Advanced Stress Echocardiography with Cardiopulmonary Exercise Testing After Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
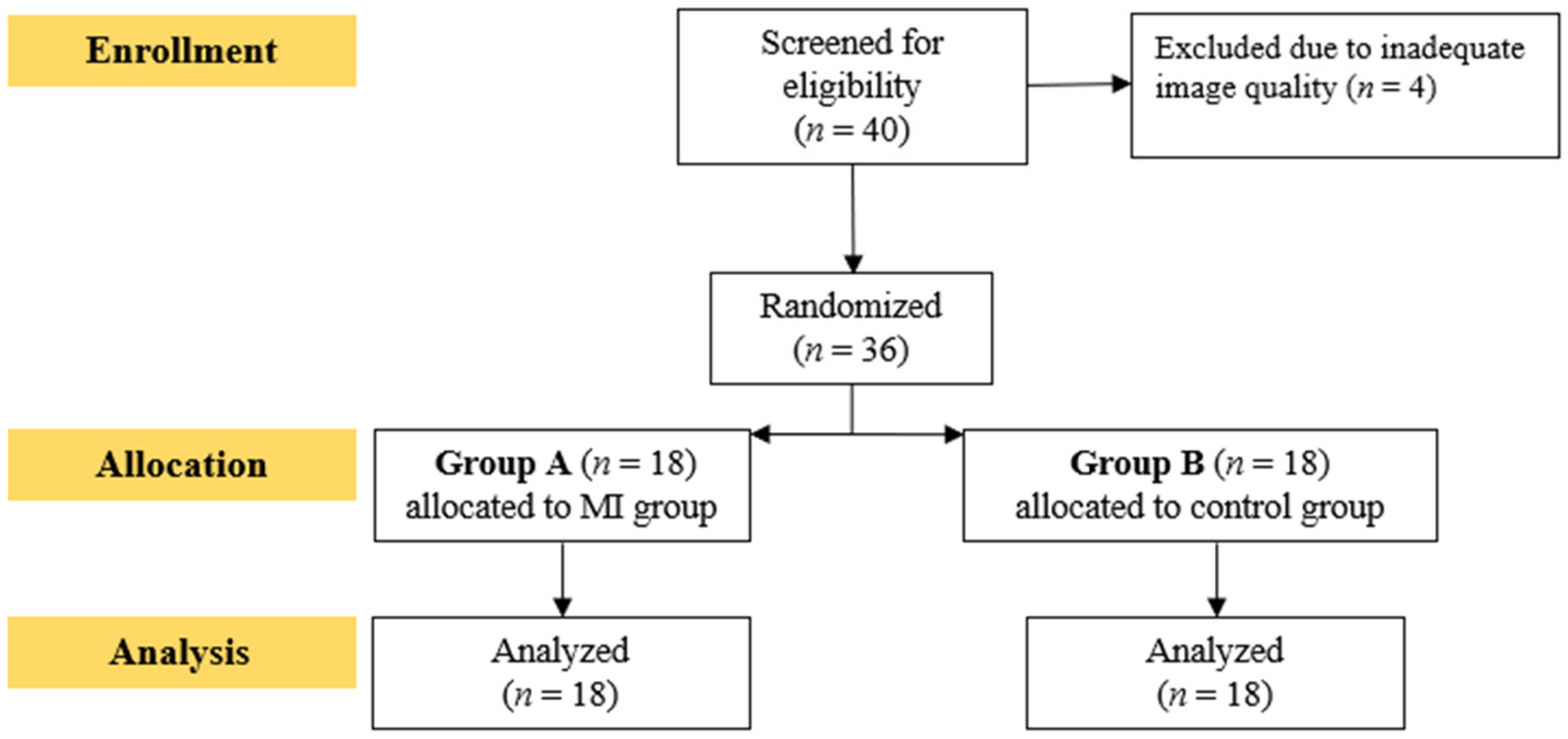
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Cardiopulmonary Exercise Testing
2.5. Dynamic Echocardiography
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Cardiorespiratory Efficiency Results
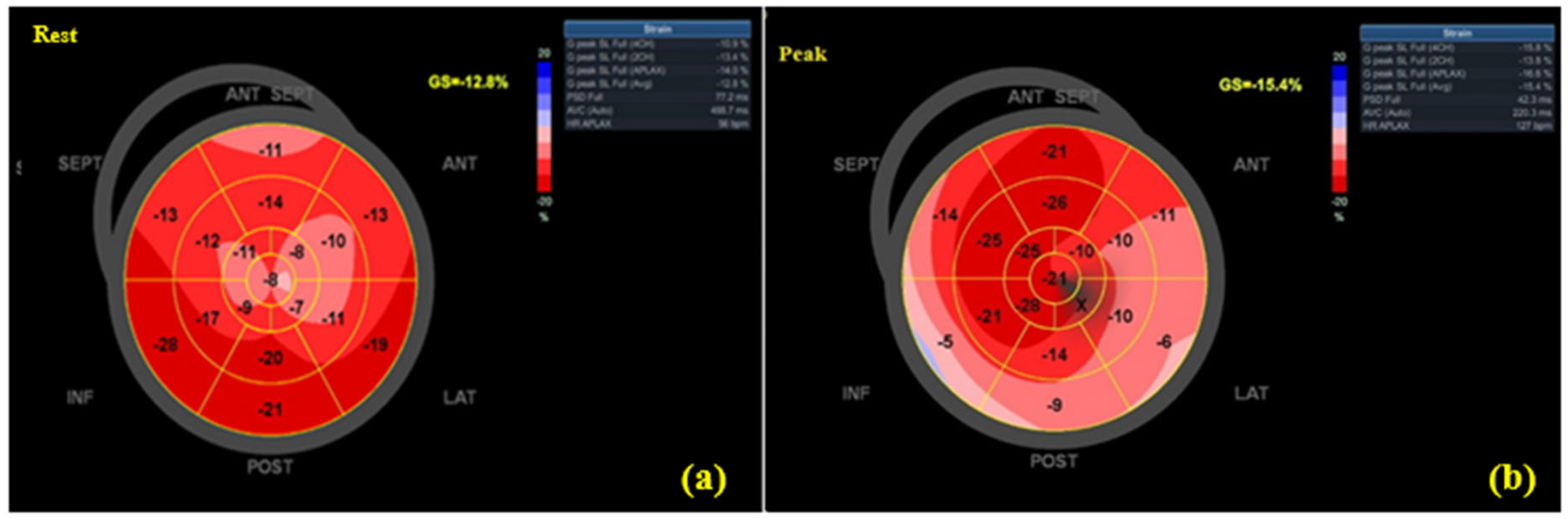
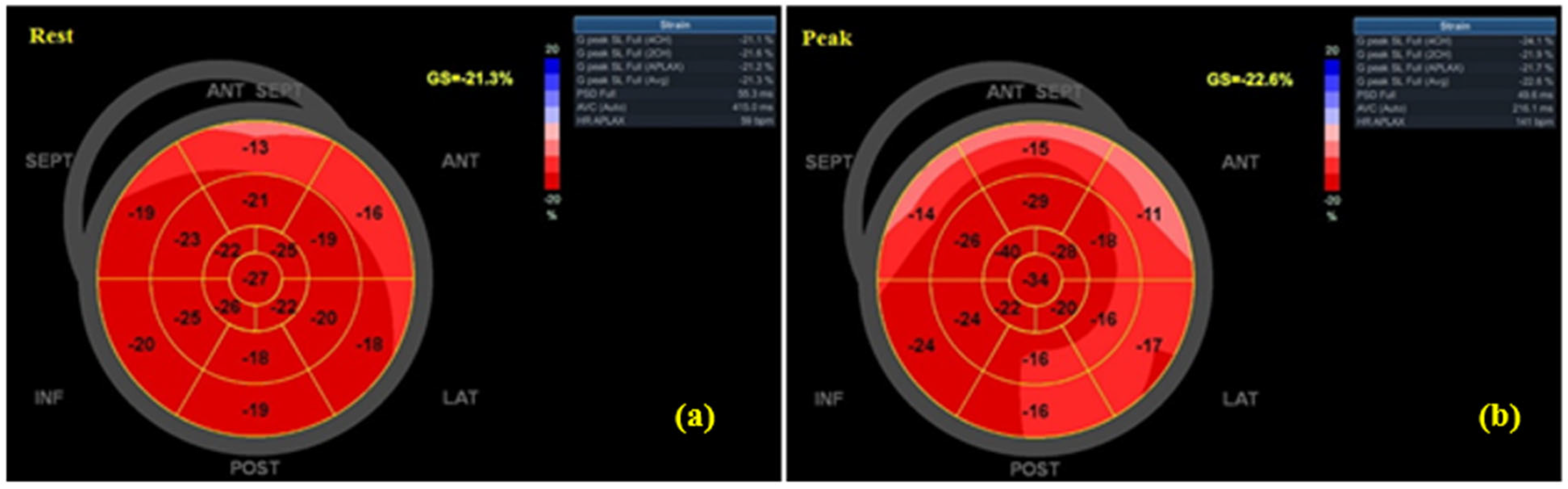
3.3. Echocardiographic Findings
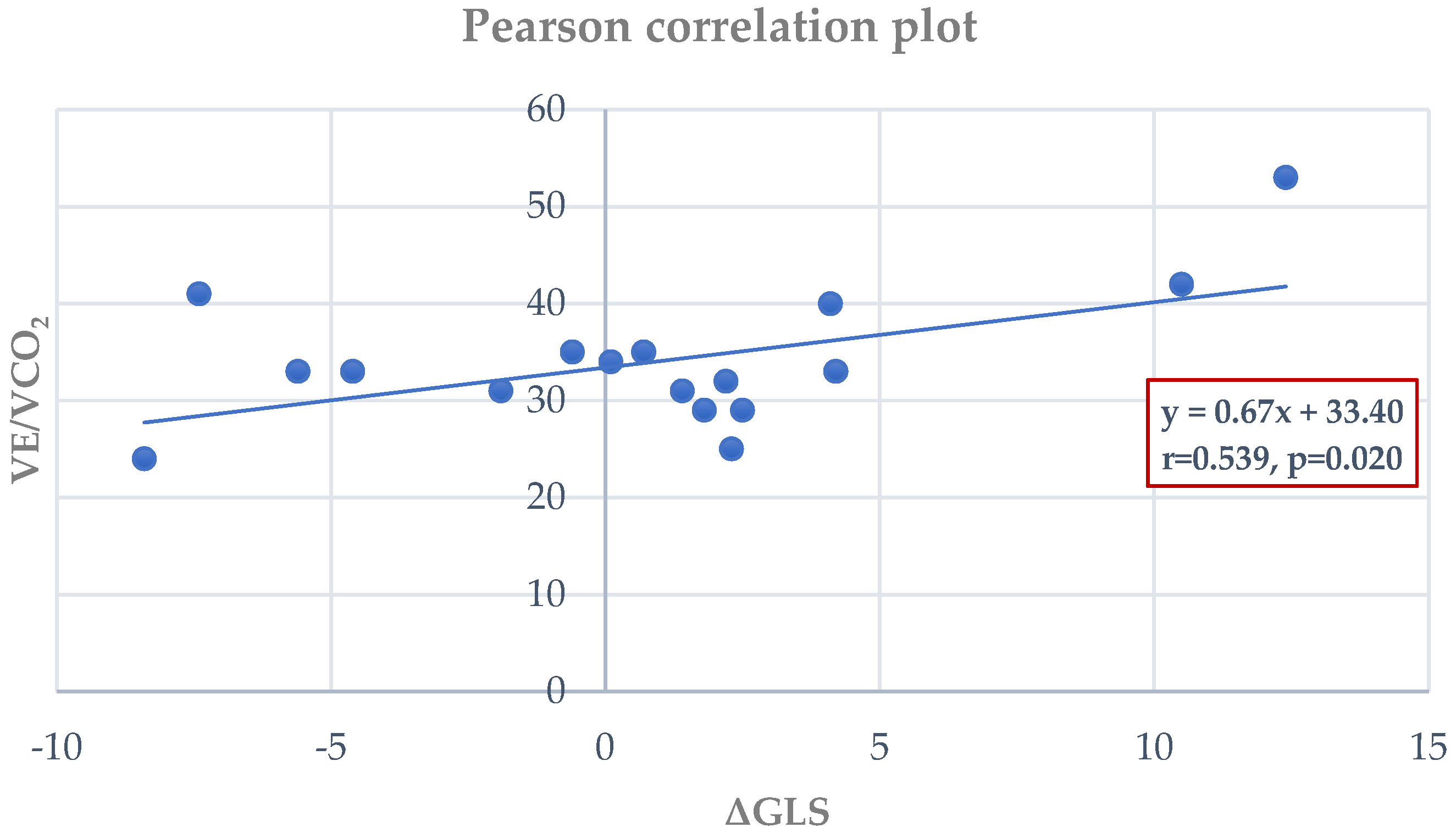
3.4. Correlations Between CPET and Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Widimsky, P.; Wijns, W.; Fajadet, J.; de Belder, M.; Knot, J.; Aaberge, L.; Andrikopoulos, G.; Baz, J.A.; Betriu, A.; Claeys, M.; et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: Description of the current situation in 30 countries. Eur. Heart J. 2010, 31, 943–957. [Google Scholar] [CrossRef]
- Turpie, A.G.G. Burden of disease: Medical and economic impact of acute coronary syndromes. Am. J. Manag. Care 2006, 12 (Suppl. S16), S430–S434. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Kripalani, S.; Zhu, Y.; Storrow, A.B.; Dittus, R.S.; Harrell, F.E.; Self, W.H. Incidence of Emergency Department Visits for ST-Elevation Myocardial Infarction in a Recent Six-Year Period in the United States. Am. J. Cardiol. 2015, 115, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Tousoulis, D. Myocardial performance versus exercise tolerance: What matters the most in patients with heart failure? Hell. J. Cardiol. 2018, 59, 336–337. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2015; pp. 1841–1875. [Google Scholar]
- Lacey, L.; Tabberer, M. Economic burden of post-acute myocardial infarction heart failure in the United Kingdom. Eur. J. Heart Fail. 2005, 7, 677–683. [Google Scholar] [CrossRef]
- Dannenberg, V.; Christiansen, F.; Schneider, M.; Kastl, S.; Hofbauer, T.M.; Scherz, T.; Mascherbauer, J.; Beitzke, D.; Testori, C.; Lang, I.M.; et al. Exploratory echocardiographic strain parameters for the estimation of myocardial infarct size in ST-elevation myocardial infarction. Clin. Cardiol. 2021, 44, 925–931. [Google Scholar] [CrossRef]
- Joseph, G.; Zaremba, T.; Johansen, M.B.; Ekeloef, S.; Heiberg, E.; Engblom, H.; Jensen, S.E.; Sogaard, P. Echocardiographic global longitudinal strain is associated with infarct size assessed by cardiac magnetic resonance in acute myocardial infarction. Echo Res. Pract. 2019, 6, 81–89. [Google Scholar] [CrossRef]
- El-Naggar, H.M.; Osman, A.S.; Ahmed, M.A.; Youssef, A.A.; Ahmed, T.A.N. Three-dimensional echocardiographic assessment of left ventricular geometric changes following acute myocardial infarction. Int. J. Cardiovasc. Imaging 2022, 39, 607–620. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Jneid, H.; Al Hajji, M.; Boubas, W.; Lerman, A. Age-Stratified Sex-Related Differences in the Incidence, Management, and Outcomes of Acute Myocardial Infarction. Mayo Clin. Proc. 2021, 96, 332–341. [Google Scholar] [CrossRef]
- Mefford, M.T.; Li, B.H.; Qian, L.; Reading, S.R.; Harrison, T.N.; Scott, R.D.; Cavendish, J.J.; Jacobsen, S.J.; Kanter, M.H.; Woodward, M.; et al. Sex-Specific Trends in Acute Myocardial Infarction Within an Integrated Healthcare Network, 2000 Through 2014. Circulation 2020, 141, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Khera, S.; Kolte, D.; Gupta, T.; Subramanian, K.S.; Khanna, N.; Aronow, W.S.; Ahn, C.; Timmermans, R.J.; Cooper, H.A.; Fonarow, G.C.; et al. Temporal Trends and Sex Differences in Revascularization and Outcomes of ST-Segment Elevation Myocardial Infarction in Younger Adults in the United States. J. Am. Coll. Cardiol. 2015, 66, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Rizza, A.; De Giorgi, A.; Negro, F.; Boari, B.; Palmieri, C.; Berti, S.; Manfredini, R. Sex-related differences and chronobiology of ST-elevation myocardial infarction: Findings from a single hub center in Italy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1540–1552. [Google Scholar] [PubMed]
- Wang, X.; Pabon, M.A.; Cikes, M.; Jering, K.; Mullens, W.; Kober, L.; Jhund, P.S.; Kovacs, A.; Merkely, B.; Zhou, Y.; et al. Sex differences in cardiac structure and function following acute myocardial infarction: Insights from the PARADISE-MI echocardiographic substudy. Eur. J. Heart Fail. 2025, 27, 788–799. [Google Scholar] [CrossRef]
- Xu, Z.; Song, T.; Yang, X.; Cong, L.; Yin, L.; Xu, Y.; Han, X.; Gao, M.; Xu, L. TMT-based proteomics reveals methylprotodioscin alleviates oxidative stress and inflammation via COX6C in myocardial infraction. Biomed. Pharmacother. 2024, 180, 117489. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Q.; Zha, L.; Zhang, L.; Huang, M.; Zhang, S.; Zhang, X.; Li, Q.; Chen, X.; Xia, N.; et al. Thymic stromal lymphopoietin modulates T cell response and improves cardiac repair post-myocardial infarction. Front Immunol. 2024, 15, 1467095. [Google Scholar] [CrossRef]
- Sumi, M.P.; Mahajan, B.; Sattar, R.S.A.; Nimisha Apurva Kumar, A.; Sharma, A.K.; Ahmad, E.; Ali, A.; Saluja, S.S. Elucidation of Epigenetic Landscape in Coronary Artery Disease: A Review on Basic Concept to Personalized Medicine. Epigenet. Insights 2021, 14, 2516865720988567. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Huang, W.M.; Chen, C.N.; Chen, Y.H.; Yen, J.H.; Tseng, T.Y.; Cheng, H.M.; Yu, W.C.; Chen, C.H.; Sung, S.H. The feasibility and safety of stepwise protocol in cardiopulmonary exercise testing-exercise stress echocardiography for subjects with heart failure. J. Chin. Med. Assoc. 2022, 85, 815–820. [Google Scholar] [CrossRef]
- Kirsch, M.; Feriel, M.; Aurelia, L.T.; Oksana, K.; Christophe, B.J.; François, L.; Pascal, C.; Vitiello, D.; Marie-Christine, I. Impact of training on combined cardiopulmonary exercise test with stress echocardiography parameters in HFrEF patients. Int. J. Cardiol. 2023, 371, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Shimiaie, J.; Sherez, J.; Aviram, G.; Megidish, R.; Viskin, S.; Halkin, A.; Ingbir, M.; Nesher, N.; Biner, S.; Keren, G.; et al. Determinants of Effort Intolerance in Patients With Heart Failure. JACC Heart Fail. 2015, 3, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; Banovic, M.; Stepanovic, J.; Giga, V.; Djordjevic-Dikic, A.; Trifunovic, D.; Nedeljkovic, M.; Petrovic, M.; Dobric, M.; Dikic, N.; et al. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur. J. Prev. Cardiol. 2016, 23, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Fabiani, I.; Santini, C.; Rovai, I.; Pedrinelli, R.; Natali, A.; Dini, F.L. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 828–836. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Conte, L.; Gargani, L.; Mazzola, M.; Fabiani, I.; Natali, A.; Dni, F.L.; Frumento, P.; Rosada, J.; et al. Cardiac Reserve and Exercise Capacity: Insights from Combined Cardiopulmonary and Exercise Echocardiography Stress Testing. J. Am. Soc. Echocardiogr. 2021, 34, 38–50. [Google Scholar] [CrossRef]
- Del Punta, L.; De Biase, N.; Balletti, A.; Filidei, F.; Pieroni, A.; Armenia, S.; Mengozzi, A.; Mazzola, M.; Di Fiore, V.; Dini, F.L.; et al. Arterial Hypertension and Cardiopulmonary Function: The Value of a Combined Cardiopulmonary and Echocardiography Stress Test. High Blood Press. Cardiovasc. Prev. 2022, 29, 145–154. [Google Scholar] [CrossRef]
- Santoro, C.; Sorrentino, R.; Esposito, R.; Lembo, M.; Capone, V.; Rozza, F.; Romano, M.; Trimarco, B.; Galderisi, M. Cardiopulmonary exercise testing and echocardiographic exam: An useful interaction. Cardiovasc. Ultrasound 2019, 17, 29. [Google Scholar] [CrossRef]
- Innocenti, F.; Caldi, F.; Tassinari, I.; Agresti, C.; Burgisser, C.; Fattirolli, F.; Baldereschi, G.J.; Marchionni, N.; Masotti, G.; Pini, R. Prognostic Value of Exercise Stress Test and D obutamine Stress Echo in Patients with Known Coronary Artery Disease. Echocardiography 2009, 26, 1–9. [Google Scholar] [CrossRef]
- Smarz, K.; Jaxa-Chamiec, T.; Zaborska, B.; Tysarowski, M.; Budaj, A. Combined use of stress echocardiography and cardiopulmonary exercise testing to assess exercise intolerance in patients treated for acute myocardial infarction. PLoS ONE 2021, 16, e0255682. [Google Scholar] [CrossRef]
- Kouris, N.T.; Kostopoulos, V.S.; Psarrou, G.A.; Kostakou, P.M.; Tzavara, C.; Olympios, C.D. Left ventricular ejection fraction and Global Longitudinal Strain variability between methodology and experience. Echocardiography 2021, 38, 582–589. [Google Scholar] [CrossRef]
- Juarez, M.; Castillo-Rodriguez, C.; Soliman, D.; Del Rio-Pertuz, G.; Nugent, K. Cardiopulmonary Exercise Testing in Heart Failure. J. Cardiovasc. Dev. Dis. 2024, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Jiang, L.; Wang, Z.; Liu, W.; Zuo, H. Diagnostic Accuracy of Global Longitudinal Strain for Detecting Exercise Intolerance in Patients with Ischemic Heart Disease. J. Cardiovasc. Dev. Dis. 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, M.P.J.; Smith, G.; Gulamhusein, S.S. Feasibility and safety of early exercise testing using the Bruce protocol after acute myocardial infarction. J. Am. Coll. Cardiol. 2000, 35, 1212–1220. [Google Scholar] [CrossRef][Green Version]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Kasiak, P.; Kowalski, T.; Klusiewicz, A.; Zdanowicz, R.; Ładyga, M.; Wiecha, S.; Mamcarz, A.; Śliż, D. Recalibrated FRIEND equation for peak oxygen pulse is accurate in endurance athletes: The NOODLE study. Sci. Rep. 2024, 14, 23133. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Paraskevaidis, I.A.; Ikonomidis, I.; Simitsis, P.; Parissis, J.; Stasinos, V.; Makavos, G.; Lekakis, J. Multidimensional contractile reserve predicts adverse outcome in patients with severe systolic heart failure: A 4-year follow-up study. Eur. J. Heart Fail. 2017, 19, 846–861. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.; Kane, G.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Picano, E.; Pierard, L.; Peteiro, J.; Djordjevic-Dikic, A.; Sade, L.E.; Cortigiani, L.; Van De Heyning, C.M.; Celutkiene, J.; Gaibazzi, N.; Ciampi, Q.; et al. The clinical use of stress echocardiography in chronic coronary syndromes and beyond coronary artery disease: A clinical consensus statement from the European Association of Cardiovascular Imaging of the ESC. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e65–e90. [Google Scholar] [CrossRef]
- Luo, B.; Luo, Z.; Zhang, X.; Xu, M.; Shi, C. Status of cognitive frailty in elderly patients with chronic kidney disease and construction of a risk prediction model: A cross-sectional study. BMJ Open 2022, 12, e060633. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B. Global Longitudinal Strain: An Additional Tool to Improve Risk Stratification in Patients After ST-Segment Elevation Myocardial Infarction? J. Am. Soc. Echocardiogr. 2024, 37, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Caunite, L.; Myagmardorj, R.; Galloo, X.; Laenens, D.; Stassen, J.; Nabeta, T.; Yedidya, I.; Meucci, M.C.; Kuneman, J.H.; van den Hoogen, I.J.; et al. Prognostic Value of Follow-up Measures of Left Ventricular Global Longitudinal Strain in Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Soc. Echocardiogr. 2024, 37, 666–673. [Google Scholar] [CrossRef]
- Luo, X.; Ge, Q.; Su, J.; Zhou, N.; Li, P.; Xiao, X.; Chen, Y.; Wang, D.; Ma, Y.; Ma, L.; et al. Normal ranges of non-invasive left ventricular myocardial work indices in healthy young people. Front. Pediatr. 2022, 10, 1000556. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, H.L.; Kim, K.J. Sex difference in the age-related decline of global longitudinal strain of left ventricle. Sci. Rep. 2023, 13, 18441. [Google Scholar] [CrossRef]
- Núñez, E.; Santas, E.; Merenciano, H.; Lorenzo-Hernández, M.; Mollar, A.; Miñana, G.; Palau, P.; Fuertes, L.; Valero, E.; de la Espriella, R.; et al. Differential sex-related effect of left ventricular ejection fraction trajectory on the risk of mortality and heart failure readmission following hospitalization for acute heart failure: A longitudinal study. Eur. J. Heart Fail. 2024, 26, 1687–1698. [Google Scholar] [CrossRef]
- Lau, E.S.; Zhao, Y.; Benjamin, E.J.; Ramachandran, V.S.; Xanthakis, V.; Cheng, S.; Ho, J.E. Sex Differences in Left Ventricular Function and Cardiac Mechanics. J. Am. Heart Assoc. 2024, 13, e035781. [Google Scholar] [CrossRef]
- Larsen, A.H.; Clemmensen, T.S.; Wiggers, H.; Poulsen, S.H. Left Ventricular Myocardial Contractile Reserve during Exercise Stress in Healthy Adults: A Two-Dimensional Speckle-Tracking Echocardiographic Study. J. Am. Soc. Echocardiogr. 2018, 31, 1116–1126.e1. [Google Scholar] [CrossRef]
- Varga, A.; Garcia, M.A.R.; Picano, E. Safety of Stress Echocardiography (from the International Stress Echo Complication Registry). Am. J. Cardiol. 2006, 98, 541–543. [Google Scholar] [CrossRef]
- Fennich, N.; Ellouali, F.; Abdelali, S.; Chaara, A.; Berrada, A.; Elhajjaji, I.; Cherradi, R.; Abir, S.; Doghmi, N.; Cherti, M. Stress echocardiography: Safety and tolerability. Cardiovasc. Ultrasound 2013, 11, 30. [Google Scholar] [CrossRef]
- Akinci Özyürek, B.; Savaş Bozbaş, Ş.; Aydinalp, A.; Bozbaş, H.; Ulubay, G. Value of cardiopulmonary exercise testing in the diagnosis of coronary artery disease. Tuberk Toraks 2019, 67, 102–107. [Google Scholar] [CrossRef]
- Popovic, D.; Martic, D.; Djordjevic, T.; Pesic, V.; Guazzi, M.; Myers, J.; Mohebi, R.; Arena, R. Oxygen consumption and carbon-dioxide recovery kinetics in the prediction of coronary artery disease severity and outcome. Int. J. Cardiol. 2017, 248, 39–45. [Google Scholar] [CrossRef]
- Popovic, D.; Guazzi, M.; Jakovljevic, D.G.; Lasica, R.; Banovic, M.; Ostojic, M.; Arena, R. Quantification of coronary artery disease using different modalities of cardiopulmonary exercise testing. Int. J. Cardiol. 2019, 285, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, R.; Shakerian, F.; Vasheghani-Farahani, A.; Halabchi, F.; Mirshahi, M.; Mansournia, M.A. The usefulness of cardiopulmonary exercise testing in assessment of patients with suspected coronary artery disease. Postgrad. Med. J. 2016, 92, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Sietsema, K.E. Cardiopulmonary Exercise Testing in the Clinical Evaluation of Patients With Heart and Lung Disease. Circulation 2011, 123, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.; Gleadall-Siddall, D.O.; Antony, R.; Clark, A.L.; Cleland, J.G.F.; Carroll, S.; Ingle, L. Estimated peak functional capacity: An accurate method for assessing change in peak oxygen consumption after cardiac rehabilitation? Clin. Physiol. Funct. Imaging 2018, 38, 681–688. [Google Scholar] [CrossRef]
- Satoh, T.; Okano, Y.; Takaki, H.; Matsumoto, T.; Yasumura, Y.; Aihara, N.; Goto, Y. Excessive Ventilation After Acute Myocardial Infarction and Its Improvement in 4 Months. Jpn. Circ. J. 2001, 65, 399–403. [Google Scholar] [CrossRef][Green Version]
- Thein, P.M.; Mirzaee, S.; Cameron, J.D.; Nasis, A. Left ventricular contractile reserve as a determinant of adverse clinical outcomes: A systematic review. Intern. Med. J. 2022, 52, 186–197. [Google Scholar] [CrossRef]
- Caniggia, C.; Amor, M.; Lowenstein, D.; Alasia, D.; Galello, M.; Darú, V.; Lowenstein, J. Feasibility and contribution of global and regional 2D strain during exercise stress echocardiography. Rev. Argent. Cardiol. 2014, 1, 110–117. [Google Scholar]
- Leitman, M.; Tyomkin, V.; Peleg, E.; Zyssman, I.; Rosenblatt, S.; Sucher, E.; Gercenshtein, V.; Vered, Z. Speckle Tracking Imaging in Normal Stress Echocardiography. J. Ultrasound Med. 2017, 36, 717–724. [Google Scholar] [CrossRef]
- Helleryd, E.; Rawshani, A.; Rawshani, A.; Hjärtstam, N.; Myredal, A.; Skoglund, K. Association between exercise load, resting heart rate, and maximum heart rate and risk of future ST-segment elevation myocardial infarction (STEMI). Open Heart 2023, 10, e002307. [Google Scholar] [CrossRef] [PubMed]
- Nishitani-Yokoyama, M.; Daida, H.; Shimada, K.; Ushijima, A.; Kida, K.; Kono, Y.; Sakata, Y.; Nagayama, M.; Furukawa, Y.; Fukuma, N.; et al. Effects of Phase II Comprehensive Cardiac Rehabilitation on Risk Factor Modification and Exercise Capacity in Patients With Acute Coronary Syndrome―Results From the JACR Registry. Circ. Rep. 2020, 2, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Smith, S.C.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Zhao, X.X. Value of high-density lipoprotein cholesterol, myocardial perfusion index, and global longitudinal strain derived from cardiac magnetic resonance imaging in predicting coronary slow flow in patients with nonobstructive coronary artery disease. Quant. Imaging Med. Surg. 2025, 15, 8491–8504. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Sentí, M.; Pérez-Fernández, S.; de Castro Moura, M.; Esteller, M.; Marrugat, J.; Elosua, R. DNA methylation biomarkers of myocardial infarction and cardiovascular disease. Clin. Epigenet. 2021, 13, 86. [Google Scholar] [CrossRef]
- Langseth, M.S.; Andersen, G.Ø.; Husebye, T.; Arnesen, H.; Zucknick, M.; Solheim, S.; Eritsland, J.; Seljeflot, I.; Opstad, T.B.; Helseth, R. Neutrophil extracellular trap components and myocardial recovery in post-ischemic acute heart failure. PLoS ONE 2020, 15, e0241333. [Google Scholar] [CrossRef]
- Cortigiani, L. The Stress Echo Prognostic Gender Gap. Eur. J. Echocardiogr. 2001, 2, 132–138. [Google Scholar] [CrossRef]


| MI-Group (nA = 18) | Healthy Controls (nB = 18) | Effect Size (Cohen’s d/Cramer’s V) | p-Value | |
|---|---|---|---|---|
| Age (years) | 53.22 ± 5.93 | 50.11 ± 10.77 | 0.36 d | p = 0.291 |
| BMI (kg/cm2) | 27.96 ± 2.24 | 26.59 ± 2.42 | 0.59 d | p = 0.090 |
| STEMI | ||||
| Yes | 10 (55.55%) | - | - | - |
| No | 8 (44.44%) | - | - | - |
| NSTEMI | ||||
| Yes | 8 (44.44%) | - | - | - |
| No | 10 (55.55%) | - | - | - |
| PCI | ||||
| Yes | 8 (44.44%) | |||
| No | 10 (55.55%) | |||
| CABG | ||||
| Yes | 3 (16.66%) | |||
| No | 7 (38.88%) | |||
| Smoking history | ||||
| Yes | 8 (44.44%) | 7 (39.00%) | OR = 1.26 (95% CI: 0.33–4.74), Cramer’s V = 0.06 | p = 0.744 |
| No | 10 (55.56%) | 11 (61.00%) | p = 0.823 | |
| HRrest (bpm) | 75.16 ± 12.34 | 76.27 ± 11.88 | 0.09 d | p = 0.874 |
| SBPrest (mmHg) | 119.72 ± 9.62 | 125.55 ± 9.68 | 0.58 d | p = 0.078 |
| DBPrest (mmHg) | 72.77 ± 4.91 | 75.55 ± 11.61 | 0.31 d | p = 0.651 |
| MI Group (nA = 18) | Healthy Controls (nB = 18) | Effect Size (Cohen’s d) | p-Value | |
|---|---|---|---|---|
| Exercise time (min) | 7.80 ± 1.67 | 9.57 ± 1.87 | 0.79 | p = 0.002 |
| VO2peak (mL/kg/min) | 29.20 ± 5.74 | 41.92 ± 13.22 | 0.98 | p = 0.0006 |
| VE/VCO2 | 34.11 ± 4.10 | 30.33 ± 6.83 | 0.67 | p = 0.114 |
| RERmax | 1.15 ± 0.16 | 1.22 ± 0.22 | 0.36 | p = 0.295 |
| HRmax (bpm) | 149.33 ± 18.99 | 162.77 ± 17.99 | 0.73 | p = 0.036 |
| SBPmax (mmHg) | 155.27 ± 15.76 | 165.27 ± 17.69 | 0.60 | p = 0.082 |
| DBPmax (mmHg) | 72.77 ± 6.90 | 75.00 ± 10.71 | 0.25 | p = 0.772 |
| MI Group (nA = 18) | Healthy Controls (nB = 18) | Effect Size (Cohen’s d) | p-Value | |
|---|---|---|---|---|
| LVEF rest (%) | 50.61 ± 6.51 | 60.72 ± 4.33 | 0.85 | p < 0.001 |
| LVEF peak (%) | 55.33 ± 8.87 | 67.44 ± 4.17 | 0.88 | p < 0.001 |
| GLS rest (%) | −14.70 ± 3.81 | −20.22 ± 1.45 | 0.72 | p = 0.003 |
| GLS peak (%) | −15.78 ± 3.61 | −22.72 ± 1.71 | 0.76 | p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afthonidis, N.L.; Michou, V.; Anyfanti, M.; Dalkiranis, A.; Panayiotou, G.; Koutlianos, N.; Kouidi, E.; Deligiannis, A. Advanced Stress Echocardiography with Cardiopulmonary Exercise Testing After Myocardial Infarction. J. Funct. Morphol. Kinesiol. 2025, 10, 393. https://doi.org/10.3390/jfmk10040393
Afthonidis NL, Michou V, Anyfanti M, Dalkiranis A, Panayiotou G, Koutlianos N, Kouidi E, Deligiannis A. Advanced Stress Echocardiography with Cardiopulmonary Exercise Testing After Myocardial Infarction. Journal of Functional Morphology and Kinesiology. 2025; 10(4):393. https://doi.org/10.3390/jfmk10040393
Chicago/Turabian StyleAfthonidis, Nektarios Lampros, Vasiliki Michou, Maria Anyfanti, Anastasios Dalkiranis, George Panayiotou, Nikolaos Koutlianos, Evangelia Kouidi, and Asterios Deligiannis. 2025. "Advanced Stress Echocardiography with Cardiopulmonary Exercise Testing After Myocardial Infarction" Journal of Functional Morphology and Kinesiology 10, no. 4: 393. https://doi.org/10.3390/jfmk10040393
APA StyleAfthonidis, N. L., Michou, V., Anyfanti, M., Dalkiranis, A., Panayiotou, G., Koutlianos, N., Kouidi, E., & Deligiannis, A. (2025). Advanced Stress Echocardiography with Cardiopulmonary Exercise Testing After Myocardial Infarction. Journal of Functional Morphology and Kinesiology, 10(4), 393. https://doi.org/10.3390/jfmk10040393









