Fifteen-Year Follow-Up of Nanos Neck-Preserving Hip Arthroplasty: An Observational Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Eligibility Criteria
2.3. Description of the Experimental Stage
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
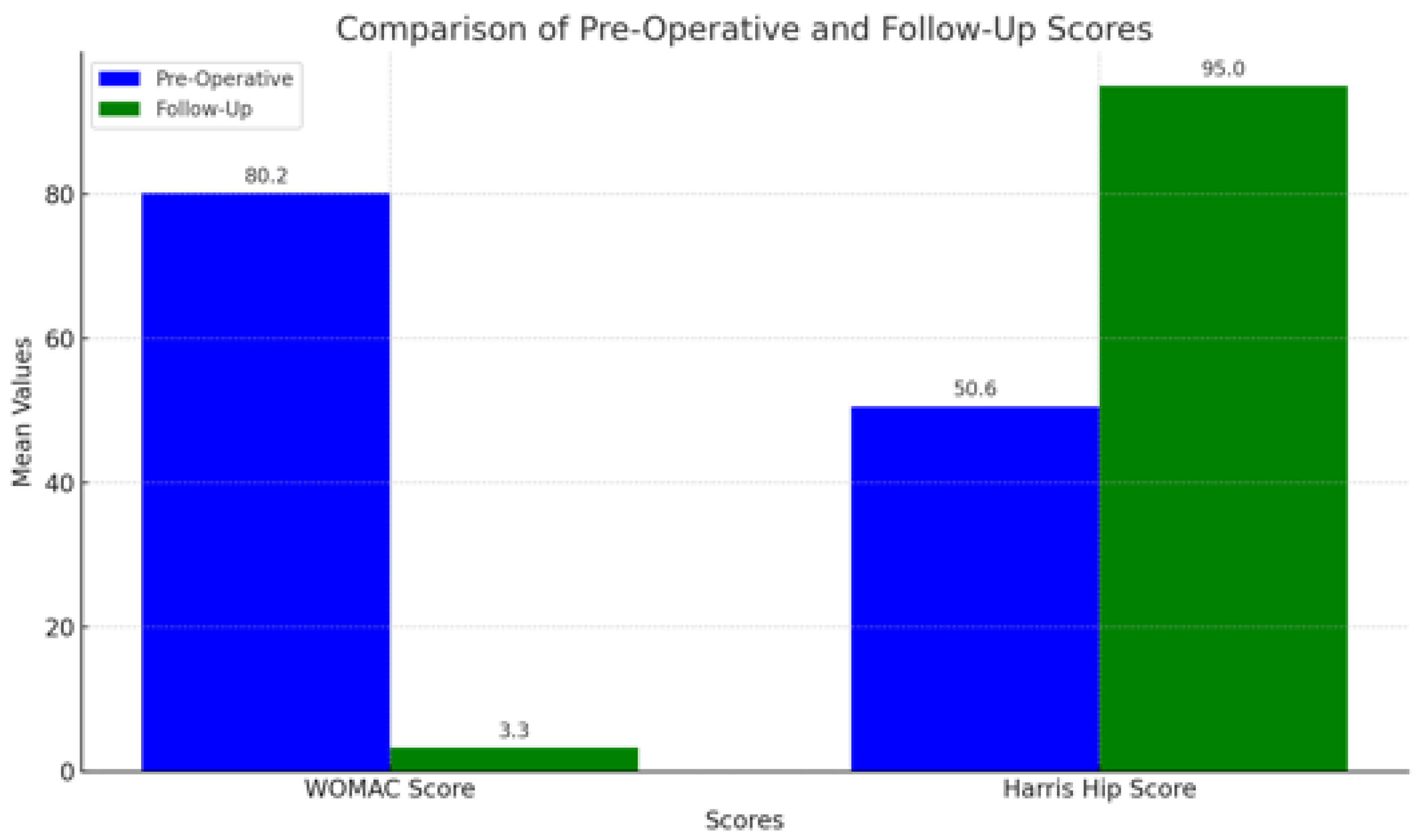
3.2. Clinical Outcomes
- HHS: Mean score of 95, indicating excellent recovery, significant functional improvement, and minimal pain.
- WOMAC: Mean score of 3.3, suggesting minimal joint pain, stiffness, and functional limitation.
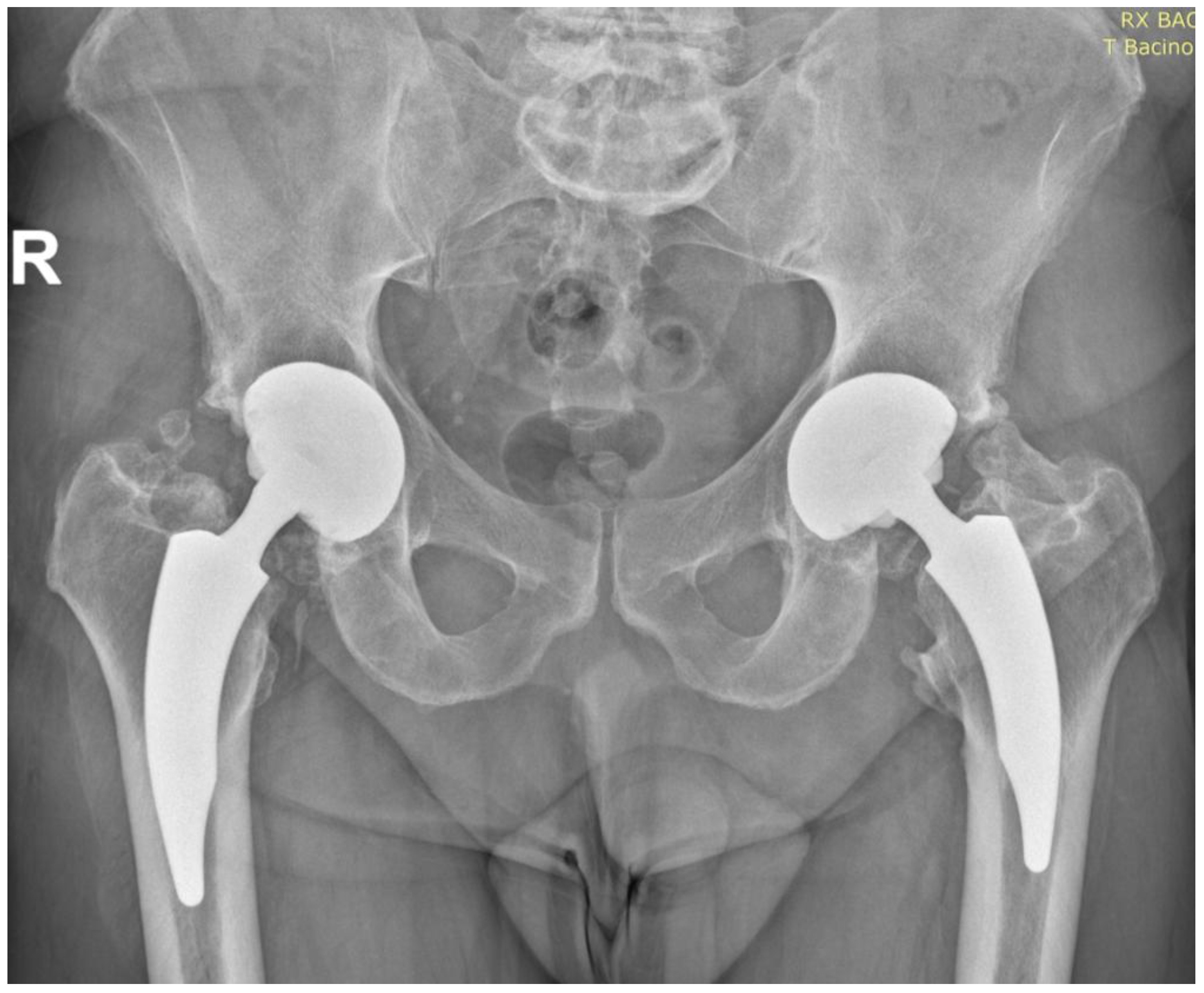
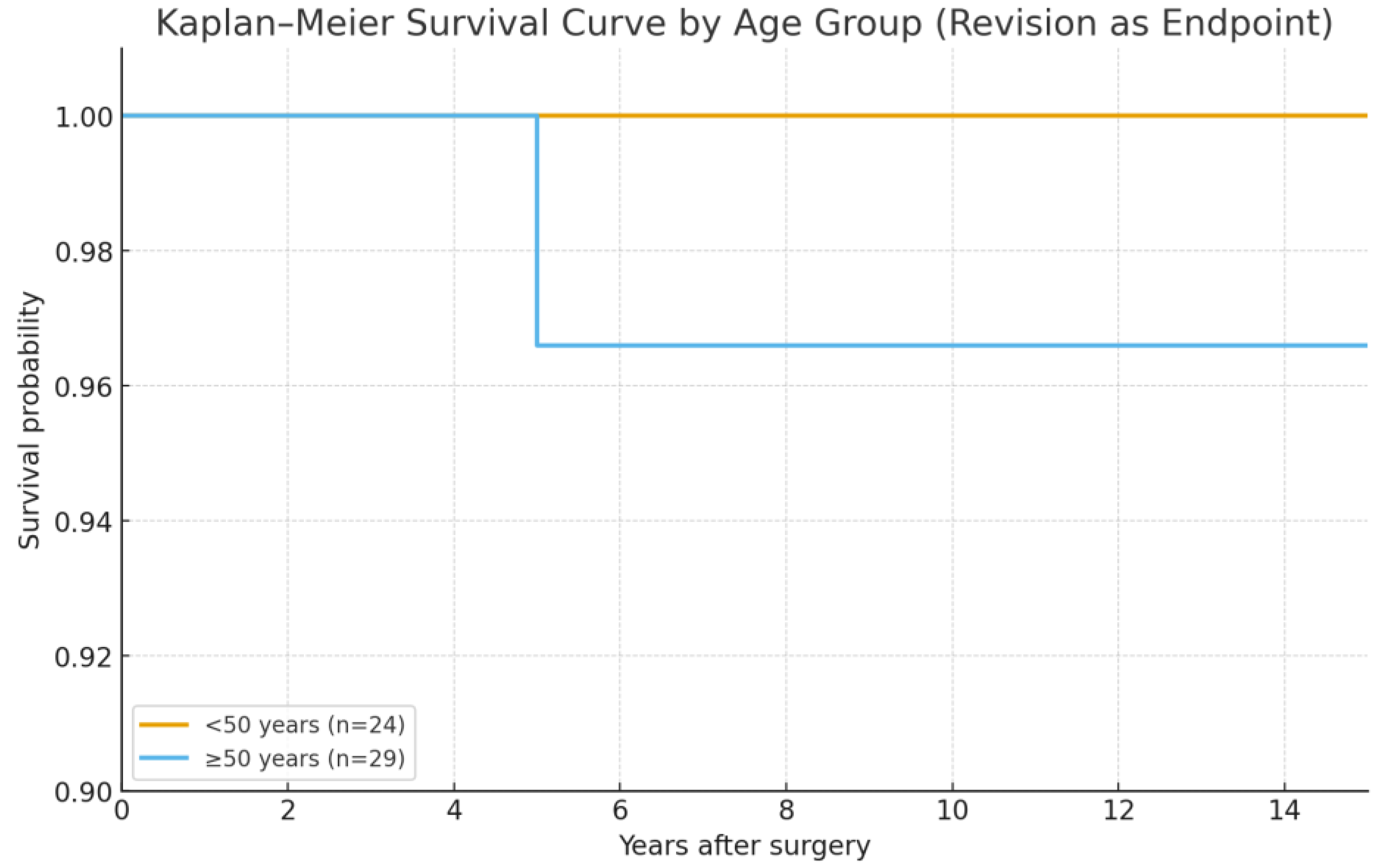
3.3. Radiographic Outcomes
- Acetabular Cup Position
- Implant Stability
- Heterotopic Ossifications (Figure 4)
3.4. Postoperative Complications
- Dislocation: Two patients experienced postoperative dislocation during bed transfers. Both patients were obese, and the dislocations occurred early in the recovery period. The dislocations were successfully managed with closed reduction, and no further dislocations were reported.
- Cortical perforation: One patient experienced perforation of the lateral femoral cortex during stem implantation. This issue went undetected initially, as the immediate postoperative X-rays were performed only in the AP view, without a lateral projection. The patient, who resided abroad, returned for follow-up after three months. Upon detection of the perforation, the patient underwent a successful revision surgery with the implantation of a Polarstem (Smith & Nephew) primary femoral stem. The postoperative complications and their corresponding details are summarized in Table 6.
4. Discussion
4.1. Advantages of Femoral Neck Preservation
4.2. Comparison with Traditional Stems
4.3. Application in Younger Patients
4.4. Surgical Technique Precision
4.5. Limitations and Bias
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THA | Total Hip Arthroplasty |
| HHS | Harris Hip Score |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
| ROM | Range of Motion |
| LLD | Leg Length Discrepancy |
| DDH | Developmental Dysplasia of the Hip |
| IRB | Institutional Review Board |
| RCT | Randomized Controlled Trial |
| SD | Standard Deviation |
| CI | Confidence Interval |
References
- Pipino, F.; Keller, A. Tissue-sparing surgery: 25 years’ experience with femoral neck-preserving hip arthroplasty. J. Orthop. Traumatol. 2006, 7, 36–41. [Google Scholar] [CrossRef]
- Capone, A.; Bienati, F.; Torchia, S.; Podda, D.; Marongiu, G. Long-term follow-up of short-stem hip arthroplasty. J. Orthop. Surg. Res. 2021, 16, 1–10. [Google Scholar]
- Bieger, R.; Kappe, T.; Wirtz, D.C. Short-stem implants in total hip arthroplasty: Biomechanics, outcomes, and potential advantages. Int. Orthop. 2020, 44, 377–389. [Google Scholar]
- Grappiolo, G.; Sancineto, C.F.; Tovaglia, V.; Cervellati, S.; Biondi, F.; Bosi, G. Periprosthetic bone preservation in short-stem hip arthroplasty. Bone Jt. J. 2019, 101-B, 897–905. [Google Scholar]
- Müller, M.; Hug, R.; Zingg, P.O. Radiographic and functional outcomes of femoral neck-preserving short-stem implants. J. Arthroplast. 2022, 37, 1154–1162. [Google Scholar]
- Ghera, S.; Pavan, L. Short stems in total hip replacement: Current concepts and clinical outcomes. Acta Biomed. 2019, 90 (Suppl. S1), 43–49. [Google Scholar]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. Br. 1987, 69, 45–55. [Google Scholar] [CrossRef]
- Slullitel, P.A.; Della Valle, A.G.; Vestri, R. Learning curve in short-stem total hip arthroplasty: A systematic review of the literature. J. Arthroplast. 2020, 35, 783–789. [Google Scholar]
- Nawabi, D.H.; Nam, D.; Cross, M.B. Short-stem implants in total hip arthroplasty: The journey so far. J. Bone Jt. Surg. Am. 2020, 102, 167–175. [Google Scholar]
- Banerjee, S.; Keshavamurthy, R.; Ramesh, C. Short-stem total hip arthroplasty in younger patients: A review of the literature. Acta Orthop. 2022, 93, 221–230. [Google Scholar]
- Santori, F.S.; Santori, N. Mid-term results of a neck-preserving total hip arthroplasty: A single surgeon experience. Hip Int. 2017, 27, 417–425. [Google Scholar]
- Capone, A.; Bloise, R.; Familiari, F. The role of short-stem implants in young patients with avascular necrosis of the femoral head. Hip Int. 2019, 29 (Suppl. S1), 10–18. [Google Scholar]
- Bieger, R.; Kappe, T.; Puhl, W. Femoral offset restoration in short-stem hip arthroplasty: Does neck preservation really matter? Orthop. Rev. 2021, 13, 146–153. [Google Scholar]
- Eftekhary, N.; Niemeier, R.J.; Connelly, J.W. Restoring femoral offset and leg length in total hip arthroplasty: A review. J. Arthroplast. 2022, 37, 1406–1413. [Google Scholar]
- Mont, M.A.; Carbone, J.J.; Fairbank, A.C. Short-stem hip implants: An analysis of clinical and functional outcomes. Clin. Orthop. Relat. Res. 2021, 479, 55–62. [Google Scholar]
- Pipino, F.; Calderale, P.M. A conservative hip prosthesis: Further developments. J. Orthop. Traumatol. 1996, 2, 55–68. [Google Scholar]
- Grappiolo, G.; Tovaglia, V.; Della Rocca, F. Femoral neck-preserving short-stem prosthesis: Design, biomechanical concepts, and clinical outcomes. Orthop. Clin. N. Am. 2021, 52, 161–178. [Google Scholar]
- Rüdiger, H.A.; Burgi, M.L.; Zingg, P.O. The role of the femoral neck in modern total hip replacement. Bone Jt. Res. 2019, 8, 496–504. [Google Scholar]
- Goodman, S.B.; Konttinen, Y.T. Revision strategies for short-stem implants: Clinical and biomechanical perspectives. J. Orthop. Res. 2020, 38, 1882–1891. [Google Scholar]
- Patel, A.; Duncan, S.; Lall, A.C. Fracture risk in neck-preserving hip implants: A review of 20 years of data. J. Bone Jt. Surg. Am. 2020, 102, 36–44. [Google Scholar]
- Muir, B.; Al Attar, A.; Abu-Rajab, R. Computer-assisted navigation in total hip replacement surgery: A review of the evidence. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 587–598. [Google Scholar]
- Falez, F.; Casella, F.; Papalia, M. Current concepts, classification, and results in short stem hip arthroplasty. Orthopedics 2015, 38 (Suppl. 3), S6–S13. [Google Scholar] [CrossRef]
- Mont, M.A.; Barrack, R.L.; Parvizi, J. New horizons in hip replacement: Perspectives for the future. J. Arthroplast. 2022, 37, 1593–1601. [Google Scholar]
- Pipino, F.; Calderale, P.M. Conservative hip implants: Initial experiences and preliminary results. Orthopedics 1994, 17, 877–885. [Google Scholar]
- Bieger, R.; Heimkes, B.; Engh, C.A. Advances in femoral neck-preserving implants: Design evolution and clinical outcomes. Int. J. Orthop. Sci. 2022, 48, 212–229. [Google Scholar]
- Eftekhary, N.; Connelly, J.W.; Malchau, H. Hip biomechanics and implant selection: Contemporary considerations in total hip arthroplasty. Bone Jt. J. 2021, 103-B, 1012–1021. [Google Scholar]
- Mont, M.A.; Schmalzried, T.P.; Frondoza, C.; Hungerford, D.S. Total hip replacement in younger patients: Biomechanical perspectives on bone preservation. J. Bone Jt. Surg. Am. 2021, 103, 214–222. [Google Scholar]
- Brooker, A.F.; Bowerman, J.W.; Robinson, R.A. Ectopic ossification following hip replacement. J. Bone Jt. Surg. Am. 1973, 55, 1629–1632. [Google Scholar] [CrossRef]
- Santori, F.S.; Manfreda, F.; Maggioni, A. Hip resurfacing and short-stem prostheses in young patients: Are they comparable? Acta Orthop. Belg. 2020, 86, 572–583. [Google Scholar]
- Hailer, N.P.; Weiss, R.J.; Stark, A. Ten-year outcomes of short-stem hip implants in the Swedish Hip Arthroplasty Register. Acta Orthop. 2022, 93, 47–55. [Google Scholar]
- Müller, M.; Spross, C.; Zingg, P.O. Cementless short-stem hip arthroplasty in young patients: Long-term outcomes. Clin. Orthop. Relat. Res. 2022, 480, 1151–1161. [Google Scholar]
- Piakong, P.; Pahl, M.; Delgado, G.; Akkaya, M.; Busch, S.M.; Salber, J.; Gehrke, T.; Citak, M. Twenty-year results of a neck-preserving short-stem prosthesis in primary total hip arthroplasty. Arch. Orthop. Trauma Surg. 2023, 143, 3481–3486. [Google Scholar] [CrossRef] [PubMed]
- Van Oldenrijk, J.; Schop, S.; Bos, P.K. Bone stock preservation with neck-preserving femoral stems: Systematic review and meta-analysis. J. Arthroplast. 2020, 35, 2822–2830. [Google Scholar]
- Ghera, S.; Beraudi, A.; Pavan, L. Osseointegration in short-stem hip implants: Role of the femoral neck in force transmission. Int Orthop. 2021, 45, 1532–1543. [Google Scholar]
- Nawabi, D.H.; Cross, M.B.; Su, E.P. Navigated short-stem total hip arthroplasty: Outcomes and surgeon learning curve. J. Bone Jt. Surg. Am. 2022, 104, 119–128. [Google Scholar]
- Falez, F.; Favetti, F.; Casella, F.; Panegrossi, G.; Barresi, C. Navigated short-stem total hip arthroplasty. Orthop. Clin. N. Am. 2021, 52, 355–369. [Google Scholar]
- Morrey, B.F.; Dobbs, R.E.; Bryan, R.S. Periprosthetic fractures in total hip arthroplasty: Management and results. Bone Jt. J. 1986, 68-B, 400–407. [Google Scholar]
- Pongsiri, S.; Hossain, M.; Scammell, B.E. Long-term outcomes of short-stem hip arthroplasty in younger patients. Bone Jt. J. 2020, 102-B, 840–847. [Google Scholar]
- Fink, B.; Oremek, D.; Singer, J. Short-stem hip arthroplasty: Preservation of bonestock and mid-term outcomes. J. Arthroplast. 2018, 33, 627–632. [Google Scholar]
- Mont, M.A.; Ragland, P.S.; Etienne, G. Hip resurfacing arthroplasty: Long-term clinical and radiographic outcomes. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S3), 98–103. [Google Scholar]
- Steinbrück, A.; Grimberg, A.W.; Elliott, J.; Melsheimer, O.; Jansson, V. Short versus conventional stem in cementless total hip arthroplasty: An evidence-based approach with registry data of mid-term survival. Orthopade 2021, 50, 296–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz-Dilernia, F.; Lucero, C.; Slullitel, P.A.; Zanotti, G.; Comba, F.; Piccaluga, F.; Buttaro, M. Medium-term outcomes of conventional versus short uncemented femoral stems for primary total hip arthroplasty in patients younger than 55 years. Hip Int. 2024, 34, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Van Veghel, M.H.W.; Hannink, G.; Van Oldenrijk, J.; Van Steenbergen, L.N.; Schreurs, B.W. A comparison of uncemented short versus standard stem length in total hip arthroplasty: Results from the Dutch Arthroplasty Register. Acta Orthop. 2023, 7, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, J.W.; Kim, J.S. Ultrashort versus Conventional Anatomic Cementless Femoral Stems in the Same Patients Younger Than 55 Years. Clin. Orthop. Relat. Res. 2016, 474, 2008–2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muir, D.C.; Christie, M.J.; Whittingham-Jones, P. The role of navigation and robotics in achieving accuracy in total hip arthroplasty. Bone Jt. J. 2020, 102-B, 199–207. [Google Scholar]
- Wilson, C.; O’Malley, J.; Langley, J. Surgeon expertise and outcomes in short-stem hip arthroplasty: A retrospective analysis. Clin. Orthop. Relat. Res. 2020, 478, 385–394. [Google Scholar]
- Dorr, L.D.; Malik, A.; Wan, Z. Precision and outcomes with navigation-assisted hip arthroplasty. J. Bone Jt. Surg. Am. 2018, 100, 613–623. [Google Scholar]
- Lucero, C.M.; Slullitel, P.A.; Díaz-Dilernia, F.; Zanotti, G.; Comba, F.; Buttaro, M. Short Femoral Stems for Primary Total Hip Arthroplasty in Young Patients: Chanical Outcomes. Rev. Asoc. Argent Ortop. Traumatol. 2023, 88, 224–251. [Google Scholar] [CrossRef]
- Mauch, M.; Brecht, H.; Clauss, M.; Stoffel, K. Use of Short Stems in Revision Total Hip Arthroplasty: A Retrospective Observational Study of 31 Patients. Medicina 2023, 59, 1822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stauss, R.; Becker, N.T.; Savov, P.; Ettinger, M.; Seeber, G.H. Analysis of Postoperative Complication and Revision Rates and Mid- to Long-Term Implant Survival in Primary Short-Stem Total Hip Arthroplasty. J. Clin. Med. 2024, 13, 3779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhen, P.; Chang, Y.; Yue, H.; Chen, H.; Zhou, S.; Liu, J.; He, X. Primary total hip arthroplasty using a short bone-conserving stem in young adult osteoporotic patients with Dorr type C femoral bone. J. Orthop. Surg. Res. 2021, 16, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Etiology | |
|---|---|
| Primary osteoarthritis | 95% |
| Avascular necrosis | 5% |
| Development dysplasia | Included in 5% |
| Acetabular fracture sequelae | Included in 5% |
| Femoral Head Size | |
|---|---|
| 28 mm | 2% |
| 32 mm | 35% |
| 36 mm | 60% |
| 46 mm | 3% |
| Clinical Parameters | Preoperative (Mean) | Postoperative (Mean) |
|---|---|---|
| HHS | 50.6 | 95 |
| WOMAC Score | 80.2 | 3.3 |
| Leg Length Discrepancy | - | 2.7 mm |
| ROM | Limited | Full |
| Squeaking | Not assessed | 3 patients (5.6%) |
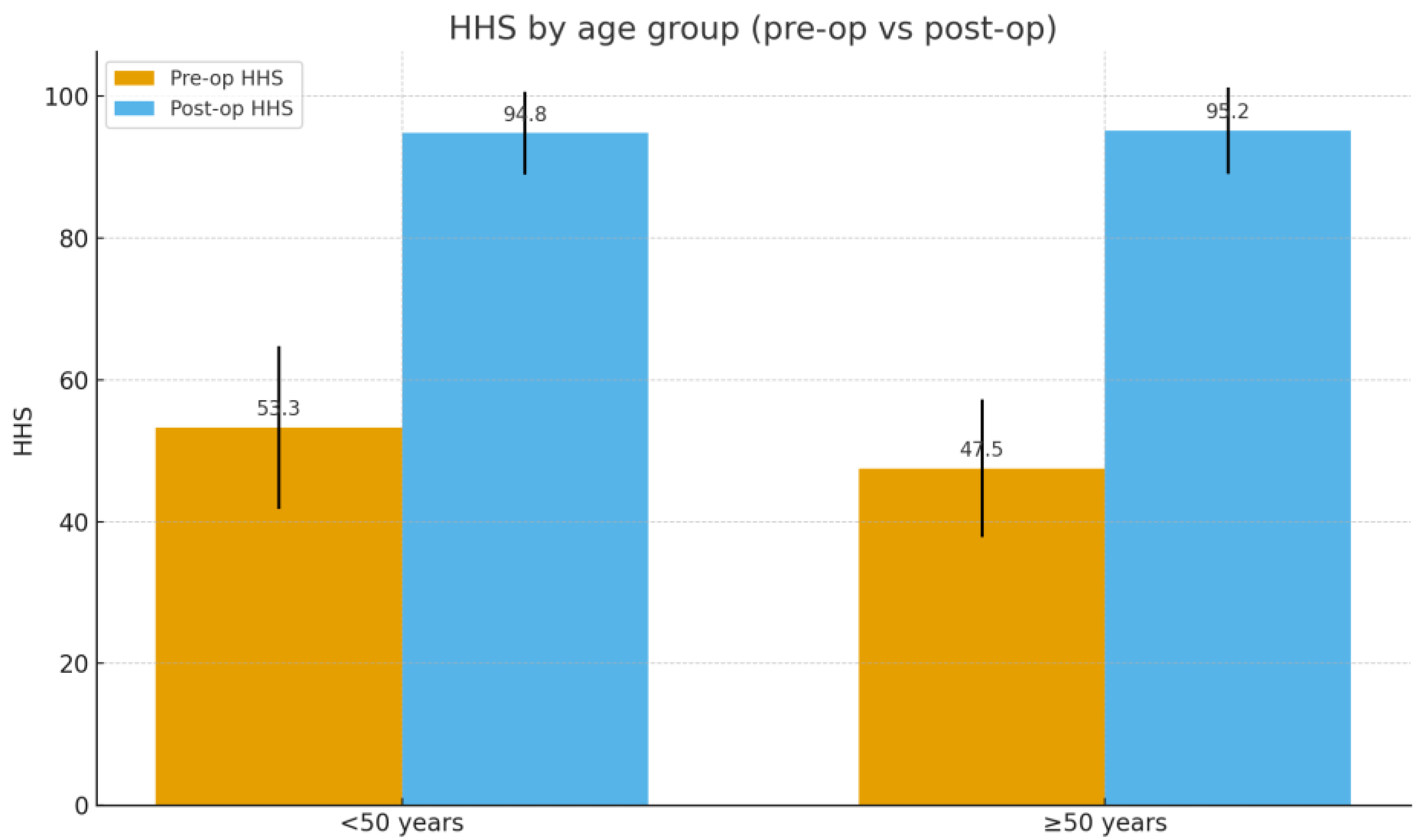
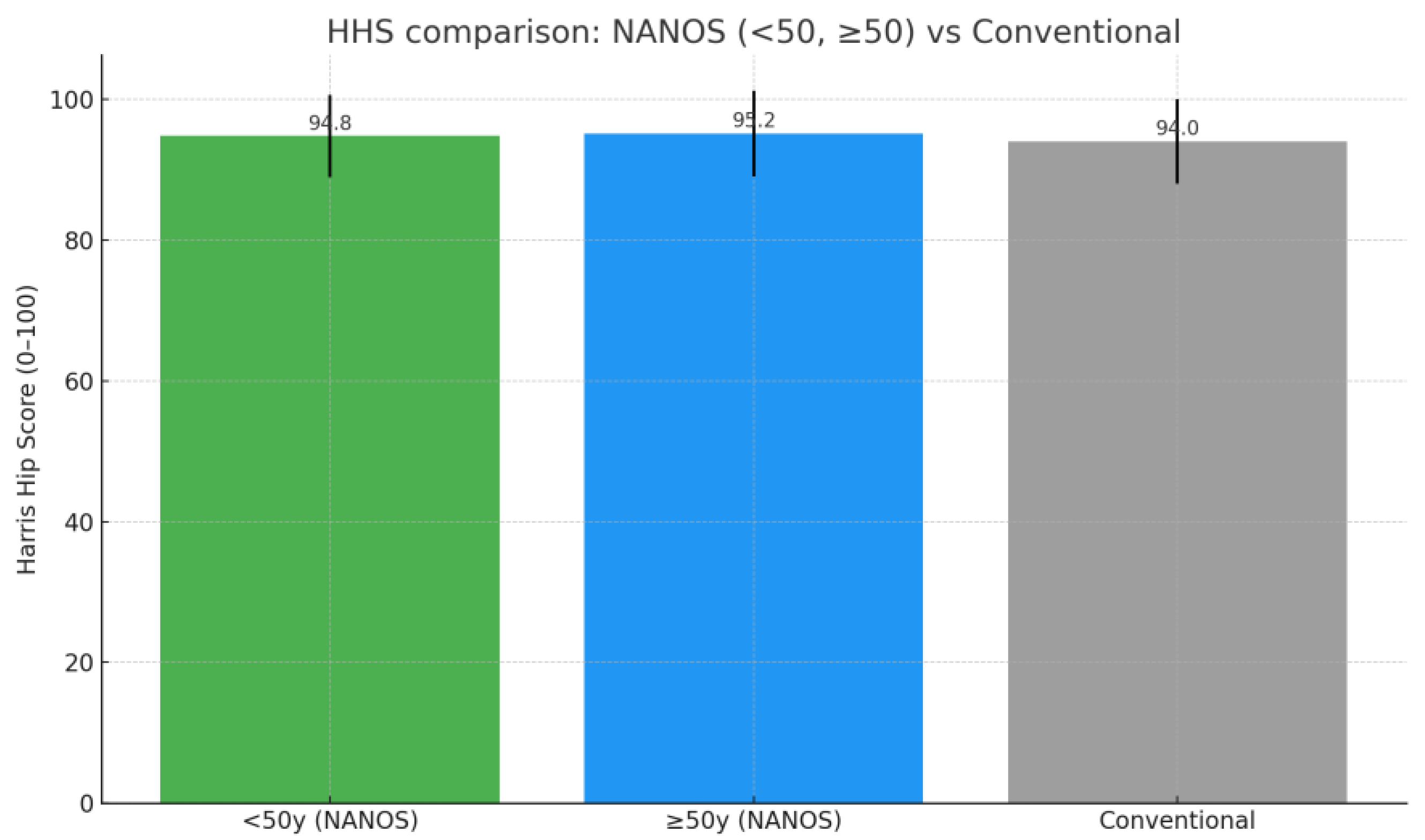
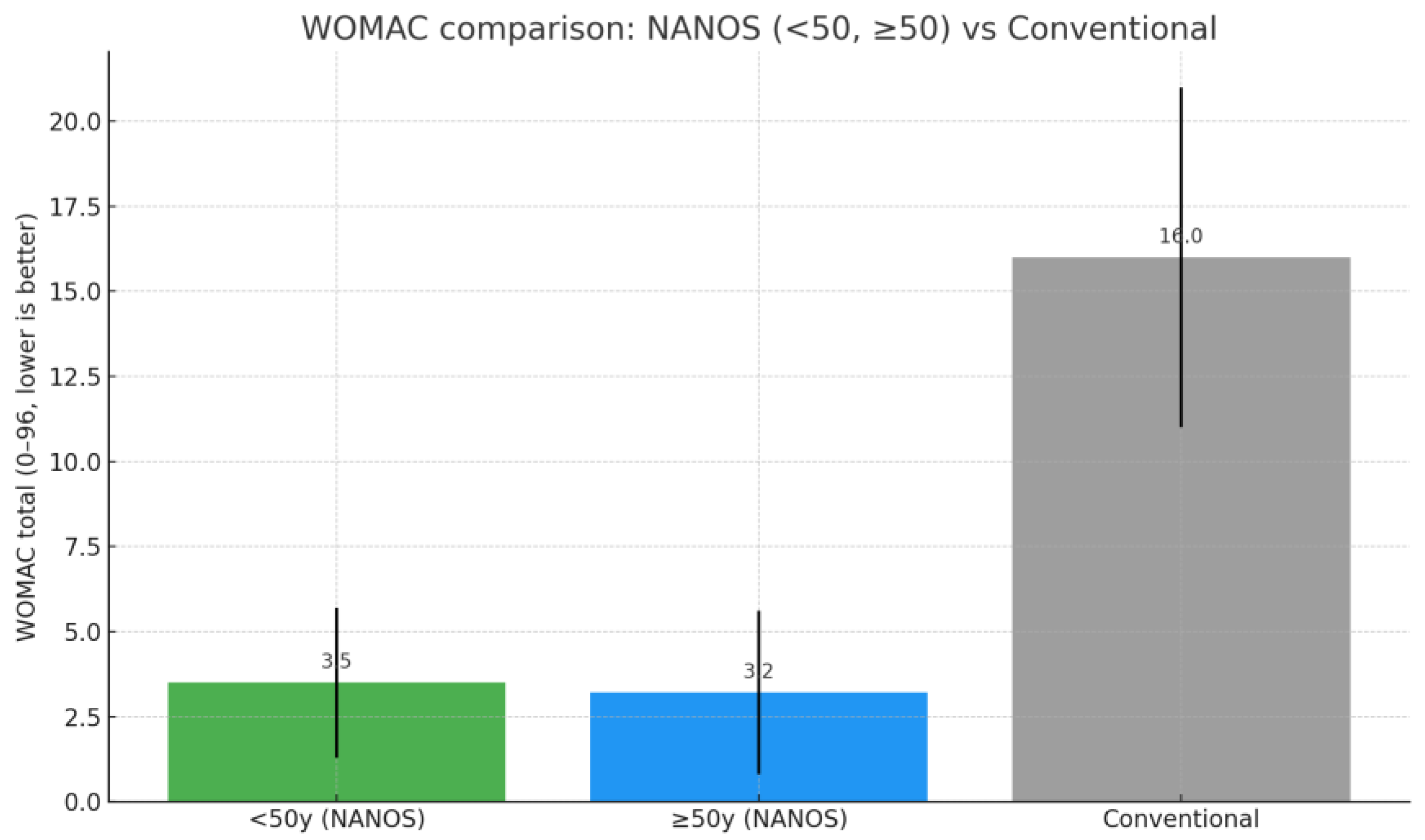
| Age Group | n | Preoperative HHS (Mean ± SD) | Postoperative HHS (Mean ± SD) | Preoperative WOMAC (Mean ± SD) | Postperative WOMAC (Mean ± SD) |
|---|---|---|---|---|---|
| <50 yrs | 24 | 53.3 ± 11.5 | 94.8 ± 5.8 | 79.9 ± 14.9 | 3.5 ± 2.2 |
| >50 yrs | 29 | 47.5 ± 9.7 | 95.2 ± 6.1 | 81.5 ± 14.0 | 3.2 ± 2.4 |
| Radiographic Parameters | Value |
|---|---|
| Acetabular inclination | 44.6° |
| Leg Length Discrepancy | 2.7 mm |
| Femoral offset | 46 mm |
| Osteolysis (Gruen zones). | None |
| Heterotopic Ossification (Grade I–II) | 5 patients (9.4%) |
| Heterotopic Ossification (Grade III–IV) | 2 patients (3.8%) |
| Type of Complication | Cases | Details |
|---|---|---|
| Dislocation. | 2 | Occurred during transfers in obese patients; managed with closed reduction |
| Cortical perforation. | 1 | Lateral femoral cortex perforation; patient revised with Polarstem Smith&Nephew |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovere, G.; Luziatelli, D.; Luziatelli, S.; Polce, G.; Pirri, P.; De Luna, V.; Liuzza, F.; Farsetti, P.; De Maio, F. Fifteen-Year Follow-Up of Nanos Neck-Preserving Hip Arthroplasty: An Observational Retrospective Study. J. Funct. Morphol. Kinesiol. 2025, 10, 389. https://doi.org/10.3390/jfmk10040389
Rovere G, Luziatelli D, Luziatelli S, Polce G, Pirri P, De Luna V, Liuzza F, Farsetti P, De Maio F. Fifteen-Year Follow-Up of Nanos Neck-Preserving Hip Arthroplasty: An Observational Retrospective Study. Journal of Functional Morphology and Kinesiology. 2025; 10(4):389. https://doi.org/10.3390/jfmk10040389
Chicago/Turabian StyleRovere, Giuseppe, Davide Luziatelli, Sandro Luziatelli, Gianluca Polce, Pierfrancesco Pirri, Vincenzo De Luna, Francesco Liuzza, Pasquale Farsetti, and Fernando De Maio. 2025. "Fifteen-Year Follow-Up of Nanos Neck-Preserving Hip Arthroplasty: An Observational Retrospective Study" Journal of Functional Morphology and Kinesiology 10, no. 4: 389. https://doi.org/10.3390/jfmk10040389
APA StyleRovere, G., Luziatelli, D., Luziatelli, S., Polce, G., Pirri, P., De Luna, V., Liuzza, F., Farsetti, P., & De Maio, F. (2025). Fifteen-Year Follow-Up of Nanos Neck-Preserving Hip Arthroplasty: An Observational Retrospective Study. Journal of Functional Morphology and Kinesiology, 10(4), 389. https://doi.org/10.3390/jfmk10040389






