Dual-Task Interference Increases Variability in Sub-Second Repetitive Motor Timing
Abstract
1. Introduction
Automatic vs. Cognitive Timing
2. Materials and Methods
2.1. Participants
2.2. Tasks and the Procedure
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Descriptives
3.2. Linear Mixed-Effects Models
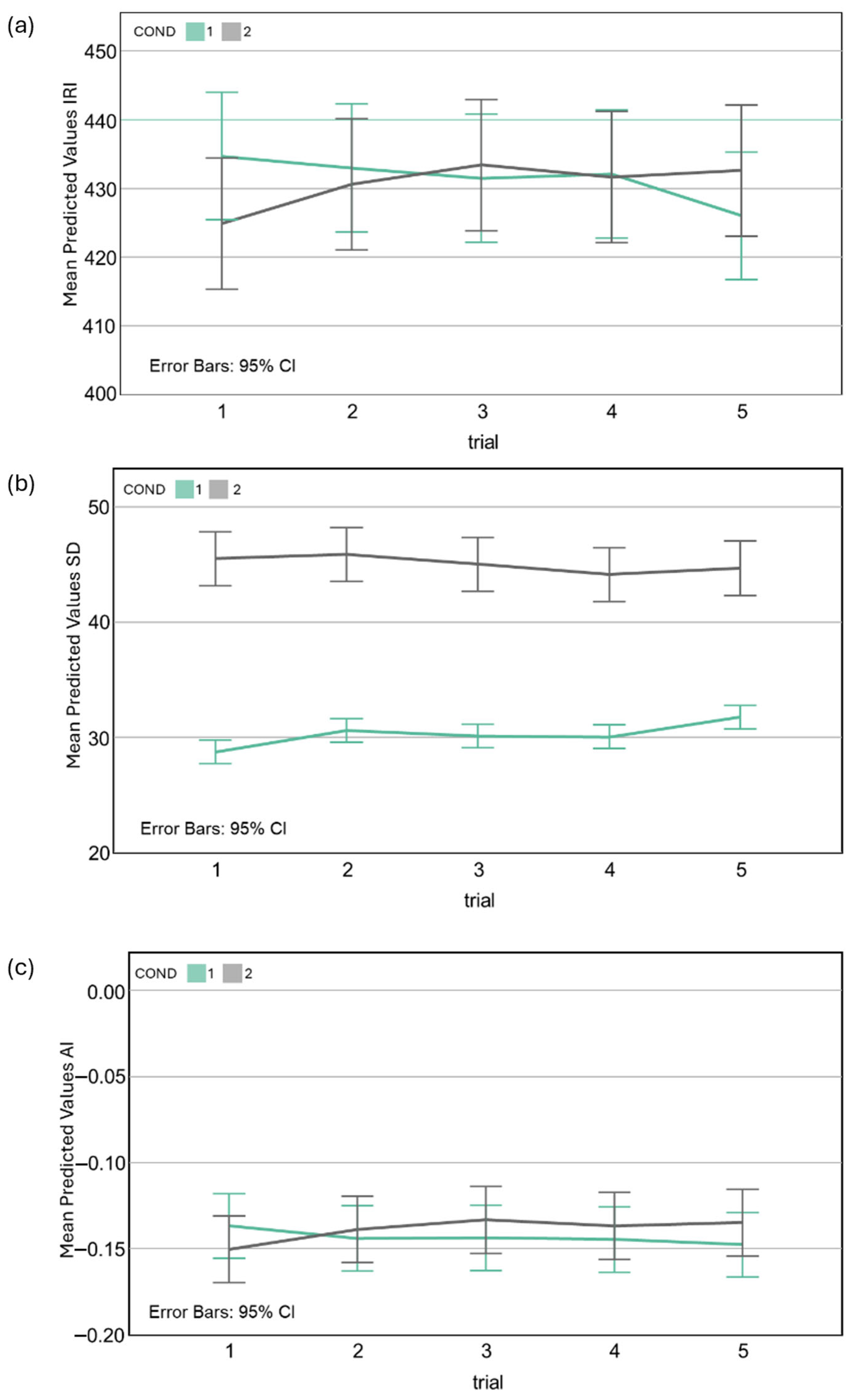
3.2.1. Interresponse Interval Duration (IRI)
3.2.2. Temporal Variability (Standard Deviation of IRIs)
3.2.3. Accuracy of Motor Timing (AI)
4. Discussion
4.1. The Negative Mean Asynchrony
4.2. Dual-Task Effects on Timing Consistency and Variability
4.3. Theoretical Integration and Practical Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IRI | inter-response intervals |
| AI | index of accuracy |
| LMM | linear mixed-effects model |
| RAS | rhythmic auditory stimulation |
| NMA | negative mean asynchrony |
| SMT | spontaneous motor tempo |
References
- Paton, J.J.; Buonomano, D.V. The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 2018, 98, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Merchant, H.; Lafuente, V. Introduction to the neurobiology of interval timing. In Neurobiology of Interval Timing; Merchant, H., Lafuente, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Wulf, G.; Lewthwaite, R. Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon. Bull. Rev. 2016, 23, 1382–1414. [Google Scholar] [CrossRef]
- Merchant, H.; Zarco, W.; Pérez, O.; Prado, L.; Bartolo, R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc. Natl. Acad. Sci. USA 2011, 108, 19784–19789. [Google Scholar] [CrossRef]
- Repp, B.H. Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef]
- Thaut, M.; Trimarchi, P.; Parsons, L. Human Brain Basis of Musical Rhythm Perception: Common and Distinct Neural Substrates for Meter, Tempo, and Pattern. Brain Sci. 2014, 4, 428–452. [Google Scholar] [CrossRef]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance Requires Hard Mental Work and Is Stressful. Hum. Factors J. Hum. Factors Ergon. Soc. 2008, 50, 433–441. [Google Scholar] [CrossRef]
- Wing, A.M.; Kristofferson, A.B. The timing of interresponse intervals. Percept. Psychophys. 1973, 13, 455–460. [Google Scholar] [CrossRef]
- Madison, G.; Karampela, O.; Ullén, F.; Holm, L. Effects of practice on variability in an isochronous serial interval production task: Asymptotical levels of tapping variability after training are similar to those of musicians. Acta Psychol. 2013, 143, 119–128. [Google Scholar] [CrossRef]
- Bobin-Begue, A.; Droit-Volet, S.; Provasi, J. Young children’s difficulties in switching from rhythm production to temporal interval production (>1 s). Front. Psychol. 2014, 5, 1346. [Google Scholar]
- Mioni, G.; Mattalia, G.; Stablum, F. Time perception in severe traumatic brain injury patients: A study comparing different methodologies. Brain Cogn. 2013, 81, 305–312. [Google Scholar] [CrossRef]
- Lewis, P.A.; Miall, R.C. A right hemispheric prefrontal system for cognitive time measurement. Behav. Process. 2006, 71, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.A.; Miall, R.C. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Curr. Opin. Neurobiol. 2003, 13, 250–255. [Google Scholar] [CrossRef]
- Karmarkar, U.R.; Buonomano, D.V. Timing in the Absence of Clocks: Encoding Time in Neural Network States. Neuron 2007, 53, 427–438. [Google Scholar] [CrossRef]
- Rammsayer, T.H.; Troche, S.J. In search of the internal structure of the processes underlying interval timing in the sub-second and the second range: A confirmatory factor analysis approach. Acta Psychol. 2014, 147, 68–74. [Google Scholar] [CrossRef]
- Herbst, S.K.; Obleser, J.; van Wassenhove, V. Implicit Versus Explicit Timing—Separate or Shared Mechanisms? J. Cogn. Neurosci. 2022, 34, 1447–1466. [Google Scholar] [CrossRef] [PubMed]
- Bareš, M.; Apps, R.; Avanzino, L.; Breska, A.; D’Angelo, E.; Filip, P.; Gerwig, M.; Ivry, R.B.; Lawrenson, C.L.; Louis, E.D.; et al. Consensus paper: Decoding the Contributions of the Cerebellum as a Time Machine. From Neurons to Clinical Applications. Cerebellum 2019, 18, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.M.; Dalal, S.S. Detection of Threshold-Level Stimuli Modulated by Temporal Predictions of the Cerebellum. eNeuro 2024, 11, ENEURO.0070-24.2024. [Google Scholar] [CrossRef]
- Brown, S.W. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Percept. Psychophys. 1997, 59, 1118–1140. [Google Scholar] [CrossRef]
- Brown, S.W. Timing and executive function: Bidirectional interference between concurrent temporal production and randomization tasks. Mem. Cognit. 2006, 34, 1464–1471. [Google Scholar] [CrossRef]
- Fortin, C.; Rousseau, R.; Bourque, P.; Kirouac, E. Time estimation and concurrent nontemporal processing: Specific interference from short-term-memory demands. Percept. Psychophys. 1993, 53, 536–548. Erratum in 1993, 53, 703. [Google Scholar] [CrossRef]
- Langsdorf, L.; Goehringer, F.; Schween, R.; Schenk, T.; Hegele, M. Additional cognitive load decreases performance but not adaptation to a visuomotor transformation. Acta Psychol. 2022, 226, 103586. [Google Scholar] [CrossRef] [PubMed]
- Kee, D.W.; Morris, K.; Bathurst, K.; Hellige, J.B. Lateralized interference in finger tapping: Comparisons of rate and variability measures under speed and consistency tapping instructions. Brain Cogn. 1986, 5, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Karampela, O.; Ullén, F.; Madison, G. Executive control and working memory are involved in sub-second repetitive motor timing. Exp. Brain Res. 2017, 235, 787–798. [Google Scholar] [CrossRef]
- Mudarris, M.A.; Krijt, R.N.; Hassell, A.M.; Murphy, T.M.; Ruitenberg, M.F.; Fokkema, M.; Schaefer, R.S. Cognitive and motor abilities predict auditory-cued finger tapping in a dual task. Front. Neurosci. 2025, 19, 1553548. [Google Scholar] [CrossRef]
- Xu, Z.; Ren, Y.; Misaki, Y.; Wu, Q.; Lu, S. Effect of Tempo on Temporal Expectation Driven by Rhythms in Dual-Task Performance. Front. Psychol. 2021, 12, 755490. [Google Scholar] [CrossRef]
- Holm, L.; Ullén, F.; Madison, G. Motor and executive control in repetitive timing of brief intervals. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 365–380. [Google Scholar] [CrossRef]
- Michon, J.A. Tapping regularity as a measure of perceptual motor load. Ergonomics 1966, 9, 401–412. [Google Scholar] [CrossRef]
- Nagasaki, H. Rhythm in periodic tapping is centrally produced. Percept. Mot. Skills 1990, 71, 985. [Google Scholar] [CrossRef]
- Pashler, H.; O’Brien, S. Dual-task interference and the cerebral hemispheres. J. Exp. Psychol. Hum. Percept. Perform. 1993, 19, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Zapién, A.L.; Velázquez-López, D.; Velázquez-Martínez, D.N. Attentional Mechanisms during the Performance of a Subsecond Timing Task. PLoS ONE 2016, 11, e0158508. [Google Scholar] [CrossRef]
- Barrouillet, P.; Bernardin, S.; Portrat, S.; Vergauwe, E.; Camos, V. Time and cognitive load in working memory. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 570–585. [Google Scholar] [CrossRef]
- Valadao, D.F.; Anderson, B.; Danckert, J. Examining the influence of working memory on updating mental models. Q. J. Exp. Psychol. 2015, 68, 1442–1456. [Google Scholar] [CrossRef] [PubMed]
- Finney, S.A. FTAP: A Linux-based program for tapping and music experiments. Behav. Res. Methods Instrum. Comput. 2001, 33, 65–72. [Google Scholar] [CrossRef]
- Oschkinat, M.; Hoole, P.; Falk, S.; Dalla Bella, S. Temporal malleability to auditory feedback perturbation is modulated by rhythmic abilities and auditory acuity. Front. Hum. Neurosci. 2022, 16, 885074. [Google Scholar] [CrossRef] [PubMed]
- Birkett, E.E.; Talcott, J.B. Interval Timing in Children: Effects of Auditory and Visual Pacing Stimuli and Relationships with Reading and Attention Variables. PLoS ONE 2012, 7, e42820. [Google Scholar] [CrossRef]
- Iversen, J.R.; Balasubramaniam, R. Synchronization and temporal processing. Curr. Opin. Behav. Sci. 2016, 8, 175–180. [Google Scholar] [CrossRef]
- Grondin, S.; McAuley, J.D. Duration Discrimination in Crossmodal Sequences. Perception 2009, 38, 1542–1559. [Google Scholar] [CrossRef]
- Mcauley, J.D.; Henry, M.J. Modality effects in rhythm processing: Auditory encoding of visual rhythms is neither obligatory nor automatic. Atten. Percept. Psychophys. 2010, 72, 1377–1389. [Google Scholar] [CrossRef]
- Thaut, M.H.; Kenyon, G.P.; Schauer, M.L.; McIntosh, G.C. The connection between rhythmicity and brain function. IEEE Eng. Med. Biol. Mag. 1999, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, A.; Vanneste, S.; Isingrini, M.; Pouthas, V. Differential involvement of internal clock and working memory in the production and reproduction of duration: A study on older adults. Acta. Psychol. 2006, 121, 285–296. [Google Scholar] [CrossRef]
- Brown, S.W.; Boltz, M.G. Attentional processes in time perception: Effects of mental workload and event structure. J. Exp. Psychol. Hum. Percept. Perform. 2002, 28, 600–615. [Google Scholar] [CrossRef]
- Mioni, G. Methodological Issues in the Study of Prospective Timing. In Timing and Time Perception: Procedures, Measures, & Applications; BRILL: Leiden, The Netherlands, 2018; pp. 79–97. [Google Scholar]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; SAGE: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, version 25.0; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Gueorguieva, R.; Krystal, J.H. Move Over ANOVA. Arch. Gen. Psychiatry 2004, 61, 310. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Molenberghs, G.; Verbeke, G. Models for Discrete Longitudinal Data; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Greene, L.S.; Williams, H.G. Age-Related Differences in Timing Control of Repetitive Movement: Application of the Wing-Kristofferson Model. Res. Q. Exerc. Sport 1993, 64, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.M.; Spencer, R.M.; Zeffiro, T.A.; Makris, N.; Spencer, T.J.; Faraone, S.V.; Biederman, J.; Seidman, L.J. Neural Substrates of Impaired Sensorimotor Timing in Adult Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2010, 68, 359–367. [Google Scholar] [CrossRef]
- Aschersleben, G. Temporal Control of Movements in Sensorimotor Synchronization. Brain Cogn. 2002, 48, 66–79. [Google Scholar] [CrossRef]
- Repp, B.H.; Su, Y.H. Sensorimotor synchronization: A review of recent research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef]
- Aschersleben, G.; Prinz, W. Synchronizing actions with events: The role of sensory information. Percept. Psychophys. 1995, 57, 305–317. [Google Scholar] [CrossRef]
- Müller, K.; Aschersleben, G.; Schmitz, F.; Schnitzler, A.; Freund, H.J.; Prinz, W. Inter-versus intramodal integration in sensorimotor synchronization: A combined behavioral and magnetoencephalographic study. Exp. Brain Res. 2008, 185, 309–318. [Google Scholar] [CrossRef][Green Version]
- Fukuda, H.; Odagaki, M.; Hiwaki, O.; Kodabashi, A.; Fujimoto, T. Brain activity during bilateral rapid alternate finger tapping measured with magnetoencephalography. J. Appl. Phys. 2009, 105, 07B313. [Google Scholar] [CrossRef]
- Lauzon, A.P.; Russo, F.A.; Harris, L.R. The influence of rhythm on detection of auditory and vibrotactile asynchrony. Exp. Brain Res. 2020, 238, 825–832. [Google Scholar] [CrossRef]
- Miyake, Y.; Onishi, Y.; Pöppel, E. Two types of anticipation in synchronization tapping. Acta Neurobiol. Exp. 2004, 64, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Konvalinka, I.; Vuust, P.; Roepstorff, A.; Frith, C.D. Follow you, Follow me: Continuous Mutual Prediction and Adaptation in Joint Tapping. Q. J. Exp. Psychol. 2010, 63, 2220–2230. [Google Scholar] [CrossRef]
- Schaefer, R.S. Auditory rhythmic cueing in movement rehabilitation: Findings and possible mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130402. [Google Scholar]
- Thaut, M.H.; McIntosh, G.C.; Rice, R.R.; Miller, R.A.; Rathbun, J.; Brault, J.M. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 1996, 11, 193–200. [Google Scholar] [CrossRef]
- Bood, R.J.; Nijssen, M.; van der Kamp, J.; Roerdink, M. The power of auditory-motor synchronization in sports: Enhancing running performance by coupling cadence with the right beats. PLoS ONE 2013, 8, e70758. [Google Scholar] [CrossRef]
- Rönnqvist, L.; McDonald, R.; Sommer, M. Influences of Synchronized Metronome Training on Soccer Players’ Timing Ability, Performance Accuracy, and Lower-Limb Kinematics. Front. Psychol. 2018, 9, 2469. [Google Scholar] [CrossRef]
- Pelzer, L.; Naefgen, C.; Gaschler, R.; Haider, H. Learning of across- and within-task contingencies modulates partial-repetition costs in dual-tasking. Psychol. Res. 2022, 86, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, Z.; Zhang, Z.; Wu, J.; Dai, J.; Funahashi, S. What Task Feature Determines the Dominant Task in Dual-Task Conditions? eNeuro 2025, 12, ENEURO.0542-24.2025. [Google Scholar] [CrossRef] [PubMed]
- Bayot, M.; Dujardin, K.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Delval, A. The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol. Clin. 2018, 48, 361–375. [Google Scholar] [CrossRef]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015, 5, 1185. [Google Scholar] [CrossRef]
- Brown, S.W. Time and attention: Review of the literature. In Psychology of Time; Grondin, S., Ed.; Emerald: Bingley, UK, 2008; pp. 111–138. [Google Scholar]
- Drake, C.; Jones, M.R.; Baruch, C. The development of rhythmic attending in auditory sequences: Attunement, referent period, focal attending. Cognition 2000, 77, 251–288. [Google Scholar] [CrossRef]
- Provasi, J.; Bobin-Bègue, A. Spontaneous motor tempo and rhythmical synchronisation in 2½- and 4-year-old children. Int. J. Behav. Dev. 2003, 27, 220–231. [Google Scholar] [CrossRef]
- Leone, C.; Feys, P.; Moumdjian, L.; D’Amico, E.; Zappia, M.; Patti, F. Cognitive-motor dual-task interference: A systematic review of neural correlates. Neurosci. Biobehav. Rev. 2017, 75, 348–360. [Google Scholar] [CrossRef]
- Guérin, S.M.R.; Boitout, J.; Delevoye-Turrell, Y.N. Attention Guides the Motor-Timing Strategies in Finger-Tapping Tasks When Moving Fast and Slow. Front. Psychol. 2021, 11, 574396. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, I.J.; Summers, J.J.; Carson, R.G.; Byblow, W.D.; Semjen, A. Attention as a modulator of coordination dynamics: Influence on the stability of multifrequency patterns. J. Exp. Psychol. Hum. Percept. Perform. 1996, 22, 672–688. [Google Scholar]
- Zakay, D.; Block, R.A. An attentional-gate model of time estimation and temporal judgment. In Time and the Dynamic Control of Behavior; De Keyser, V., d’Ydewalle, G., Vandierendonck, A., Eds.; Université de Liège: Liège, Belgium, 1995; pp. 167–178. [Google Scholar]
- Taatgen, N.A.; van Rijn, H.; Anderson, J. An integrated theory of prospective time interval estimation: The role of cognition, attention, and learning. Psychol. Rev. 2007, 114, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Grahn, J.A.; London, J. The Psychology of Music: Rhythm and Movement. Annu. Rev. Psychol. 2018, 69, 51–75. [Google Scholar] [CrossRef]
- Grahn, J.A.; Brett, M. Rhythm and Beat Perception in Motor Areas of the Brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef]
- Piras, F.; Piras, F.; Ciullo, V.; Danese, E.; Caltagirone, C.; Spalletta, G. Time Dysperception Perspective for Acquired Brain Injury. Front. Neurol. 2014, 4, 217. [Google Scholar] [CrossRef] [PubMed]
- Pacella, V.; Scandola, M.; Bà, M.; Smania, N.; Beccherle, M.; Rossato, E.; Volpe, D.; Moro, V. Temporal judgments of actions following unilateral brain damage. Sci. Rep. 2022, 12, 21668. [Google Scholar] [CrossRef]
- van Merriënboer, J.J.G.; Kester, L.; Paas, F. Teaching complex rather than simple tasks: Balancing intrinsic and germane load to enhance transfer of learning. Appl. Cogn. Psychol. 2006, 20, 343–352. [Google Scholar] [CrossRef]
- Hsieh, S. Task Shifting in Dual-Task Settings. Percept. Mot. Skills 2002, 94, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Šerbetar, I.; Petanjek, Z.; Zarevski, P. Repetitive Movement Timing of Preschool Children and Young Adults Assessed by the Wing- Kristofferson Model. Croat. J. Educ. 2024, 26, 163–184. [Google Scholar] [CrossRef]
- Ivry, R.B.; Spencer, R.M. The neural representation of time. Curr. Opin. Neurobiol. 2004, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.; Nobre, A. Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol. 2008, 18, 137–144. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Lowenthal, J.; Herman, T.; Gruendlinger, L.; Peretz, C.; Giladi, N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur. J. Neurosci. 2007, 26, 2369–2375. [Google Scholar] [CrossRef]
- Stöckel, T.; Wunsch, K.; Hughes, C.M.L. Age-Related Decline in Anticipatory Motor Planning and Its Relation to Cognitive and Motor Skill Proficiency. Front. Aging Neurosci. 2017, 9, 283. [Google Scholar] [CrossRef]
- Singh, A.; Cole, R.C.; Espinoza, A.I.; Evans, A.; Cao, S.; Cavanagh, J.F.; Narayanan, N.S. Timing variability and midfrontal ~4 Hz rhythms correlate with cognition in Parkinson’s disease. NPJ Parkinson’s Dis. 2021, 7, 14. [Google Scholar] [CrossRef]
- Mainka, S.; Wissel, J.; Völler, H.; Evers, S. The Use of Rhythmic Auditory Stimulation to Optimize Treadmill Training for Stroke Patients: A Randomized Controlled Trial. Front. Neurol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Tsunoda, Y.; Kakei, S. Anticipation of future events improves the ability to estimate elapsed time. Exp. Brain Res. 2011, 214, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Barbaresi, M.; Nardo, D.; Fagioli, S. Physiological Entrainment: A Key Mind–Body Mechanism for Cognitive, Motor and Affective Functioning, and Well-Being. Brain Sci. 2024, 15, 3. [Google Scholar] [CrossRef]
- McPherson, G.E.; Davidson, J.W.; Faulkner, R. Music in Our Lives: Rethinking Musical Ability, Development, and Identity; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Krause, V.; Schnitzler, A.; Pollok, B. Functional network interactions during sensorimotor synchronization in musicians and non-musicians. Neuroimage 2010, 52, 245–251. [Google Scholar] [CrossRef]
- Bååth, R. Estimating the distribution of sensorimotor synchronization data: A Bayesian hierarchical modeling approach. Behav. Res. Methods 2016, 48, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Warm, J.S.; Reinerman, L.E.; Langheim, L.K.; Saxby, D.J. Task Engagement, Attention, and Executive Control. In Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control; Springer: New York, NY, USA, 2010; pp. 205–230. [Google Scholar]

| Condition | N | Mean | SD | SE | |
|---|---|---|---|---|---|
| IRI | ST | 103 | 432.29 | 54.90 | 2.43 |
| DT | 103 | 430.65 | 54.17 | 2.39 | |
| IRI.SD | ST | 103 | 30.33 | 8.70 | 0.39 |
| DT | 103 | 45.06 | 16.53 | 0.73 | |
| AI | ST | 103 | −0.143 | 0.111 | 0.0049 |
| DT | 103 | −0.138 | 0.108 | 0.0048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šerbetar, I.; Mamen, A. Dual-Task Interference Increases Variability in Sub-Second Repetitive Motor Timing. J. Funct. Morphol. Kinesiol. 2025, 10, 366. https://doi.org/10.3390/jfmk10040366
Šerbetar I, Mamen A. Dual-Task Interference Increases Variability in Sub-Second Repetitive Motor Timing. Journal of Functional Morphology and Kinesiology. 2025; 10(4):366. https://doi.org/10.3390/jfmk10040366
Chicago/Turabian StyleŠerbetar, Ivan, and Asgeir Mamen. 2025. "Dual-Task Interference Increases Variability in Sub-Second Repetitive Motor Timing" Journal of Functional Morphology and Kinesiology 10, no. 4: 366. https://doi.org/10.3390/jfmk10040366
APA StyleŠerbetar, I., & Mamen, A. (2025). Dual-Task Interference Increases Variability in Sub-Second Repetitive Motor Timing. Journal of Functional Morphology and Kinesiology, 10(4), 366. https://doi.org/10.3390/jfmk10040366






