The Effect of Ammonia Inhalants on Mental-Fatigue-Related Force Loss
Abstract
1. Introduction
2. Materials and Methods
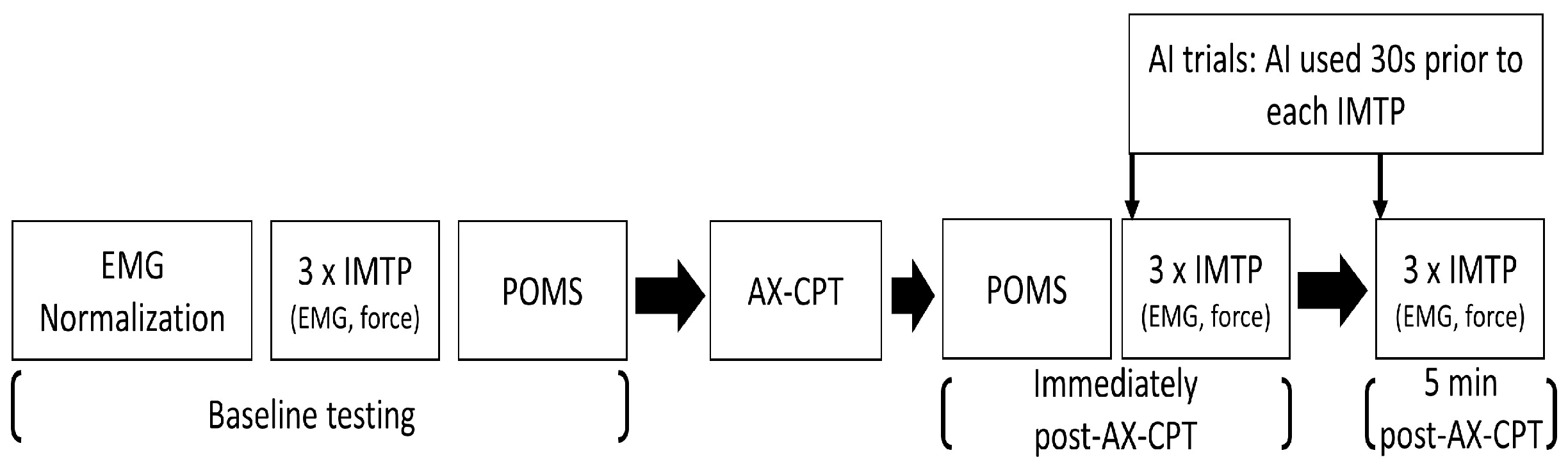
2.1. Experimental Design
2.2. Participants
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Mood Disturbance
3.2. IMTP Force
3.3. EMG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Ammonia inhalant |
| AX-CPT | AX-continuous performance test |
| CON | Control |
| EMG | Electromyography |
| IMTP | Isometric midthigh pull |
| MVIC | Maximum voluntary contraction |
| POMS | Profile of mood states |
| TMD | Total mood disturbance |
References
- Cusimano, K.; Freeman, P.; Moran, J. Identifying the Psyching-Up Strategies Used in Strength Sports: A Concept Mapping Approach. J. Strength Cond. Res. 2025, 39, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, K.; Freeman, P.; Pawaar, J.; Moran, J. The effects of psyching-up on maximal force production: A systematic review. Strength Cond. J. 2024, 46, 468–484. [Google Scholar] [CrossRef]
- Sapuppo, M.; Oberlin, D.; Burke, R.; Pinero, A.; Mohan, A.; Augustin, F.; Coleman, M.; Korakakis, P.; Nuckols, G.; Schoenfeld, B. Preparation for powerlifting competition: A case study. Int. J. Strength Cond. 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Pritchard, H.J.; Stannard, S.R.; Barnes, M.J. Ammonia inhalant and stimulant use among powerlifters: Results from an international survey. J. Aust. Strength Cond. 2014, 22, 52–54. [Google Scholar]
- Qi, P.; Ru, H.; Gao, L.; Zhang, X.; Zhou, T.; Tian, Y.; Thakor, N.; Bezerianos, A.; Li, J.; Sun, Y. Neural mechanisms of mental fatigue revisited: New insights from the brain connectome. Engineering 2019, 5, 276–286. [Google Scholar] [CrossRef]
- Staiano, W.; Bonet, L.R.S.; Romagnoli, M.; Ring, C. Mental fatigue: The cost of cognitive loading on weight lifting, resistance training, and cycling performance. Int. J. Sports Physiol. Perform. 2023, 18, 465–473. [Google Scholar] [CrossRef]
- Martin, K.; Meeusen, R.; Thompson, K.G.; Keegan, R.; Rattray, B. Mental fatigue impairs endurance performance: A physiological explanation. Sports Med. 2018, 48, 2041–2051. [Google Scholar] [CrossRef]
- Kunasegaran, K.; Ismail, A.M.H.; Ramasamy, S.; Gnanou, J.V.; Caszo, B.A.; Chen, P.L. Understanding mental fatigue and its detection: A comparative analysis of assessments and tools. PeerJ 2023, 11, e15744. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Grgic, J.; Jiménez-Martínez, P.; Baz-Valle, E.; Balsalobre-Fernández, C. Effects of mental fatigue on strength endurance: A systematic review and meta-analysis. Mot. Control 2022, 27, 442–461. [Google Scholar] [CrossRef]
- De Salles Painelli, V.; Lienbenberger, C.A.; Zorek, L.; Pires, F.O. Mental fatigue impairs strength endurance performance in trained individuals. Sport Sci. Health 2024, 20, 789–796. [Google Scholar] [CrossRef]
- Yang, H.S.; Atkins, L.T.; James, C.R. Effects of mental fatigue on isometric mid-thigh pull performance and muscle activities. PLoS ONE 2025, 20, e0318238. [Google Scholar] [CrossRef] [PubMed]
- Habay, J.; Van Cutsem, J.; Verschueren, J.; De Bock, S.; Proost, M.; De Wachter, J.; Tassignon, B.; Meeusen, R.; Roelands, B. Mental fatigue and sport-specific psychomotor performance: A systematic review. Sports Med. 2021, 51, 1527–1548, Erratum in Sports Med. 2021, 51, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Soh, K.G.; Roslan, S.; Wazir, M.R.W.N.; Soh, K.L. Does mental fatigue affect skilled performance in athletes? A systematic review. PLoS ONE 2021, 16, e0258307. [Google Scholar] [CrossRef] [PubMed]
- Budini, F.; Lowery, M.; Durbaba, R.; De Vito, G. Effect of mental fatigue on induced tremor in human knee extensors. J. Electromyogr. Kinesiol. 2014, 24, 412–418. [Google Scholar] [CrossRef]
- Jacquet, T.; Poulin-Charronnat, B.; Bard, P.; Lepers, R. Effect of mental fatigue on hand force production capacities. PLoS ONE 2024, 19, e0298958. [Google Scholar] [CrossRef]
- Kowalski, K.L.; Tierney, B.C.; Christie, A.D. Mental fatigue does not substantially alter neuromuscular function in young, healthy males and females. Physiol. Behav. 2022, 253, 113855. [Google Scholar] [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does mental exertion alter maximal muscle activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef]
- Bender, J.M.; Popkin, C.A. Ammonia inhalants: Use, misuse, and role in sports performance. Sports Health 2024, 16, 706–710. [Google Scholar] [CrossRef]
- Campbell, A.K.; Williamson, C.E.; Macgregor, L.J.; Hamilton, D.L. Elevated arousal following acute ammonia inhalation is not associated with increased neuromuscular performance. Eur. J. Sport Sci. 2022, 22, 1391–1400. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Smith, M.R.; Chai, R.; Nguyen, H.T.; Marcora, S.M.; Coutts, A.J. Comparing the effects of three cognitive tasks on indicators of mental fatigue. J. Psychol. 2019, 153, 759–783. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG. A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2005; Volume 1, pp. 1–60. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Escamilla, R.F.; Francisco, A.C.; Kayes, A.V.; Speer, K.P.; Moorman, C.T. An electromyographic analysis of sumo and conventional style deadlifts. Med. Sci. Sports Exerc. 2002, 34, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Prentice, C.; Stannard, S.R.; Barnes, M.J. The effects of binge drinking behaviour on recovery and performance after a rugby match. J. Sci. Med. Sport 2014, 17, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, N.; Rossi, S.J.; Justice, B.D.; Haff, E.E.; Pistilli, E.E.; O’Bryant, H.S.; Stone, M.H.; Haff, G.G. Peak force and rate of force development during isometric and dynamic mid-thigh clean pulls performed at various intensities. J. Strength Cond. Res. 2006, 20, 483–491. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Braver, T.S.; Gray, J.R.; Burgess, G.C. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Var. Work. Mem. 2007, 75, 76–106. [Google Scholar] [CrossRef]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef]
- Azevedo, R.; Silva-Cavalcante, M.D.; Gualano, B.; Lima-Silva, A.E.; Bertuzzi, R. Effects of caffeine ingestion on endurance performance in mentally fatigued individuals. Eur. J. Appl. Physiol. 2016, 116, 2293–2303. [Google Scholar] [CrossRef]
- Perry, B.G.; Pritchard, H.J.; Barnes, M.J. Cerebrovascular, cardiovascular and strength responses to acute ammonia inhalation. Eur. J. Appl. Physiol. 2016, 116, 583–592. [Google Scholar] [CrossRef]
- Yagin, F.H.; Pinar, A.; de Sousa Fernandes, M.S. Statistical effect sizes in sports science. J. Exerc. Sci. Phys. Act. Rev. 2024, 2, 164–171. [Google Scholar] [CrossRef]
- Boksem, M.A.; Tops, M. Mental fatigue: Costs and benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef]
- Alix-Fages, C.; González-Cano, H.; Baz-Valle, E.; Balsalobre-Fernández, C. Effects of mental fatigue induced by stroop task and by social media use on resistance training performance, movement velocity, perceived exertion, and repetitions in reserve: A randomized and double-blind crossover trial. Mot. Control 2023, 27, 645–659. [Google Scholar] [CrossRef]
- Fortes, L.S.; Fonseca, F.S.; Nakamura, F.Y.; Barbosa, B.T.; Gantois, P.; de Lima-Júnior, D.; Ferreira, M.E. Effects of mental fatigue induced by social media use on volleyball decision-making, endurance, and countermovement jump performance. Percept. Mot. Ski. 2021, 128, 2745–2766. [Google Scholar] [CrossRef]
- Gantois, P.; Lima-Júnior, D.d.; Fortes, L.d.S.; Batista, G.R.; Nakamura, F.Y.; Fonseca, F.d.S. Mental fatigue from smartphone use reduces volume-load in resistance training: A randomized, single-blinded cross-over study. Percept. Mot. Ski. 2021, 128, 1640–1659. [Google Scholar] [CrossRef]
- Yasin, S.; Altunisik, E.; Tak, A.Z.A. Digital danger in our pockets: Effect of smartphone overuse on mental fatigue and cognitive flexibility. J. Nerv. Ment. Dis. 2023, 211, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Schampheleer, E.; Roelands, B. Mental fatigue in sport—From impaired performance to increased injury risk. Int. J. Sports Physiol. Perform. 2024, 19, 1158–1166. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, C.; He, F.; Zhao, X.; Qi, H.; Zhou, P.; Zhang, L.; Ming, D. How physical activities affect mental fatigue based on EEG energy, connectivity, and complexity. Front. Neurol. 2018, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Umeda, T.; Nakaji, S.; Sugawara, K. Position related analysis of the appearance of and relationship between post-match physical and mental fatigue in university rugby football players. Br. J. Sports Med. 2004, 38, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; Lima-Junior, D.; Faro, H.; Roelands, B.; Fortes, L.S. Prolonged cognitive effort impairs inhibitory control and causes significant mental fatigue after an endurance session with an auditive distractor in professional soccer players. Psychol. Sport Exerc. 2024, 70, 102533. [Google Scholar] [CrossRef]
- Ruaro, M.F.; Procopio, K.F.; Gusmao, N.; de Franca, E.; Doro, M.R.; Izaias, J.E.; Santana, J.O.; Sanches, I.C.; De Sa, C.A.; Caperuto, E.C. Acute Effects of Strength Training with Blood Flow Restriction on Different Cuffs on Mood Profile in Active Adults. J. Exerc. Physiol. Online 2020, 23, 15–24. [Google Scholar]
- Cavarretta, D.J.; Hall, E.E.; Bixby, W.R. The acute effects of resistance exercise on affect, anxiety, and mood–practical implications for designing resistance training programs. Int. Rev. Sport Exerc. Psychol. 2019, 12, 295–324. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S. The effects of mental fatigue on sport performance: An update. In Motivation and Self-Regulation in Sport and Exercise; Routledge: Oxford, UK, 2021; pp. 134–148. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Jiménez-Martínez, P.; de Oliveira, D.S.; Möck, S.; Balsalobre-Fernández, C.; Del Vecchio, A. Mental fatigue impairs physical performance but not the neural drive to the muscle: A preliminary analysis. Eur. J. Appl. Physiol. 2023, 123, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Lochbaum, M.; Zanatta, T.; Kirschling, D.; May, E. The Profile of Moods States and athletic performance: A meta-analysis of published studies. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.R.; Beardsley, K.G.; Cumbie, P.E.; Ballmann, C.G. Ammonia inhalants enhance psychophysiological responses and performance during repeated high intensity exercise. Res. Q. Exerc. Sport 2023, 94, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Nigro, F.; Gubellini, L.; Semprini, G.; Ciacci, S.; Hoffman, J.R.; Merni, F. Acute effects of ammonia inhalants on strength and power performance in trained men. J. Strength Cond. Res. 2018, 32, 244–247. [Google Scholar] [CrossRef]
- Vigil, J.N.; Sabatini, P.L.; Hill, L.C.; Swain, D.P.; Branch, J.D. Ammonia inhalation does not increase deadlift 1-repetition maximum in college-aged male and female weight lifters. J. Strength Cond. Res. 2018, 32, 3383–3388. [Google Scholar] [CrossRef]
- Richmond, S.R.; Potts, A.C.; Sherman, J.R. The impact of ammonia inhalants on strength performance in resistance trained males. J. Exerc. Physiol. Online 2014, 17, 60–66. [Google Scholar]
- Maleček, J.; Omcirk, D.; Skálová, K.; Pádecký, J.; Janikov, M.T.; Obrtel, M.; Jonáš, M.; Kolář, D.; Michalička, V.; Sýkora, K. Effects of 36 hours of sleep deprivation on military-related tasks: Can ammonium inhalants maintain performance? PLoS ONE 2023, 18, e0293804. [Google Scholar] [CrossRef]
- Selvi, Y.; Kilic, S.; Aydin, A.; Özdemir, P.G. The effects of sleep deprivation on dissociation and profiles of mood, and its association with biochemical changes. Nöro Psikiyatr. Arş. 2015, 52, 83. [Google Scholar] [CrossRef]
- Kaida, K.; Niki, K. Total sleep deprivation decreases flow experience and mood status. Neuropsychiatr. Dis. Treat. 2013, 10, 19–25. [Google Scholar] [CrossRef]
- Herrick, R.T.; Herrick, S. Allergic reaction to aromatic ammonia inhalant ampule: A case report. Am. J. Sports Med. 1983, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Dean, B.; Krenzelok, E. Oral exposure to ammonia inhalants: A report of 8 cases. Vet. Hum. Toxicol. 1988, 30, 350. [Google Scholar] [CrossRef]
- Aou, S.; Mizuno, M.; Matsunaga, Y.; Kubo, K.; Li, X.-L.; Hatanaka, A. Green odor reduces pain sensation and fatigue-like responses without affecting sensorimotor function. Chem. Senses 2005, 30 (Suppl. S1), i262–i263. [Google Scholar] [CrossRef]
- Saito, N.; Yamano, E.; Ishii, A.; Tanaka, M.; Nakamura, J.; Watanabe, Y. Involvement of the olfactory system in the induction of anti-fatigue effects by odorants. PLoS ONE 2018, 13, e0195263. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Endo, H.; Kobayakawa, T.; Kato, K.; Kitazaki, S. Effects of intermittent odours on cognitive-motor performance and brain functioning during mental fatigue. Ergonomics 2012, 55, 1–11. [Google Scholar] [CrossRef]
- Kontaris, I.; East, B.S.; Wilson, D.A. Behavioral and neurobiological convergence of odor, mood and emotion: A review. Front. Behav. Neurosci. 2020, 14, 35. [Google Scholar] [CrossRef]
- Proost, M.; Habay, J.; De Wachter, J.; De Pauw, K.; Rattray, B.; Meeusen, R.; Roelands, B.; Van Cutsem, J. How to tackle mental fatigue: A systematic review of potential countermeasures and their underlying mechanisms. Sports Med. 2022, 52, 2129–2158. [Google Scholar] [CrossRef]
| Control | Ammonia Inhalation | |||||||
|---|---|---|---|---|---|---|---|---|
| Subscale | Pre-AX-CPT | Post-AX-CPT | p | Cohen’s d [95% CI] | Pre-AX-CPT | Post-AX-CPT | p | Cohen’s d [95% CI] |
| Tension | 10.62 ± 8.65 | 12.02 ± 6.84 | 0.336 | 0.18 [−1.13, 1.49] | 9.78 ± 8.00 | 13.48 ± 8.45 | 0.006 | 0.45 [−0.87, 1.77] |
| Anger | 7.89 ± 8.46 | 16.15 ± 10.68 | 0.099 | 0.86 [−0.51, 2.22] | 7.67 ± 9.40 | 17.95 ± 10.94 | 0.028 | 1.01 [−0.38, 2.40] |
| Vigor | 16.64 ± 3.72 | 8.30 ± 5.71 | 0.003 | −1.73 [−3.26, −0.20] | 16.47 ± 7.94 | 8.84 ± 4.86 | 0.004 | −1.16 [−2.57, 0.25] |
| Depression | 9.73 ± 10.47 | 14.10 ± 8.58 | 0.176 | 0.46 [−0.87, 1.78] | 8.77 ± 9.49 | 15.90 ± 9.45 | 0.012 | 0.75 [−0.60, 2.11] |
| Confusion | 8.14 ± 7.19 | 10.66 ± 6.08 | 0.126 | 0.38 [−0.94, 1.70] | 6.38 ± 5.98 | 10.71 ± 6.70 | 0.005 | 0.68 [−0.66, 2.03] |
| Fatigue | 11.63 ± 8.86 | 18.46 ± 6.17 | 0.012 | 0.90 [−0.48, 2.27] | 6.61 ± 6.65 | 16.68 ± 5.24 | 0.002 | 1.68 [0.16, 3.20] |
| TMD | 31.43 ± 42.40 | 63.04 ± 30.39 | 0.031 | 0.86 [−0.51, 2.22] | 22.73 ± 35.37 | 65.88 ± 35.54 | <0.001 | 1.22 [−0.21, 2.64] |
| Baseline | Immediately Post AX-CPT | p | Cohen’s d [95% CI] | 5 min Post AX-CPT | p | Cohen’s d [95% CI] | |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| EMG (%) | 35.8 ± 9.0 | 33.6 ± 18.5 | 1.00 | −0.15 [−1.46, 1.16] | 34.2 ± 13.0 | 1.00 | −0.14 [−1.45, 1.17] |
| IMTP (N) | 1624 ± 301 | 1511 ± 342 | 0.482 | −0.35 [−1.67, 0.97] | 1523 ± 356 | 0.402 | −0.31 [−1.62, 1.01] |
| IMTP (%Δ) | - | −6.6 ± 12.7 | 1.00 | −0.74 [−2.09, 0.62] | −6.5 ± 10.0 | 1.00 | −0.92 [−2.29, 0.46] |
| Ammonia Inhalation | |||||||
| EMG (%) | 39.5 ± 11.0 | 34.5 ± 10.9 | 0.206 | −0.46 [−1.78, 0.87] | 38.9 ± 18.4 | 1.00 | −0.04 [−1.35, 1.27] |
| IMTP (N) | 1774 ± 387 | 1531 ± 327 | 0.042 | −0.69 [−2.02, 0.67] | 1688 ± 365 | 0.707 | −0.23 [−1.54, 1.08] |
| IMTP (%Δ) | - | −12.6 ± 12.3 | 0.859 | −1.45 [−2.92, 0.02] | −4.1 ± 10.1 | 1.00 | −0.57 [−1.91, 0.76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, M.J.; O’Connor, E.; van Zanten, J. The Effect of Ammonia Inhalants on Mental-Fatigue-Related Force Loss. J. Funct. Morphol. Kinesiol. 2025, 10, 406. https://doi.org/10.3390/jfmk10040406
Barnes MJ, O’Connor E, van Zanten J. The Effect of Ammonia Inhalants on Mental-Fatigue-Related Force Loss. Journal of Functional Morphology and Kinesiology. 2025; 10(4):406. https://doi.org/10.3390/jfmk10040406
Chicago/Turabian StyleBarnes, Matthew J., Emma O’Connor, and Jason van Zanten. 2025. "The Effect of Ammonia Inhalants on Mental-Fatigue-Related Force Loss" Journal of Functional Morphology and Kinesiology 10, no. 4: 406. https://doi.org/10.3390/jfmk10040406
APA StyleBarnes, M. J., O’Connor, E., & van Zanten, J. (2025). The Effect of Ammonia Inhalants on Mental-Fatigue-Related Force Loss. Journal of Functional Morphology and Kinesiology, 10(4), 406. https://doi.org/10.3390/jfmk10040406





