Advances in Non-Pharmacological Strategies for DOMS: A Scoping and Critical Review of Recent Evidence
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Practical Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kancherla, A. Delayed Onset Muscle Soreness (DOMS): Management Update. Ann. Innov. Med. 2023, 1. [Google Scholar] [CrossRef]
- Sonkodi, B.; Hegedűs, Á.; Kopper, B.; Berkes, I. Significantly Delayed Medium-Latency Response of the Stretch Reflex in Delayed-Onset Muscle Soreness of the Quadriceps Femoris Muscles Is Indicative of Sensory Neuronal Microdamage. J. Funct. Morphol. Kinesiol. 2022, 7, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Heiss, R.; Höger, S.A.; Uder, M.; Hotfiel, T.; Hanspach, J.; Laun, F.B.; Nagel, A.M.; Roemer, F.W. Early functional and morphological changes of calf muscles in delayed onset muscle soreness (DOMS) assessed with 7T MRI. Ann. Anat. 2024, 251, 152181. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Jacobson, J.A.; Fessell, D.P.; Mautner, K. Ultrasound Findings of Delayed-Onset Muscle Soreness. J. Ultrasound Med. 2016, 35, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Hotfiel, T.; Freiwald, J.; Hoppe, M.W.; Lutter, C.; Forst, R.; Grim, C.; Bloch, W.; Hüttel, M.; Heiss, R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sport. Sportschaden 2018, 32, 243–250. (In English) [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hao, Y.; Yang, X.; Zhang, C.; Xu, J.; Wu, X.; Deng, Z.; Li, N. Effect of Kinesio tape and Compression sleeves on delayed onset of muscle soreness: A single-blinded randomized controlled trial. BMC Musculoskelet. Disord. 2023, 24, 392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolska, B.; Domagała, Ł.; Kisilewicz, A.; Hassanlouei, H.; Makar, P.; Kawczyński, A.; Klich, S. Multiple cryosauna sessions for post-exercise recovery of delayed onset muscle soreness (DOMS): A randomized control trial. Front. Physiol. 2023, 14, 1253140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizumura, K.; Taguchi, T. Neurochemical mechanism of muscular pain: Insight from the study on delayed onset muscle soreness. J. Physiol. Sci. 2024, 74, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiecha, S.; Posadzki, P.; Prill, R.; Płaszewski, M. Physical Therapies for Delayed Onset Muscle Soreness: A Protocol for an Umbrella and Mapping Systematic Review with Meta-Meta-Analysis. J. Clin. Med. 2024, 13, 2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilke, J.; Behringer, M. Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort. Int. J. Mol. Sci. 2021, 22, 9482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleckenstein, J.; Neuberger, E.W.I.; Bormuth, P.; Comes, F.; Schneider, A.; Banzer, W.; Fischer, L.; Simon, P. Investigation of the Sympathetic Regulation in Delayed Onset Muscle Soreness: Results of an RCT. Front. Physiol. 2021, 12, 697335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akehurst, H.; Grice, J.E.; Angioi, M.; Morrissey, D.; Migliorini, F.; Maffulli, N. Whole-body vibration decreases delayed onset muscle soreness following eccentric exercise in elite hockey players: A randomised controlled trial. J. Orthop. Surg. Res. 2021, 16, 589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.; Ma, X.; Ma, X.; Cui, C. The effects of hydrotherapy and cryotherapy on recovery from acute post-exercise induced muscle damage—A network meta-analysis. BMC Musculoskelet. Disord. 2024, 25, 749. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, M.; Moretti, S.; Vezzoli, A.; Genitoni, V.; Giardini, G.; Balestra, C.; Bosco, G.; Pratali, L.; Spagnolo, E.; Montorsi, M.; et al. Electrical stimulation and DOMS recovery. J. Funct. Morphol. Kinesiol. 2023, 8, 146. [Google Scholar] [CrossRef]
- Iodice, P.; Ripari, P.; Pezzulo, G. Local high-frequency vibration therapy following eccentric exercises reduces muscle soreness perception and posture alterations in elite athletes. Eur. J. Appl. Physiol. 2019, 119, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Freitas, T.T.; Martínez-Noguera, F.J.; Laurent, C.; Gaillet, S.; Chung, L.H.; Alcaraz, P.E.; Cases, J. Supplementation with a Polyphenol-Rich Extract, TensLess®, Attenuates Delayed Onset Muscle Soreness and Improves Muscle Recovery from Damages After Eccentric Exercise. Phytother. Res. 2017, 31, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, E.M.; Sayer, B.L.; Church, J.B.; Bryant, L.G.; Přibyslavská, V. Effects of foam rolling for delayed onset muscle soreness on loaded military task performance and perceived recovery. J. Exerc. Sci. Fit. 2021, 19, 166–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farias Junior, L.F.; Browne, R.A.V.; Frazão, D.T.; Dantas, T.C.B.; Silva, P.H.M.; Freitas, R.P.A.; Aoki, M.S.; Costa, E.C. Effect of Low-Volume High-Intensity Interval Exercise and Continuous Exercise on Delayed-Onset Muscle Soreness in Untrained Healthy Males. J. Strength Cond. Res. 2019, 33, 774–782. [Google Scholar] [CrossRef]
- Doma, K.; Burt, D.; Connor, J.D. The acute effect of a multi-modal plyometric training session on field-specific performance measures. J. Sports Med. Phys. Fitness 2021, 61, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.G.; Dias, S.S.; de Angelis, T.R.; Fernandes, E.V.; Bernardes, A.G.; Milanez, V.F.; Jussiani, E.I.; de Paula Ramos, S. The use of BCAA to decrease delayed-onset muscle soreness after a single bout of exercise: A systematic review and meta-analysis. Amino Acids 2021, 53, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Sanno, M.; Ellenberg, K.; Frick, H.; Böhm, E.; Haiduck, B.; Goldmann, J.P.; Achtzehn, S.; Brüggemann, G.P.; Mester, J.; et al. Aqua Cycling Does Not Affect Recovery of Performance, Damage Markers, and Sensation of Pain. J. Strength Cond. Res. 2017, 31, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.; Bloch, W.; Hirschmüller, A.; Engelhardt, M.; Grim, C.; Heiss, R.; Hotfiel, T. DOMS—Muscle biomechanics and therapy update. Dtsch. Z. Sportmed. 2024, 75, 189–194. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Luo, L.; Zhang, J.; Cheng, P.; Wu, Q.; Wen, X. The effect of percussion massage therapy on the recovery of delayed onset muscle soreness in physically active young men-a randomized controlled trial. Front. Public Health 2025, 13, 1561970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, L.; Jiang, Y.; He, B. Effects of vibration training combined with kinesio taping on delayed onset muscle soreness of athletes’ knee joints post-DOMS induction: A randomised controlled trial. J. Front. Bioeng. Biotechnol. 2025, 13, 1561309. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.D.; de Noronha, M.; Kamper, S.J. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst. Rev. 2011, CD004577. [Google Scholar] [CrossRef] [PubMed]
- Szajkowski, S.; Pasek, J.; Cieślar, G. Foam Rolling or Percussive Massage for Muscle Recovery: Insights into Delayed-Onset Muscle Soreness (DOMS). J. Funct. Morphol. Kinesiol. 2025, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, B. Effects of vibration foam rolling on pain, fatigue, and range of motion in individuals with muscle fatigue: A systematic review. Healthcare 2025, 13, 1391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antonassi, D.P.; Rodacki, C.L.N.; Lodovico, A.; Ugrinowitsch, C.; Rodacki, A.L.F. Immediate Effects of Acupuncture on Force and Delayed Onset of Muscle Soreness. Med. Acupunct. 2021, 33, 203–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, T.R.; Greene, D.A.; Baker, M.K. Effects of Cold Water Immersion and Contrast Water Therapy for Recovery from Team Sport: A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2017, 31, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
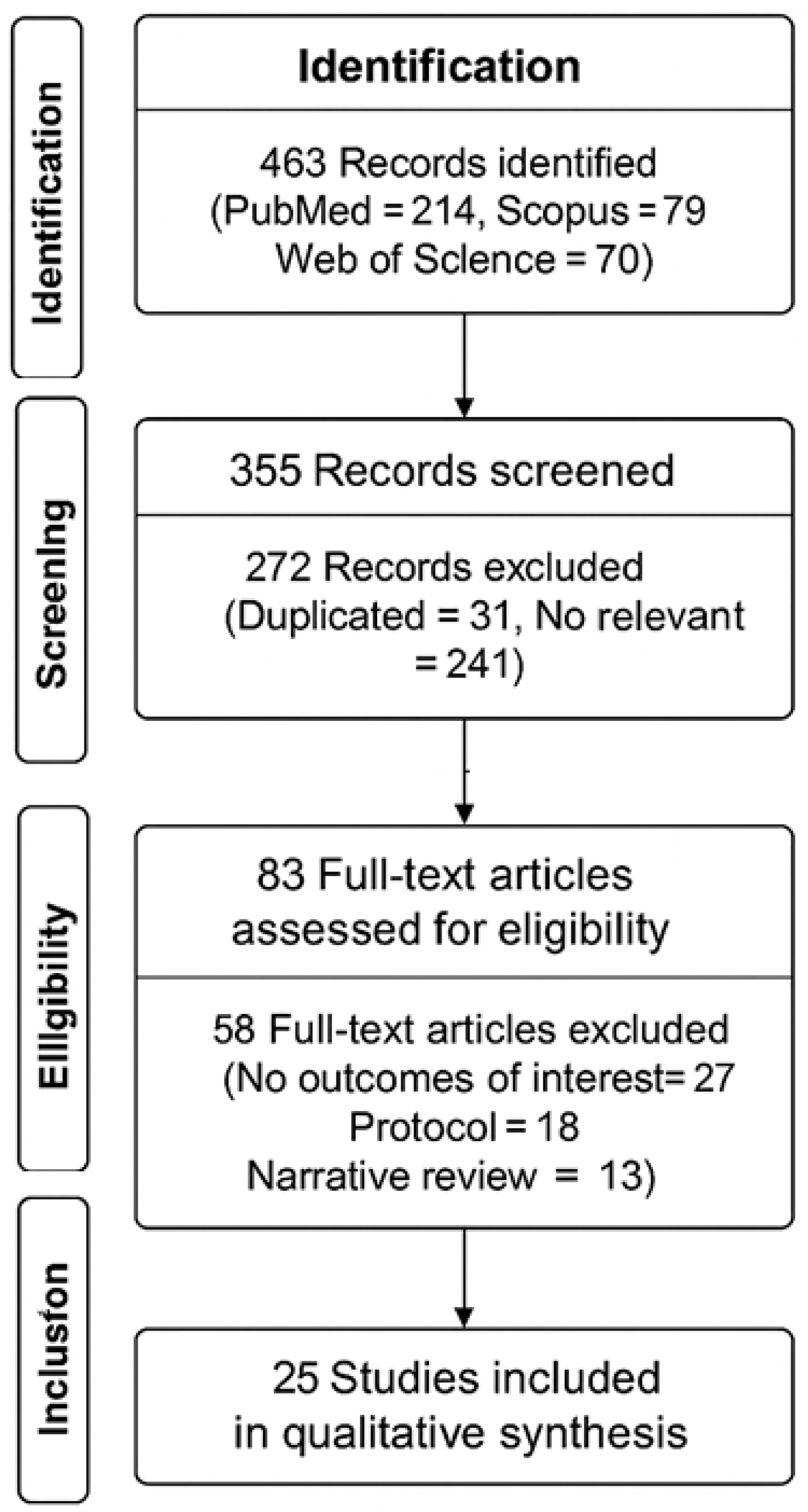
| Stage | Description | Number of Records |
|---|---|---|
| Identification | Records identified through database searching (PubMed, Scopus, Web of Science) | 461 |
| Screening | Records after duplicates removed | 355 |
| Records screened by title and abstract | 355 | |
| Records excluded (not related to DOMS or not meeting inclusion criteria) | 272 | |
| Eligibility | Full-text articles assessed for eligibility | 83 |
| Full-text articles excluded (e.g., protocol only, pharmacological-only, incomplete outcomes) | 58 | |
| Included | Studies included in qualitative synthesis | 23 |
| Author (Year) | Journal | Study Type | Intervention/Focus | Key Findings/Contribution | Thematic Category |
|---|---|---|---|---|---|
| Kancherla A (2023) [1] | Ann Innov Med | Management Update | Overview of DOMS management | Summarizes recent trends and therapeutic directions | General Overview |
| Sonkodi B et al. (2022) [2] | J Funct Morphol Kinesiol | Research | Neuronal microdamage & reflex delay | Highlights sensory neuron injury in DOMS | Neurophysiology |
| Heiss R et al. (2024) [5] | Ann Anat | Imaging Study | 7T MRI for early detection | Demonstrates advanced imaging sensitivity to microdamage | Imaging/Biomarkers |
| Longo V et al. (2016) [6] | J Ultrasound Med | Imaging Study | Ultrasound markers | Shows fascial thickening and echogenicity in DOMS | Imaging/Biomarkers |
| Hotfiel T et al. (2018) [7] | Sportverletz Sportschaden | Review | Pathogenesis & diagnostics | Reviews DOMS etiology, structural markers | Pathophysiology |
| Xue X et al. (2023) [8] | BMC Musculoskelet Disord | RCT | Kinesio taping + compression sleeves | Reduces pain and improves recovery markers | Mechanical Therapy |
| Wolska B et al. (2023) [9] | Front Physiol | RCT | Cryosauna (−110 °C) | Reduces muscle damage markers and stiffness | Thermal Therapy |
| Mizumura K, Taguchi T (2024) [10] | J Physiol Sci | Review | Neurochemical mechanisms | Explores pain mediators and DOMS-related discomfort | Neurophysiology |
| Wiecha S et al. (2024) [11] | J Clin Med | Umbrella Review Protocol | Physical therapy strategies | Maps physical modalities for DOMS management | Mechanical Therapy |
| Wilke J, Behringer M (2021) [12] | Int J Mol Sci | Review | Fascia involvement | Reframes DOMS pathogenesis from a fascial perspective | Fascial/Connective Tissue |
| Fleckenstein J et al. (2021) [13] | Front Physiol | RCT | Sympathetic system role | Shows autonomic modulation of DOMS symptoms | Neurophysiology |
| Akehurst H et al. (2021) [14] | J Orthop Surg Res | RCT | Whole-body vibration | Improves soreness and neuromuscular performance | Mechanical Therapy |
| Chen R et al. (2024) [15] | BMC Musculoskelet Disord | Meta-analysis | Cryotherapy & hydrotherapy | Confirms benefit of cryo/hydrotherapy in DOMS | Thermal Therapy |
| Hotfiel T et al. (2018) [7] | Sportverletz Sportschaden | Review | Prevention & treatment of DOMS | Updates recommendations on intervention timing | General Overview |
| Gussoni M et al. (2023) [16] | J Funct Morphol Kinesiol | RCT | Electrical stimulation | No significant effects vs. placebo | Neurostimulation |
| Iodice P et al. (2019) [17] | Eur J Appl Physiol | Therapy Study | High-frequency vibration | Reduces pain and posture alterations | Mechanical Therapy |
| Romain C et al. (2017) [18] | Phytother Res | Nutritional Study | TensLess® (polyphenols) | Reduces soreness, improves recovery | Nutritional Therapy |
| Scudamore EM et al. (2021) [19] | J Exerc Sci Fit | Performance Study | Foam rolling | Improves task performance, unclear pain effect | Mechanical Therapy |
| Farias-Junior LF et al. (2019) [20] | Physiol Behav | Comparative Study | HIIE vs. MICE exercise effects | Similar DOMS outcomes across modalities | Exercise Modality |
| Doma K et al. (2021) [21] | J Sports Med Phys Fitness | Acute Effects Study | Plyometric training | Increases DOMS, impairs neuromuscular performance | Exercise Modality |
| Weber MG et al. (2021) [22] | Amino Acids | Meta-analysis | BCAA supplementation | Moderate benefit on soreness and muscle recovery | Nutritional Therapy |
| Wahl P et al. (2017) [23] | J Strength Cond Res | RCT | Aqua cycling | No added benefit over passive recovery | Exercise Recovery Modality |
| Schroeter S et al. (2024) [24] | Dtsch Z Sportmed | Review | Compression & thermotherapies | Effective when applied early, for both prevention and treatment | Thermal & Mechanical |
| Author (Year) | Study Type | Quality Assessment Tool | Score/Level | Quality Rating | Notes |
|---|---|---|---|---|---|
| Kancherla A (2023) [1] | Management Update | — | Scoping summary | Moderate | Lacks systematic methodology |
| Sonkodi B et al. (2022) [2] | Research | — | Experimental study | Moderate | Good mechanistic insight, but no control group |
| Heiss R et al. (2024) [5] | Imaging Study | — | Descriptive imaging | Moderate | High technical quality; lacks comparison group |
| Longo V et al. (2016) [6] | Imaging Study | — | Descriptive imaging | Moderate | Ultrasound focused; no longitudinal follow-up |
| Hotfiel T et al. (2018) [7] | Review | AMSTAR 2 | 6/11 | Moderate | Good background, lacks structured bias assessment |
| Xue X et al. (2023) [8] | RCT | PEDro | 7/10 | High | Good methodology, limited blinding |
| Wolska B et al. (2023) [9] | RCT | PEDro | 6/10 | Moderate | Missing dropout reporting, no power analysis |
| Mizumura K, Taguchi T (2024) [10] | Review | AMSTAR 2 | 7/11 | Moderate–High | Strong theoretical background, weak search strategy |
| Wiecha S et al. (2024) [11] | Umbrella Review Protocol | ROBIS | Low risk of bias | High | Systematic protocol, registered and reproducible |
| Wilke J, Behringer M (2021) [12] | Review | AMSTAR 2 | 8/11 | High | Includes fascia-specific pathophysiology, well-structured |
| Fleckenstein J et al. (2021) [13] | RCT | PEDro | 7/10 | High | Well-controlled autonomic analysis |
| Akehurst H et al. (2021) [14] | RCT | PEDro | 6/10 | Moderate | Limited by small sample and short follow-up |
| Chen R et al. (2024) [15] | Meta-analysis | ROBIS | Low risk of bias | High | Robust synthesis, adequate heterogeneity control |
| Hotfiel T et al. (2018) [7] | Review | AMSTAR 2 | 6/11 | Moderate | Lacks explicit inclusion/exclusion criteria |
| Gussoni M et al. (2023) [16] | RCT | PEDro | 5/10 | Moderate | Null results; minimal reporting of participant flow |
| Iodice P et al. (2019) [17] | Therapy Study | PEDro (adapted) | 6/10 | Moderate | Applied vibration, lacks control arm |
| Romain C et al. (2017) [18] | Nutritional Study | PEDro | 5/10 | Moderate | Small sample; plausible results; well reported |
| Scudamore EM et al. (2021) [19] | Performance Study | PEDro (adapted) | 6/10 | Moderate | Functional outcomes only; weak blinding |
| Farias-Junior LF et al. (2019) [20] | Comparative Study | PEDro (adapted) | 5/10 | Moderate | Lacks pre-specified hypothesis |
| Doma K et al. (2021) [21] | Acute Effects Study | PEDro | 6/10 | Moderate | Good experimental setup; no long-term follow-up |
| Weber MG et al. (2021) [22] | Meta-analysis | ROBIS | Low risk of bias | High | Well-conducted with clear inclusion/exclusion criteria |
| Wahl P et al. (2017) [23] | RCT | PEDro | 6/10 | Moderate | Well conducted; no superiority found |
| Schroeter S et al. (2024) [24] | Review | AMSTAR 2 | 7/11 | High | Good methodological structure, solid synthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, L.; Forte, A.M.; Agosti, V.; Forte, F.; Lanciano, T.; Pirraglia, N.; D’Avanzo, C. Advances in Non-Pharmacological Strategies for DOMS: A Scoping and Critical Review of Recent Evidence. J. Funct. Morphol. Kinesiol. 2025, 10, 452. https://doi.org/10.3390/jfmk10040452
Di Lorenzo L, Forte AM, Agosti V, Forte F, Lanciano T, Pirraglia N, D’Avanzo C. Advances in Non-Pharmacological Strategies for DOMS: A Scoping and Critical Review of Recent Evidence. Journal of Functional Morphology and Kinesiology. 2025; 10(4):452. https://doi.org/10.3390/jfmk10040452
Chicago/Turabian StyleDi Lorenzo, Luigi, Alfonso Maria Forte, Valeria Agosti, Francesco Forte, Tiziana Lanciano, Nicola Pirraglia, and Carmine D’Avanzo. 2025. "Advances in Non-Pharmacological Strategies for DOMS: A Scoping and Critical Review of Recent Evidence" Journal of Functional Morphology and Kinesiology 10, no. 4: 452. https://doi.org/10.3390/jfmk10040452
APA StyleDi Lorenzo, L., Forte, A. M., Agosti, V., Forte, F., Lanciano, T., Pirraglia, N., & D’Avanzo, C. (2025). Advances in Non-Pharmacological Strategies for DOMS: A Scoping and Critical Review of Recent Evidence. Journal of Functional Morphology and Kinesiology, 10(4), 452. https://doi.org/10.3390/jfmk10040452







