220. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Yarasheski, K.E. Exercise, aging, and muscle protein metabolism. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M918–M922. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.; Bassey, E.J.; Butterworth, S.; Hardy, R.; Wadsworth, M.E.; Musculoskeletal Study Team. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: Associations with physical activity, health status, and socioeconomic conditions. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 224–231. [Google Scholar]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Makanae, Y.; Fujita, S. Role of Exercise and Nutrition in the Prevention of Sarcopenia. J. Nutr. Sci. Vitaminol. (Tokyo) 2015, 61, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.W.G.; Slater, C.; Preston, T.; Barber, M.D.; Fearon, K.C.H. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancercan be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br. J. Cancer 2004, 90, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Georgiou, D.; Cianci, G.; Purcaro, A. 10-year exercise training in chronic heart failure: A randomized controlled trial. J. Am. Coll. Cardiol. 2012, 60, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar] [PubMed]
- Nicastro, H.; Zanchi, N.E.; Da Luz, C.R.; Lancha, A.H. Functional and morphological effects of resistance exercise on disuse-induced skeletal muscle atrophy. Braz. J. Med. Biol. Res. 2011, 44, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Mac Intyre, D.L.; Narici, M.V.; Maganaris, C.N.; Reid, W.D. Preservation of eccentric strength in older adults: Evidence, mechanisms and implications for training and rehabilitation. Exp. Gerontol. 2010, 45, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Anderson, J.M.; Armstrong, L.E.; Nindl, B.C.; Volek, J.S.; Maresh, C.M. Resistance exercise biology: Manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008, 38, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Verdijk, L.B.; Van Loon, L.J.C. Exercise and nutritional interventions to combat age-related muscle loss. In Sarcopenia—Age-Related Muscle Wasting and Weakness; Lynch, G., Ed.; Springer: Berlin, Germany, 2011; pp. 289–315. [Google Scholar]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Rogan, S.; Hilfiker, R.; Herren, K.; Radlinger, L.; De Bruin, E.D. Effects of whole-body vibration on postura control in elderly: A systematic review and meta-analysis. BMC Geriatr. 2011, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The Use of Vibration as Physical Exercise and Therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef]
- Porcari, J.P.; Mclean, K.P.; Foster, C.; Kernozek, T.; Crenshaw, B.; Swenson, C. Effects of electrical muscle stimulation on body composition, muscle strength, and physical appearance. J. Strength Cond. Res. 2002, 16, 165–172. [Google Scholar] [PubMed]
- Lexell, J.; Taylor, C.; Sjostrom, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Abercromby, A.F.; Amonette, W.E.; Layne, C.S.; Mcfarlin, B.K.; Hinman, M.R.; Paloski, W.H. Variation in neuromuscular responses during acute whole-body vibration exercise. Med. Sci. Sports Exerc. 2007, 39, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J. Vibration exercise: The potential benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Bosco, C. The use of vibration as an exercise intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Von Stengel, S. Alternative exercise technologies to fight against sarcopenia at old age: A series of studies and review. J. Aging Res. 2012, 2012, 109013. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Loreto, C.; Castorina, S.; Pichler, K.; Weinberg, A.M. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int. J. Mol. Sci. 2013, 14, 15767–15784. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Apoptotic signaling in skeletal muscle fibers during atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Tudoraşcu, I.; Sfredel, V.; Riza, A.L.; Dănciulescu Miulescu, R.; Ianoşi, S.L.; Dănoiu, S. Motor Unit Changes in Normal Aging: A Brief Review. Rom. J. Morphol. Embryol. 2014, 55, 1295–1301. [Google Scholar] [PubMed]
- Marzetti, E.; Wohlgemutz, S.E.; Lees, H.A.; Chung, H.; Giovannini, S.; Leeuwenburgh, C. Age-Related Activation of Mitochondrial Caspase-Independent Apoptotic Signaling in Rat Gastrocnemius Muscle. Mech. Ageing Dev. 2008, 129, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; van Loon, L.J. Aging, Exercise and Muscle Protein Metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Hofer, T.; Seo, A.T.; Leeuwenburgh, C. Molecular Mechanisms of Life- and Health-Span Extension: Role of Calorie Restriction and Exercise Intervention. Appl. Physiol. Nutr. Metab. 2007, 32, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-Virgin Olive Oil Diet and Mild Physical Activity Prevent Cartilage Degeneration in an Osteoarthritis Model: An in Vivo and in Vitro Study on Lubricin Expression. J. Nutr. Biochem. 2013, 24, 2064–2975. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Imbesi, R.; Castrogiovanni, P. Effects of Dietary Extra-Virgin Olive Oil on Oxidative Stress Resulting from Exhaustive Exercise in Rat Skeletal Muscle: A Morphological Study. Acta Histochem. 2014, 116, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.J.; Yu, L.J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2009, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kwak, H.B.; Lawler, J.M. Exercise Training Attenuates Age-Induced Changes in Apoptotic Signaling in Rat Skeletal Muscle. Antioxid. Redox Signal. 2006, 8, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, J.S.; Cheng, B.; Rubin, D.C.; Yarasheski, K.E.; Semenkovich, C.F. Resistance Exercise Decreases Skeletal Muscle Tumor Necrosis Factor Alpha in Frail Elderly Humans. FASEB J. 2001, 15, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Adhihetty, P.J.; O’Leary, M.F.; Chabi, B.; Wicks, K.L.; Hood, D.A. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007, 102, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M. Muscle apoptotic response to denervation, disuse, and aging. Med. Sci. Sports Exerc. 2009, 41, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Cvecka, J.; Tirpakova, V.; Sedliak, M.; Kern, H.; Mayr, W.; Hamar, D. Physical Activity in Elderly. Eur. J. Transl. Myol. 2015, 25, 249–252. [Google Scholar] [CrossRef] [PubMed]
| Presence of angiotensin-converting enzyme D allele |
| Age-related loss of muscle fiber |
| Atherosclerosis |
| Diabetes mellitus |
| Decreased physical activity |
| Obesity in some individuals |
| Decreased food intake including protein and creatine |
| Decreased testosterone level |
| Decreased intake of vitamin D |
| Decreased insulin-like growth factor-1 |
| Mechano-growth factor |
| Increased cytokines (tumor necrosis factor-α, interleukin-6) |
| Decreased motor unit acuity with a decrease in ciliary neurotrophic factor |
| Loss of muscle mass and strength |
| Overexpression of myostatin, a transforming growth factor |
| Fracture with low bone mass, or both |
| Osteoporosis and Osteoarthritis |
| Cancer |
| Cardiorespiratory and dismetabolic disorders |
| Chronic kidney disease |
| Chronic liver disease |
| Malnutrition |
| Amyotrophic lateral sclerosis |
| Atherosclerosis |
| Anorexia |
| Primary depression |
| Malabsorption |
| Hyperthyroidism |
| Inflammation |
| Insulin resistance |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musumeci, G. Sarcopenia and Exercise “The State of the Art”. J. Funct. Morphol. Kinesiol. 2017, 2, 40. https://doi.org/10.3390/jfmk2040040
Musumeci G. Sarcopenia and Exercise “The State of the Art”. Journal of Functional Morphology and Kinesiology. 2017; 2(4):40. https://doi.org/10.3390/jfmk2040040
Chicago/Turabian StyleMusumeci, Giuseppe. 2017. "Sarcopenia and Exercise “The State of the Art”" Journal of Functional Morphology and Kinesiology 2, no. 4: 40. https://doi.org/10.3390/jfmk2040040
APA StyleMusumeci, G. (2017). Sarcopenia and Exercise “The State of the Art”. Journal of Functional Morphology and Kinesiology, 2(4), 40. https://doi.org/10.3390/jfmk2040040





