Moderate Exercise Stimulates PACAP-Mediated Neurogenesis in Rat Dentate Gyrus and Cerebellar Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
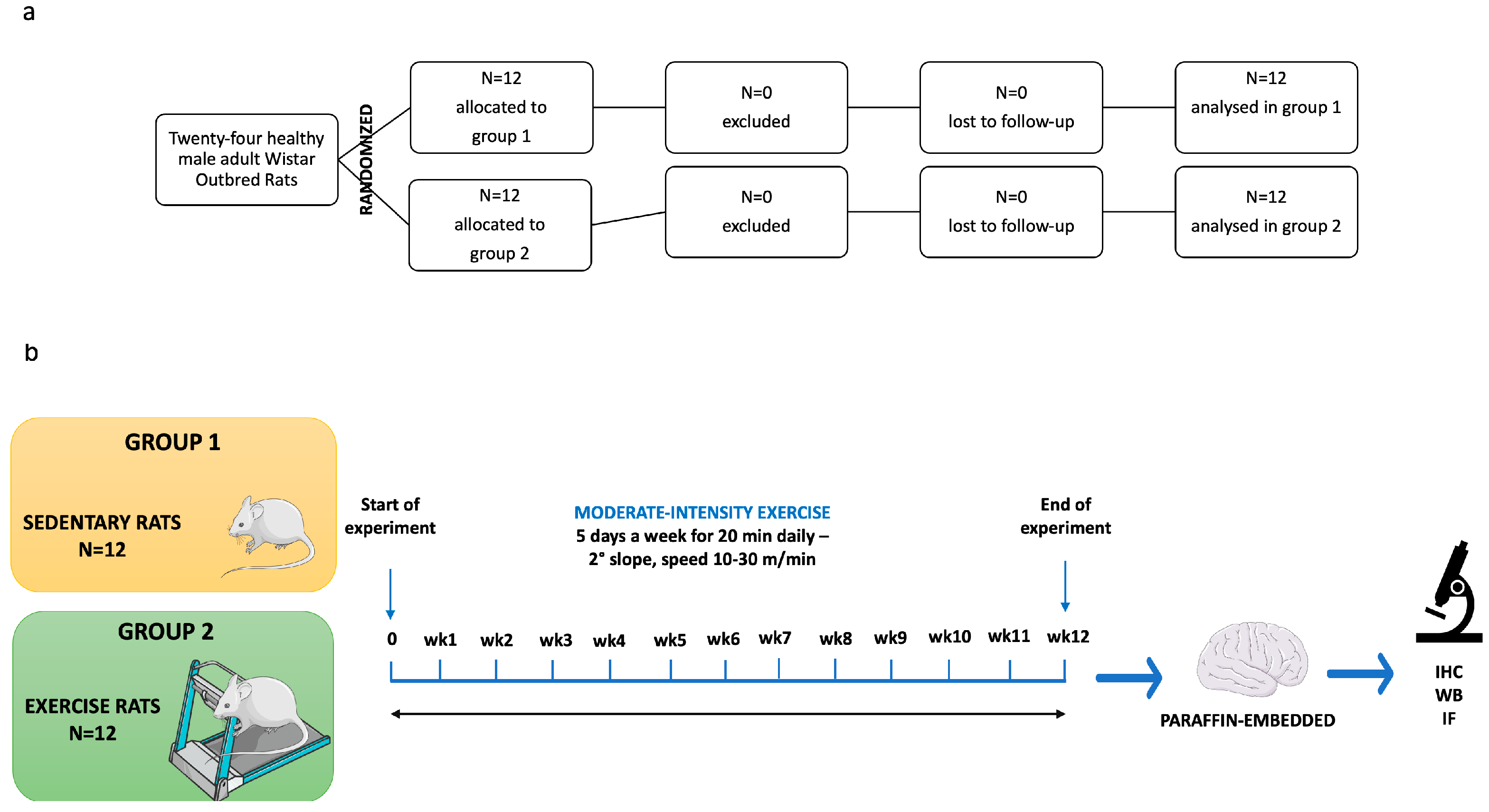
2.3. Experimental Design
2.4. Immunohistochemistry (IHC) Analysis
2.5. FFPE Tissue Samples
2.6. Western Blot Analysis
2.7. Immunofluorescence Analysis
2.8. Statistical Analysis
3. Results
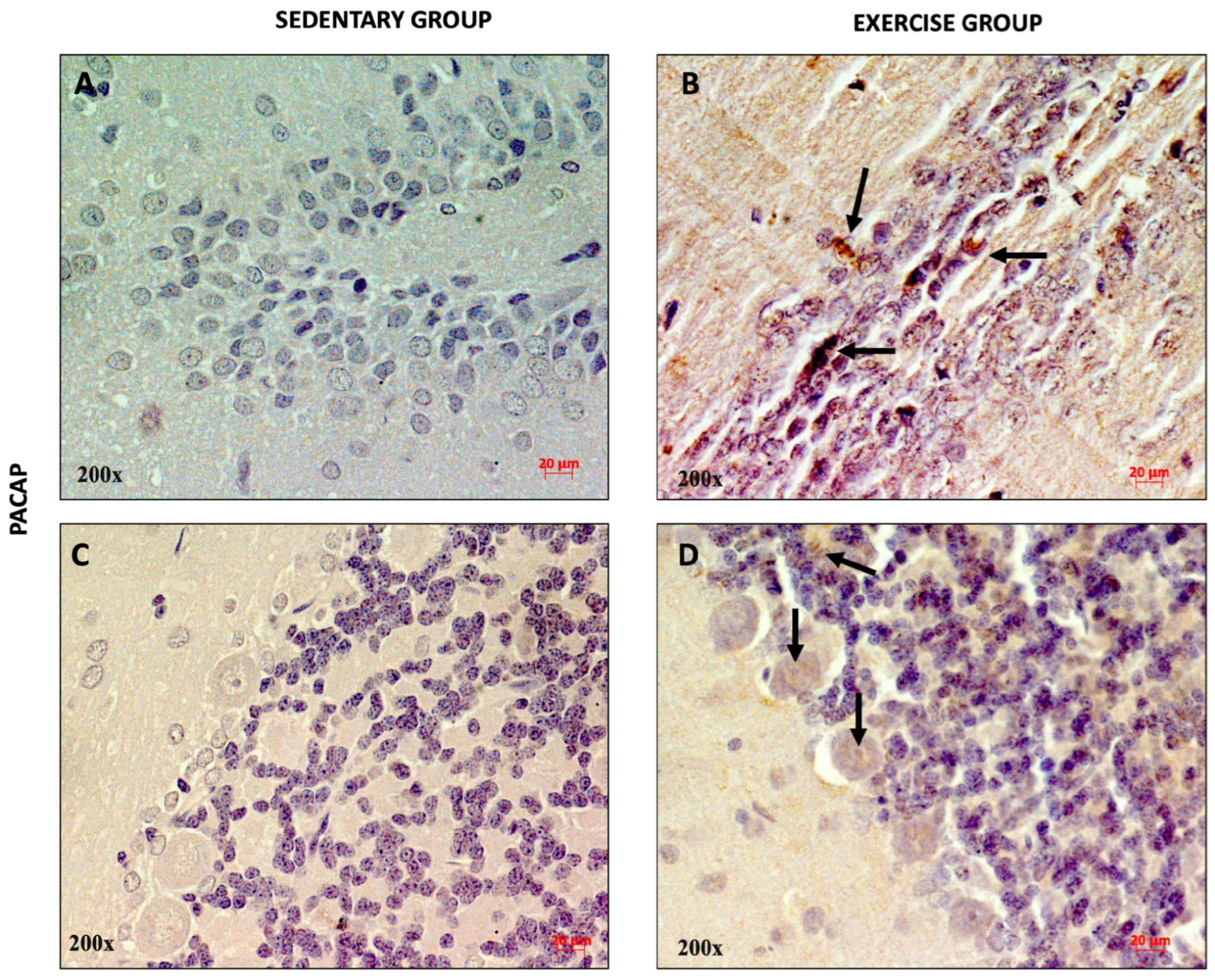
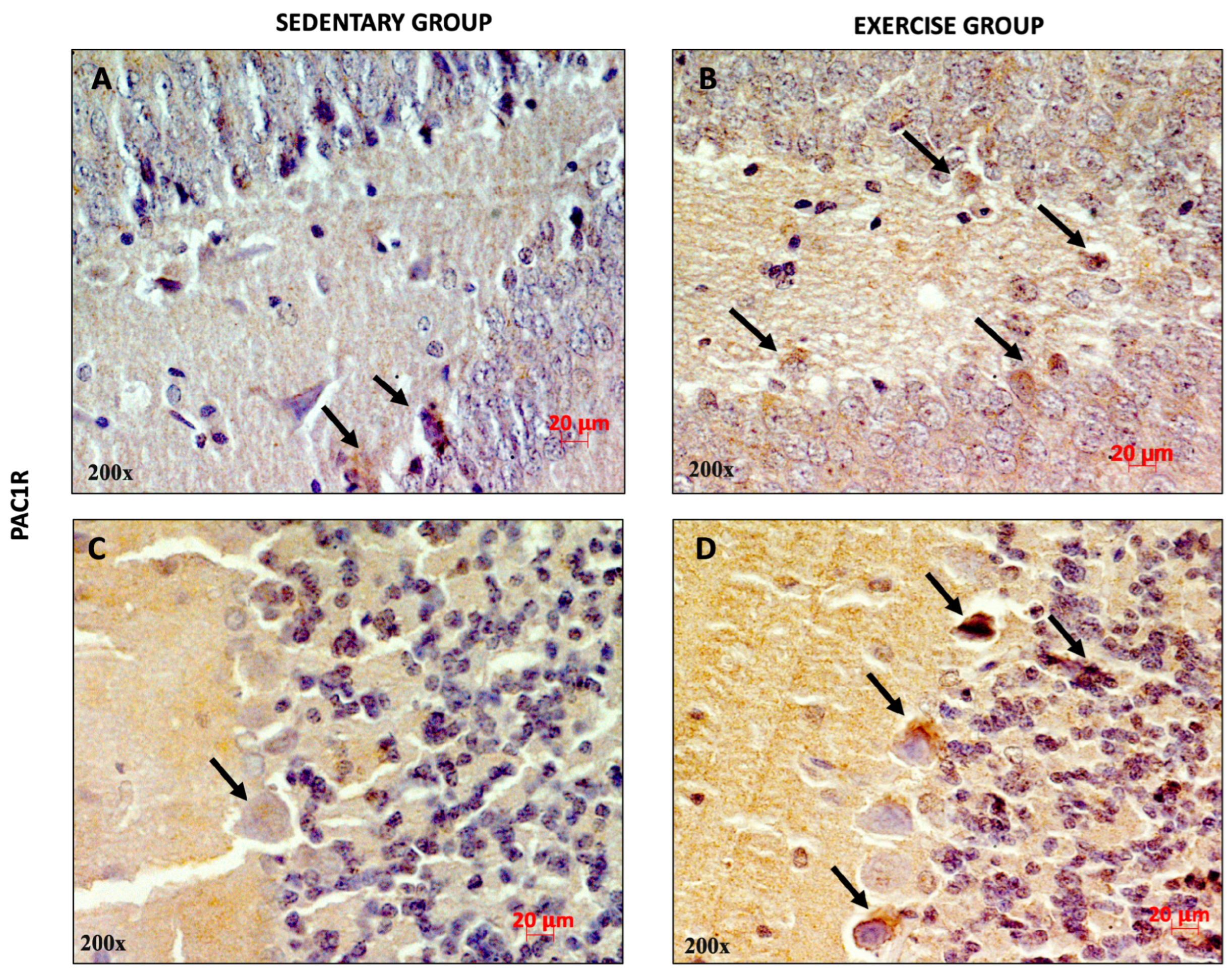
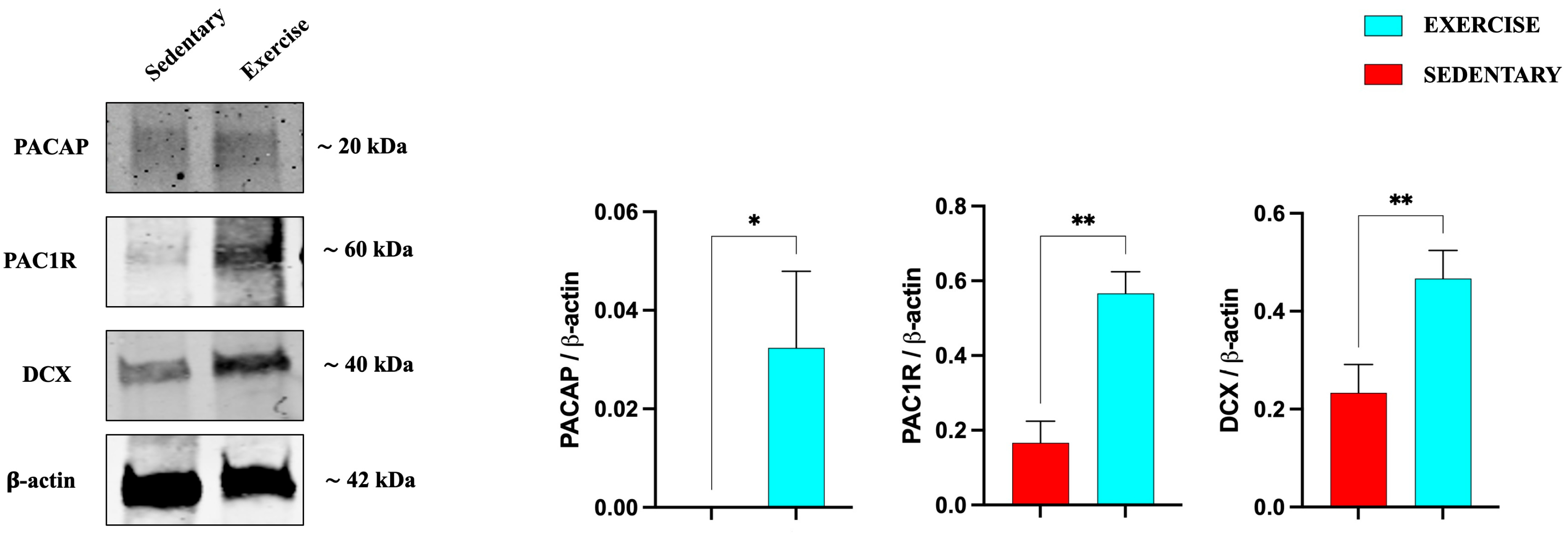
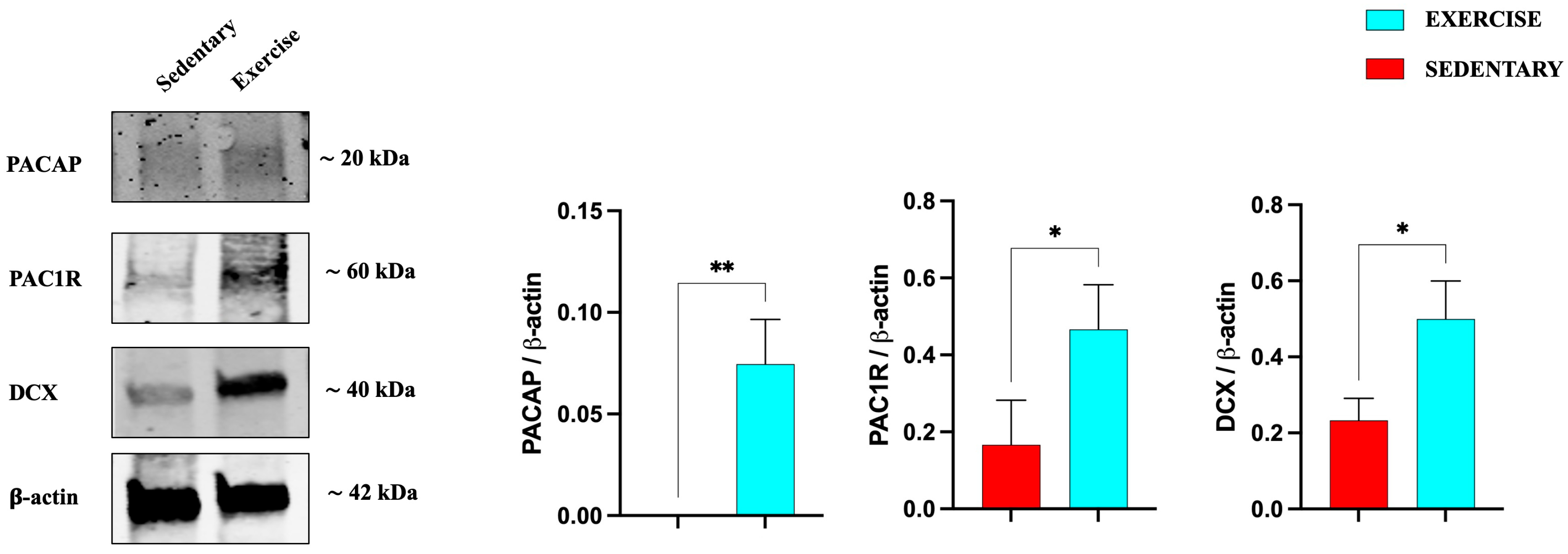
3.1. Effect of Moderate Training on PACAP and PAC1R Expression in the Rat DG and Cerebellum
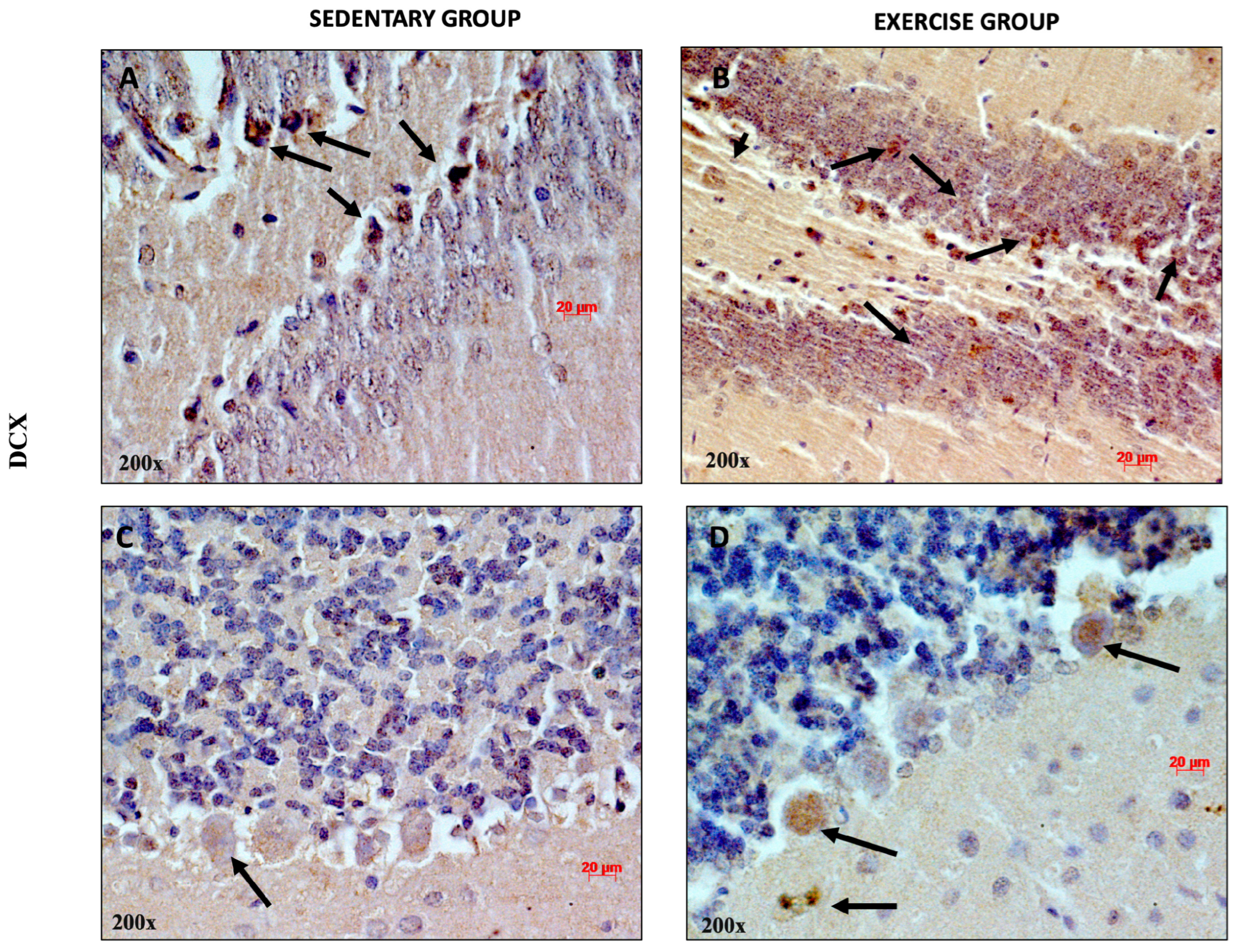
3.2. Moderate Training Promotes Adult Neurogenesis
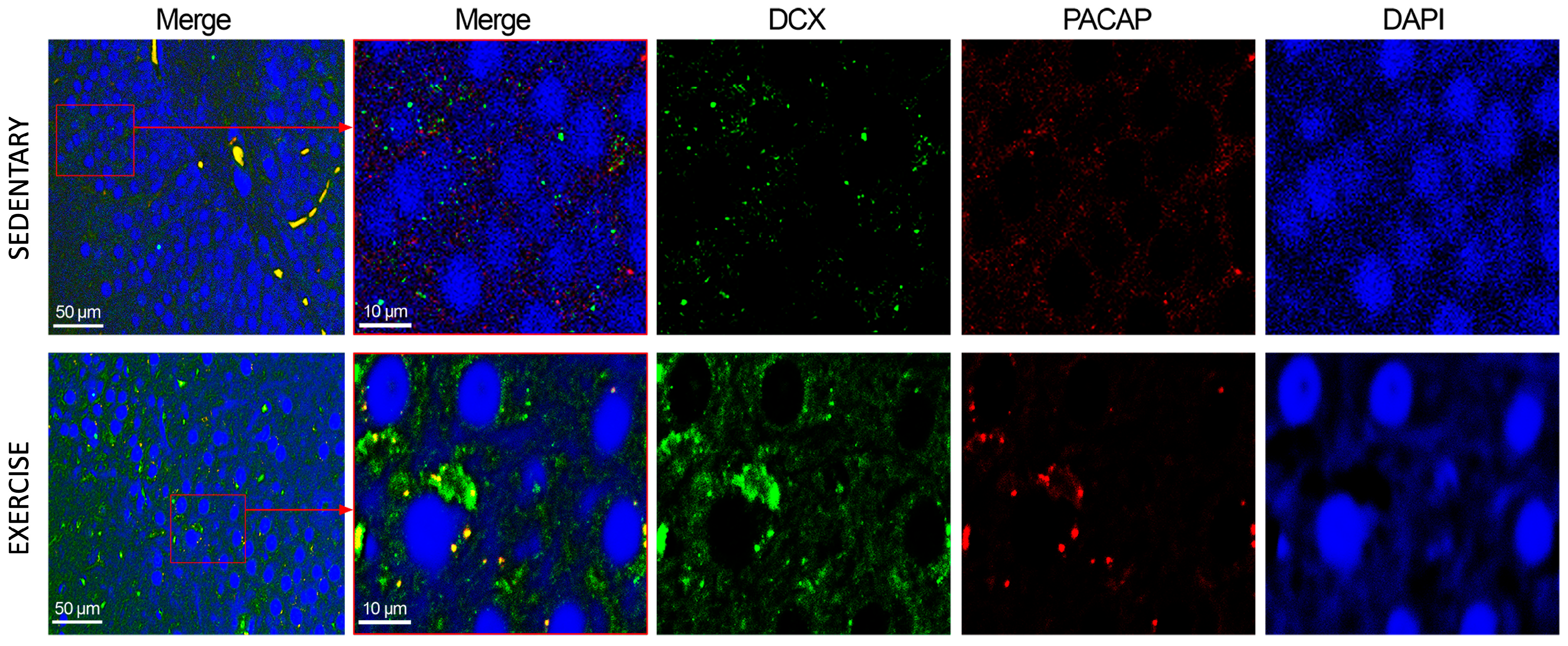
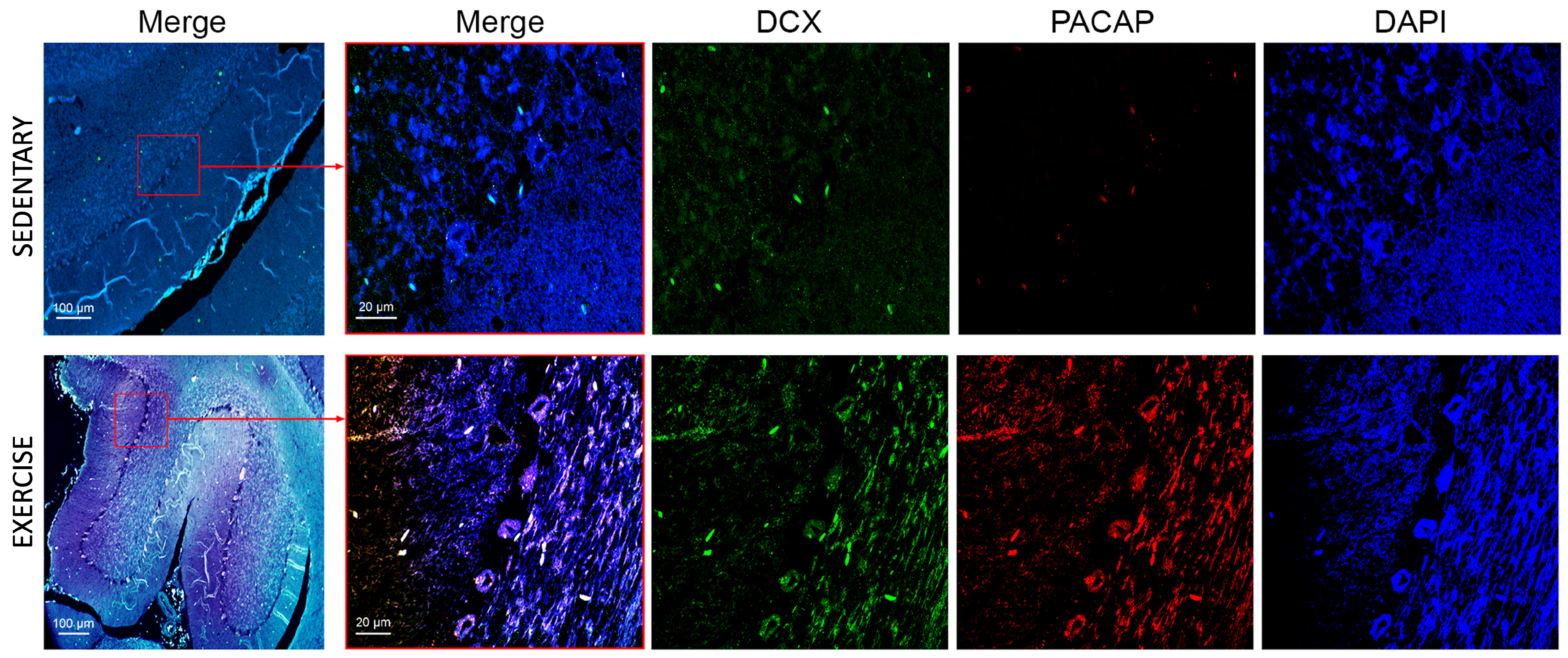
3.3. Moderate Training Promotes the Co-Localization of PACAP and DCX in Rat DG and Cerebellum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| AD | Alzheimer’s disease |
| BDNF | Brain-derived neurotrophic factor |
| cAMP | Cyclic adenosine monophosphate |
| CNS | Central nervous system |
| DAB | Diaminobenzidine solution |
| DCX | Doublecortin |
| DG | Dentate gyrus |
| IF | Immunofluorescence |
| LTP | Long-term potentiation |
| MAPK | mitogen-activated protein kinases |
| PA | Moderate physical activity |
| PAC1R | Pituitary adenylate cyclase-activating polypeptide type I receptor |
| PACAP | Pituitary adenylate cyclase-activating polypeptide |
| PD | Parkinson’s disease |
| PKA | Protein kinase A |
| SGZ | Subgranular zone |
| SVZ | Subventricular zone |
| UV-A | Ultraviolet radiation-A |
| VIP | Vasoactive intestinal polypeptide |
| VPAC1 | Vasoactive intestinal peptide receptor 1 |
| VPAC2 | Vasoactive intestinal peptide receptor 2 |
References
- Maugeri, G.; D’amico, A.G.; Federico, C.; Saccone, S.; D’agata, V.; Musumeci, G. Moderate Physical Activity Increases the Expression of ADNP in Rat Brain. Int. J. Mol. Sci. 2024, 25, 4382. [Google Scholar] [CrossRef]
- Makizako, H.; Liu-Ambrose, T.; Shimada, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Uemura, K.; Suzuki, T. Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 480–486. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Shoenfeld, Y.; Hoffman, J.R. The Effect of Exercise on Neurogenesis in the Brain. Isr. Med. Assoc. J. 2022, 24, 533–538. [Google Scholar]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.M.; Yoo, D.Y.; Jung, H.Y.; Kim, I.Y.; Hwang, I.K.; Seong, J.K.; Yoon, Y.S. Comparison of Adult Hippocampal Neurogenesis and Susceptibility to Treadmill Exercise in Nine Mouse Strains. Neural Plast. 2017, 2017, 5863258. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V. Effects of Physical Activity on Amyotrophic Lateral Sclerosis. J. Funct. Morphol. Kinesiol. 2020, 5, 29. [Google Scholar] [CrossRef]
- Sujkowski, A.; Hong, L.; Wessells, R.; Todi, S.V. The protective role of exercise against age-related neurodegeneration. Ageing Res. Rev. 2022, 74, 101543. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Rolland, Y.; de Souto Barreto, P. Protective Effects of Physical Exercise in Alzheimer’s Disease and Parkinson’s Disease: A Narrative Review. J. Clin. Neurol. 2015, 11, 212–219. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Inoue, K.; Okamoto, M.; Liu, Y.F.; Matsui, T.; Yook, J.S.; Soya, H. Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load-free running. Neurosci. Lett. 2013, 537, 6–10. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The effects of aerobic activity on brain structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef]
- Gomes da Silva, S.; Arida, R.M. Physical activity and brain development. Expert. Rev. Neurother. 2015, 15, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef] [PubMed]
- Uda, M.; Ishido, M.; Kami, K.; Masuhara, M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006, 1104, 64–72. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Leal-Galicia, P.; Chávez-Hernández, M.E.; Mata, F.; Mata-Luévanos, J.; Rodríguez-Serrano, L.M.; Tapia-De-Jesús, A.; Buenrostro-Jáuregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int. J. Mol. Sci. 2021, 22, 11489. [Google Scholar] [CrossRef]
- Zhao, X.; van Praag, H. Steps towards standardized quantification of adult neurogenesis. Nat. Commun. 2020, 11, 4275. [Google Scholar] [CrossRef]
- Carletti, B.; Rossi, F. Neurogenesis in the cerebellum. Neuroscientist 2008, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, J.P.; Prazeres, P.H.; Magno, L.A.; Romano-Silva, M.A.; Mintz, A.; Birbrair, A. Neurogenesis in the postnatal cerebellum after injury. Int. J. Dev. Neurosci. 2018, 67, 33–36. [Google Scholar] [CrossRef]
- Wizeman, J.W.; Guo, Q.; Wilion, E.M.; Li, J.Y. Specification of diverse cell types during early neurogenesis of the mouse cerebellum. Elife 2019, 8, e42388. [Google Scholar] [CrossRef]
- Rauf, S.; Soesatyo, M.H.; Agustiningsih, D.; Partadiredja, G. Moderate intensity intermittent exercise upregulates neurotrophic and neuroprotective genes expression and inhibits Purkinje cell loss in the cerebellum of ovariectomized rats. Behav. Brain Res. 2020, 382, 112481. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, S.; Serra, L.; Nicolis, S.K. More than just Stem Cells: Functional Roles of the Transcription Factor Sox2 in Differentiated Glia and Neurons. Int. J. Mol. Sci. 2019, 20, 4540. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.; Rönnholm, H.; Holmberg, J.; Lundh, H.; Heidrich, J.; Zachrisson, O.; Ossoinak, A.; Frisén, J.; Patrone, C. PACAP promotes neural stem cell proliferation in adult mouse brain. J. Neurosci. Res. 2004, 76, 205–215. [Google Scholar] [CrossRef]
- D’amico, A.G.; Maugeri, G.; Musumeci, G.; Reglodi, D.; D’agata, V. PACAP and NAP: Effect of Two Functionally Related Peptides in Diabetic Retinopathy. J. Mol. Neurosci. 2021, 71, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nakamachi, T.; Watanabe, J.; Sugiyama, K.; Ohtaki, H.; Murai, N.; Sasaki, S.; Xu, Z.; Hashimoto, H.; Seki, T.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Involved in Adult Mouse Hippocampal Neurogenesis After Stroke. J. Mol. Neurosci. 2016, 59, 270–279. [Google Scholar] [CrossRef]
- Basille, M.; Gonzalez, B.J.; Desrues, L.; Demas, M.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates adenylyl cyclase and phospholipase C activity in rat cerebellar neuroblasts. J. Neurochem. 1995, 65, 1318–1324. [Google Scholar] [CrossRef]
- Falluel-Morel, A.; Chafai, M.; Vaudry, D.; Basille, M.; Cazillis, M.; Aubert, N.; Louiset, E.; DeJouffrey, S.; Le Bigot, J.F.; Fournier, A.; et al. The neuropeptide pituitary adenylate cyclase-activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: Focus on cerebellar granule neurones and embryonic stem cells. J. Neuroendocrinol. 2007, 19, 321–327. [Google Scholar] [CrossRef]
- Botia, B.; Basille, M.; Allais, A.; Raoult, E.; Falluel-Morel, A.; Galas, L.; Jolivel, V.; Wurtz, O.; Komuro, H.; Fournier, A.; et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides 2007, 28, 1746–1752. [Google Scholar] [CrossRef]
- Ago, Y.; Yoneyama, M.; Ishihama, T.; Kataoka, S.; Kawada, K.; Tanaka, T.; Ogita, K.; Shintani, N.; Hashimoto, H.; Baba, A.; et al. Role of endogenous pituitary adenylate cyclase-activating polypeptide in adult hippocampal neurogenesis. Neuroscience 2011, 172, 554–561. [Google Scholar] [CrossRef]
- Ogata, K.; Shintani, N.; Hayata-Takano, A.; Kamo, T.; Higashi, S.; Seiriki, K.; Momosaki, H.; Vaudry, D.; Vaudry, H.; Galas, L.; et al. PACAP enhances axon outgrowth in cultured hippocampal neurons to a comparable extent as BDNF. PLoS ONE 2015, 10, e0120526. [Google Scholar] [CrossRef]
- Vaudry, D.; Hamelink, C.; Damadzic, R.; Eskay, R.L.; Gonzalez, B.; Eiden, L.E. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides 2005, 26, 2518–2524. [Google Scholar] [CrossRef][Green Version]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell Biochem. 2019, 120, 10097–10105. [Google Scholar]
- Deguil, J.; Jailloux, D.; Page, G.; Fauconneau, B.; Houeto, J.; Philippe, M.; Muller, J.; Pain, S. Neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in MPP+-induced alteration of translational control in Neuro-2a neuroblastoma cells. J. Neurosci. Res. 2007, 85, 2017–2025. [Google Scholar]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. Faseb J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef]
- Maugeri, G.; D’aMico, A.G.; Morello, G.; Reglodi, D.; Cavallaro, S.; D’aGata, V. Differential Vulnerability of Oculomotor Versus Hypoglossal Nucleus During ALS: Involvement of PACAP. Front. Neurosci. 2020, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef]
- Nielsen, H.S.; Hannibal, J.; Fahrenkrug, J. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. Neuroreport 1998, 9, 2639–2642. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Castrogiovanni, P.; Trovato, F.M.; Malatino, L.; Ravalli, S.; Imbesi, R. Adapted Moderate Training Exercise Decreases the Expression of Ngal in the Rat Kidney: An Immunohistochemical Study. Appl. Sci. 2019, 9, 1041. [Google Scholar] [CrossRef]
- D’amico, A.G.; Scuderi, S.; Maugeri, G.; Cavallaro, S.; Drago, F.; D’agata, V. NAP reduces murine microvascular endothelial cells proliferation induced by hyperglycemia. J. Mol. Neurosci. 2014, 54, 405–413. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell Physiol. 2019, 234, 5230–5240. [Google Scholar]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Yau, S.-Y.; Gil-Mohapel, J.; Christie, B.R.; So, K.-F. Physical exercise-induced adult neurogenesis: A good strategy to prevent cognitive decline in neurodegenerative diseases? Biomed. Res. Int. 2014, 2014, 403120. [Google Scholar] [PubMed]
- Deuel, T.A.; Liu, J.S.; Corbo, J.C.; Yoo, S.-Y.; Rorke-Adams, L.B.; Walsh, C.A. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron 2006, 49, 41–53. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Karniushko, O.A. Expression of doublecortin and neun in the developing cerebellar neurons in rat. Morfologiia 2016, 149, 38–42. [Google Scholar] [PubMed]
- Ahlfeld, J.; Filser, S.; Schmidt, F.; Wefers, A.K.; Merk, D.J.; Glaß, R.; Herms, J.; Schüller, U. Neurogenesis from Sox2 expressing cells in the adult cerebellar cortex. Sci. Rep. 2017, 7, 6137. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Sosner, P.; Guiraud, T.; Gremeaux, V.; Arvisais, D.; Herpin, D.; Bosquet, L. The ambulatory hypotensive effect of aerobic training: A reappraisal through a meta-analysis of selected moderators. Scand. J. Med. Sci. Sports 2017, 27, 327–341. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Rêgo, M.L.; Cabral, D.A.; Costa, E.C.; Fontes, E.B. Physical Exercise for Individuals with Hypertension: It Is Time to Emphasize its Benefits on the Brain and Cognition. Clin. Med. Insights Cardiol. 2019, 13, 1179546819839411. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Parducci, P.; Livingston, J.; Taylor, B.A. A Systematically Assembled Signature of Genes to be Deep-Sequenced for Their Associations with the Blood Pressure Response to Exercise. Genes 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Mooren, F.C.; Pilat, C. The Immunomodulatory Effects of Physical Activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- Ben Ezzdine, L.; Dhahbi, W.; Dergaa, I.; Ceylan, H.; Guelmami, N.; Ben Saad, H.; Chamari, K.; Stefanica, V.; El Omri, A. Physical activity and neuroplasticity in neurodegenerative disorders: A comprehensive review of exercise interventions, cognitive training, and AI applications. Front. Neurosci. 2025, 19, 1502417. [Google Scholar] [CrossRef]
- Farmand, S.; Du Preez, A.; Kim, C.; de Lucia, C.; Ruepp, M.-D.; Stubbs, B.; Thuret, S. Cognition on the move: Examining the role of physical exercise and neurogenesis in counteracting cognitive aging. Ageing Res. Rev. 2025, 107, 102725. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Takács, J.; Zaninetti, R.; Víg, J.; Vastagh, C.; Hámori, J. Postnatal expression of Doublecortin (Dcx) in the developing cerebellar cortex of mouse. Acta Biol. Hung. 2008, 59, 147–161. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; A Flanagan, L.; A Walsh, C. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Boseret, G.; Ball, G.F.; Balthazart, J. The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries. J. Chem. Neuroanat. 2007, 33, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef]
- Mansouri, S.; Agartz, I.; Ögren, S.-O.; Patrone, C.; Lundberg, M. PACAP Protects Adult Neural Stem Cells from the Neurotoxic Effect of Ketamine Associated with Decreased Apoptosis, ER Stress and mTOR Pathway Activation. PLoS ONE 2017, 12, e0170496. [Google Scholar] [CrossRef] [PubMed]
- Stumm, R.; Kolodziej, A.; Prinz, V.; Endres, M.; Wu, D.; Höllt, V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J. Neurochem. 2007, 103, 1666–1681. [Google Scholar] [CrossRef]
- Riek-Burchardt, M.; Kolodziej, A.; Henrich-Noack, P.; Reymann, K.G.; Höllt, V.; Stumm, R. Differential regulation of CXCL12 and PACAP mRNA expression after focal and global ischemia. Neuropharmacology 2010, 58, 199–207. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chiu, L.; Lee, H.-T.; Chiang, C.-W.; Liu, S.-P.; Hsu, Y.-H.; Lin, S.-Z.; Hsu, C.Y.; Hsieh, C.-H.; Shyu, W.-C. PACAP38/PAC1 signaling induces bone marrow-derived cells homing to ischemic brain. Stem Cells 2015, 33, 1153–1172. [Google Scholar]
- Skoglösa, Y.; Lewén, A.; Takei, N.; Hillered, L.; Lindholm, D. Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: Comparison with brain-derived neurotrophic factor and the induction of neuronal cell death. Neuroscience 1999, 90, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Gregg, C.; Weiss, S. Pituitary adenylate cyclase-activating polypeptide regulates forebrain neural stem cells and neurogenesis in vitro and in vivo. J. Neurosci. Res. 2006, 84, 1177–1186. [Google Scholar]
- Waschek, J.A.; Casillas, R.A.; Nguyen, T.B.; DiCicco-Bloom, E.M.; Carpenter, E.M.; Rodriguez, W.I. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: Potential role in patterning and neurogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9602–9607. [Google Scholar] [CrossRef]
- Perényi, H.; Szegeczki, V.; Horváth, G.; Hinnah, B.; Tamás, A.; Radák, Z.; Ábrahám, D.; Zákány, R.; Reglodi, D.; Juhász, T. Physical Activity Protects the Pathological Alterations of Alzheimer’s Disease Kidneys via the Activation of PACAP and BMP Signaling Pathways. Front. Cell Neurosci. 2020, 14, 243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Maugeri, G.; Di Bartolo, S.; Palmeri, N.; D’Amico, A.G.; Brancato, D.; Federico, C.; D’Agata, V.; Musumeci, G. Moderate Exercise Stimulates PACAP-Mediated Neurogenesis in Rat Dentate Gyrus and Cerebellar Cortex. J. Funct. Morphol. Kinesiol. 2026, 11, 37. https://doi.org/10.3390/jfmk11010037
Maugeri G, Di Bartolo S, Palmeri N, D’Amico AG, Brancato D, Federico C, D’Agata V, Musumeci G. Moderate Exercise Stimulates PACAP-Mediated Neurogenesis in Rat Dentate Gyrus and Cerebellar Cortex. Journal of Functional Morphology and Kinesiology. 2026; 11(1):37. https://doi.org/10.3390/jfmk11010037
Chicago/Turabian StyleMaugeri, Grazia, Salvatore Di Bartolo, Nicoletta Palmeri, Agata Grazia D’Amico, Desiree Brancato, Concetta Federico, Velia D’Agata, and Giuseppe Musumeci. 2026. "Moderate Exercise Stimulates PACAP-Mediated Neurogenesis in Rat Dentate Gyrus and Cerebellar Cortex" Journal of Functional Morphology and Kinesiology 11, no. 1: 37. https://doi.org/10.3390/jfmk11010037
APA StyleMaugeri, G., Di Bartolo, S., Palmeri, N., D’Amico, A. G., Brancato, D., Federico, C., D’Agata, V., & Musumeci, G. (2026). Moderate Exercise Stimulates PACAP-Mediated Neurogenesis in Rat Dentate Gyrus and Cerebellar Cortex. Journal of Functional Morphology and Kinesiology, 11(1), 37. https://doi.org/10.3390/jfmk11010037










