Increased Corticospinal Excitability and Muscular Activity in a Lower Limb Reaction Task under Psychological Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
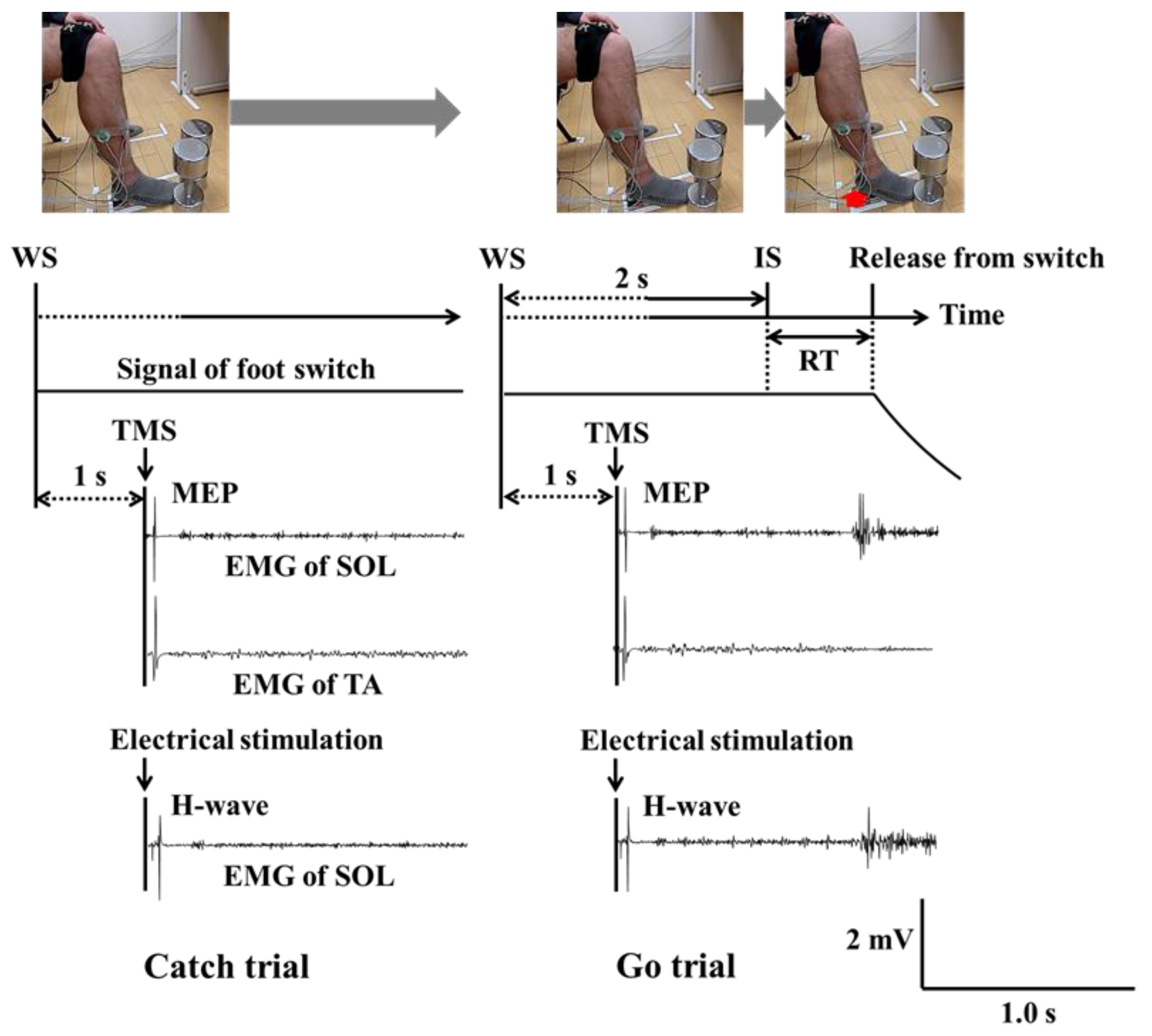
2.2. Task
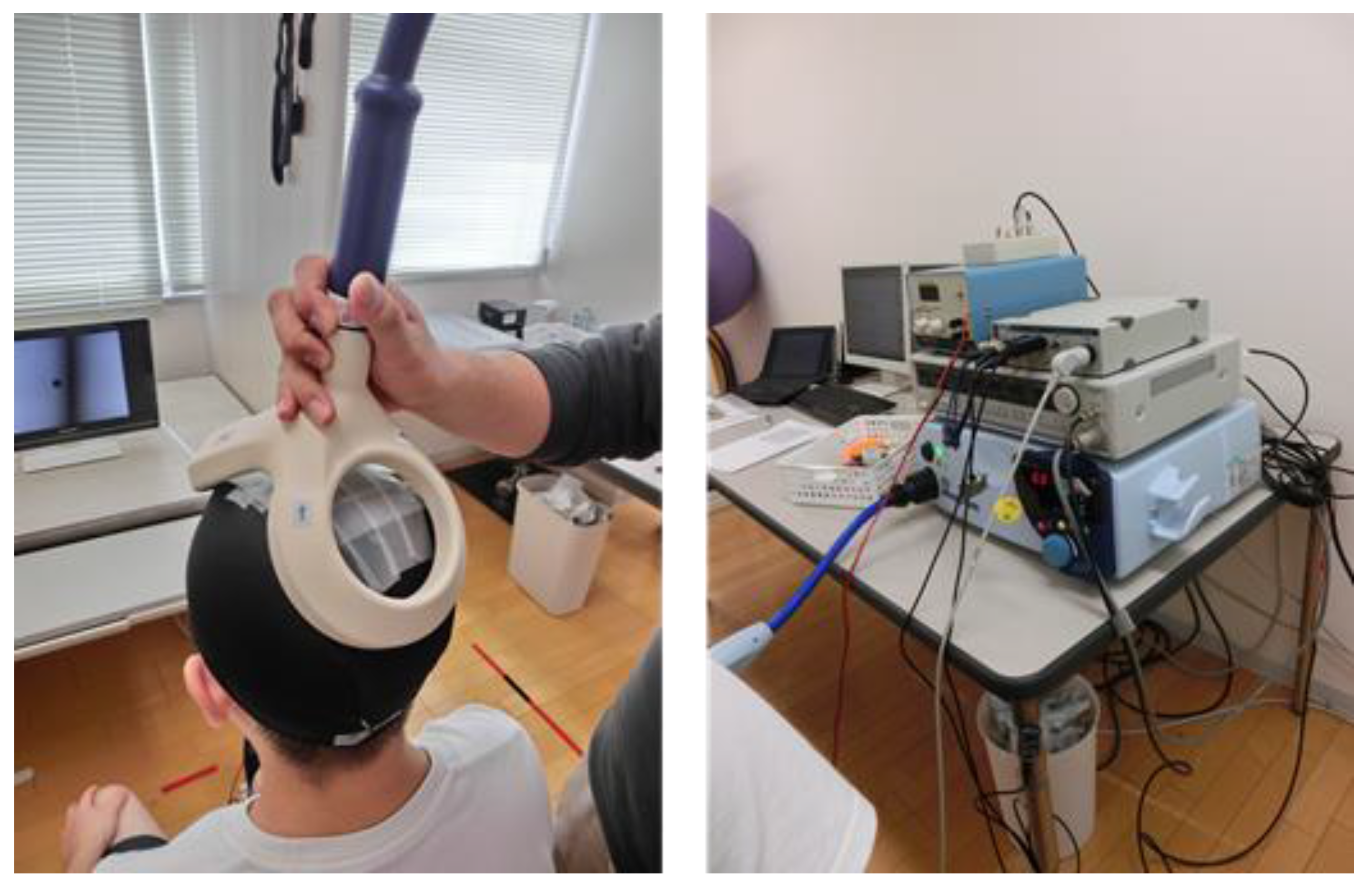
2.3. Induction and Recording of Physiological Indices
2.3.1. Corticospinal Excitability (MEP)
2.3.2. Spinal Reflex Excitability (H-reflex) and Heart Rate
2.4. Procedure
2.5. Dependent Variables
2.5.1. State Anxiety and Mental Effort
2.5.2. Heart Rate
2.5.3. MEP, H-Reflex, and EMG Amplitudes
2.5.4. Task Performance
2.6. Statistical Analysis
3. Results
3.1. Pressure Manipulation Check
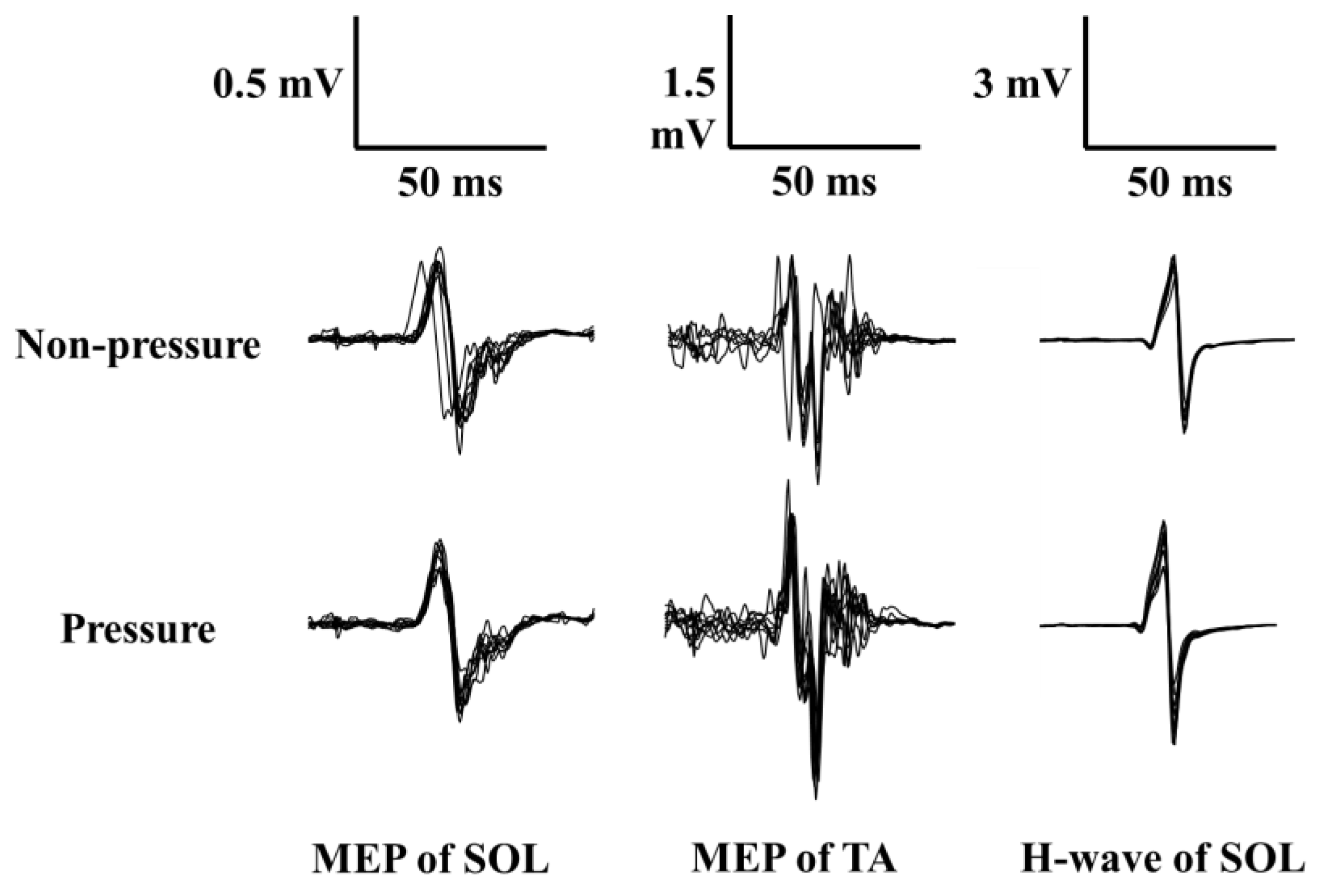
3.2. MEP, H-Reflex, and bEMG during Motor Preparation Phase
3.3. EMG Amplitude and Reaction Time during Task Execution Phase
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumeister, R.F. Choking under pressure: Self-consciousness and paradoxical effects of incentives on skillful performance. J. Pers. Soc. Psychol. 1984, 46, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.P.; Janelle, C.M. Event-related potential evidence for the processing efficiency theory. J. Sports Sci. 2007, 25, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, J.C.; Goodman, R.N.; King, B.R.; Lo, L.C.; Contreras-Vidal, J.L.; Hatfield, B.D. Cerebral cortical dynamics and the quality of motor behavior during social evaluative challenge. Psychophysiology 2011, 48, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Poolton, J.M.; Wilson, M.R.; Maxwell, J.P.; Masters, R.S.W. Neural co-activation as a yardstick of implicit motor learning and the propensity for conscious control of movement. Biol. Psychol. 2011, 87, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, B.D.; Costanzo, M.E.; Goodman, R.N.; Lo, L.C.; Oh, H.; Rietschel, J.C.; Saffer, M.; Bradberry, T.; Contreras-Vidal, J.; Haufler, A. The influence of social evaluation on cerebral cortical activity and motor performance: A study of “Real-Life” competition. Int. J. Psychophysiol. 2013, 90, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Funase, K.; Sekiya, H.; Sasaki, J.; Tanaka, Y.M. Psychological pressure facilitates corticospinal excitability: Motor preparation processes and EMG activity in a choice reaction task. Int. J. Sport Exerc. Psychol. 2014, 12, 287–301. [Google Scholar] [CrossRef]
- Tanaka, Y.; Funase, K.; Sekiya, H.; Murayama, T. Modulation of corticospinal motor tract excitability during a fine finger movement under psychological pressure: A TMS study. Int. J. Sport Health Sci. 2012, 10, 39–49. [Google Scholar] [CrossRef]
- Rollnik, J.D.; Schubert, M.; Dengler, R. Effects of a competitive stressor on motor cortex excitability: A pilot study. Stress. Med. 2000, 16, 49–54. [Google Scholar] [CrossRef]
- Cooke, A.; Kavussanu, M.; McIntyre, D.; Ring, C. Effects of competition on endurance performance and the underlying psychological and physiological mechanisms. Biol. Psychol. 2011, 86, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, M.; Kudo, K.; Ohtsuki, T. Effects of psychological stress on state anxiety, electromyographic activity, and arpeggio performance in pianists. Med. Probl. Perform. Art. 2008, 23, 120–132. [Google Scholar]
- Yoshie, M.; Kudo, K.; Murakoshi, T.; Ohtsuki, T. Music performance anxiety in skilled pianists: Effects of social-evaluative performance situation on subjective, autonomic, and electromyographic reactions. Exp. Brain Res. 2009, 199, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Kavussanu, M.; McIntyre, D.; Ring, C. Psychological, muscular and kinematic factors mediate performance under pressure. Psychophysiology 2010, 47, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Funase, K.; Sekiya, H.; Sasaki, J.; Takemoto, T. Multiple EMG activity and intracortical inhibition and facilitation during a fine finger movement under pressure. J. Mot. Behav. 2011, 43, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Carpenter, M.G.; Perry, J.C.; Frank, J.S. Effects of postural anxiety on the soleus H-reflex. Hum. Mov. Sci. 2007, 26, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.A.; Koceja, D.M. The effects of vision and task complexity on Hoffmann reflex gain. Brain Res. 1995, 700, 303–307. [Google Scholar] [CrossRef]
- Elias, L.J.; Bryden, M.P. Footedness is a better predictor of language lateralization that handedness. Laterality 1998, 3, 41–51. [Google Scholar] [PubMed]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Mynark, R.G.; Koceja, D.M. Comparison of soleus H-reflex gain from prone to standing in dancers and controls. Electroencephalogr. Clin. Neurophysiol. 1997, 105, 135–140. [Google Scholar] [CrossRef]
- Cella, D.F.; Perry, S.W. Reliability and concurrent validity of three visual-analogue mood scales. Psychol. Rep. 1986, 59, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Spinal reflexes during postural control under psychological pressure. Motor Control 2015, 19, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 5th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 4th ed.; Sage Publications: London, UK, 2013. [Google Scholar]
- Yang, F.A.; Gorassini, M. Spinal and brain control of human walking: Implications for retraining of walking. Neuroscientist 2006, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Sawaguchi, T.; Oishi, T.; Ueki, K.; Kubota, K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J. Neurophysiol. 1991, 65, 1542–1553. [Google Scholar] [PubMed]
- Tokimura, H.; Di Lazzaro, V.; Tokimura, Y.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Short-latency inhibition of human motor cortex by somatosensory input from the hand. J. Physiol. 2000, 523, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Surround inhibition. Suppl. Clin. Neurophysiol. 2003, 56, 153–159. [Google Scholar] [PubMed]
- Osu, R.; Franklin, D.W.; Kato, H.; Gomi, H.; Domen, K.; Yoshioka, T.; Kawato, M. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J. Neurophysiol. 2002, 88, 991–1004. [Google Scholar] [PubMed]
- Coombes, S.A.; Tandonnet, C.; Fujiyama, H.; Janelle, C.M.; Cauraugh, J.H.; Summers, J.J. Emotion and motor preparation: A transcranial magnetic stimulation study of corticospinal motor tract excitability. Cogn. Affect. Behav. Neurosci. 2009, 9, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Vall-Solé, J.; Rothwell, J.C.; Goulart, F.; Cossu, G.; Muňoz, E. Patterned ballistic movements triggered by a startle in healthy humans. J. Physiol. 1999, 516, 931–938. [Google Scholar] [CrossRef]
- Siegmund, G.P.; Inglis, J.T.; Sanderson, D.J. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J. Physiol. 2001, 535, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Nijhus, L.B.O.; Janssen, L.; Bloem, B.R.; van Dijk, J.G.; Gielen, S.C.; Borm, G.F.; Overeem, S. Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J. Physiol. 2007, 584, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Koceja, D.M.; Burke, J.R.; Kamen, G. Organization of segmental reflexes in trained dancers. Int. J. Sports Med. 1991, 12, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Crone, C.; Hultborn, H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.H.; Koceja, D.M. Modulation of the triceps surae H-reflex with training. Int. J. Neurosci. 1994, 76, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Mynark, R.G.; Koceja, D.M. Down training of the elderly soleus H reflex with the use of a spinally induced balance perturbation. J. Appl. Physiol. 2002, 93, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherboune, C.D. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Mchorney, C.A.; Ware, J.E.; Raczek, A.E. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1994, 31, 247–263. [Google Scholar] [CrossRef]
- Mchorney, C.A.; Ware, J.E.; Raczek, A.E. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Martens, R.; Landers, D.M. Motor performance under stress: A test of the inverted-U hypothesis. J. Pers. Soc. Psychol. 1970, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.B.; Ogston, K.M.; Miles, T.S. Age and sex differences in human motor cortex input–output characteristics. J. Physiol. 2003, 546, 605–613. [Google Scholar] [CrossRef] [PubMed]
| Dependent variables | Non-Pressure | Pressure |
|---|---|---|
| Subjective state anxiety (mm) | 53.11 ± 8.87 | 83.11 ± 5.09 * |
| Mental effort (mm) | 74.78 ± 4.38 | 93.56 ± 2.03 ** |
| Heart rate (bpm) | 88.30 ± 4.76 | 91.16 ± 5.27 * |
| Dependent Variables | Non-Pressure | Pressure |
|---|---|---|
| MEP amplitude of SOL (mV) | 2.80 ± 0.81 | 2.98 ± 1.48 |
| MEP amplitude of TA (mV) | 2.28 ± 0.35 | 2.57 ± 0.35 * |
| H-reflex amplitude of SOL (mV) | 5.13 ± 1.23 | 4.63 ± 1.56 |
| bEMG amplitude of SOL in the TMS block (%MVC) | 3.60 ± 0.62 | 4.83 ± 0.81 * |
| bEMG amplitude of TA in the TMS block (%MVC) | 4.42 ± 0.93 | 4.99 ± 1.09 * |
| bEMG amplitude of SOL in the H-reflex block (%MVC) | 4.19 ± 0.73 | 4.86 ± 1.13 |
| Dependen Variables | Non-Pressure | Pressure |
|---|---|---|
| Maximum EMG of SOL (%MVC) | 51.70 ± 3.77 | 64.68 ± 4.81 ** |
| Maximum EMG of TA (%MVC) | 7.89 ± 0.93 | 8.63 ± 1.07 * |
| Co-contraction rate (%) | 0.80 ± 0.08 | 0.90 ± 0.11 * |
| RT (ms) | 236.91 ± 15.98 | 230.38 ± 12.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Shimo, T. Increased Corticospinal Excitability and Muscular Activity in a Lower Limb Reaction Task under Psychological Pressure. J. Funct. Morphol. Kinesiol. 2017, 2, 14. https://doi.org/10.3390/jfmk2020014
Tanaka Y, Shimo T. Increased Corticospinal Excitability and Muscular Activity in a Lower Limb Reaction Task under Psychological Pressure. Journal of Functional Morphology and Kinesiology. 2017; 2(2):14. https://doi.org/10.3390/jfmk2020014
Chicago/Turabian StyleTanaka, Yoshifumi, and Tatsunori Shimo. 2017. "Increased Corticospinal Excitability and Muscular Activity in a Lower Limb Reaction Task under Psychological Pressure" Journal of Functional Morphology and Kinesiology 2, no. 2: 14. https://doi.org/10.3390/jfmk2020014
APA StyleTanaka, Y., & Shimo, T. (2017). Increased Corticospinal Excitability and Muscular Activity in a Lower Limb Reaction Task under Psychological Pressure. Journal of Functional Morphology and Kinesiology, 2(2), 14. https://doi.org/10.3390/jfmk2020014





