Impact of a 24-Week Workplace Physical Activity Program on Oxidative Stress Markers, Metabolic Health, and Physical Fitness: A Pilot Study in a Real-World Academic Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Inclusion and Exclusion Criteria
2.4. Intervention
2.5. Outcome Measures
2.6. Oxidative Stress
2.7. Anthropometry, Metabolic, and Behavioral Variables
2.8. Physical Fitness
2.9. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics and Health Habits
3.2. Metabolic and Physical Fitness
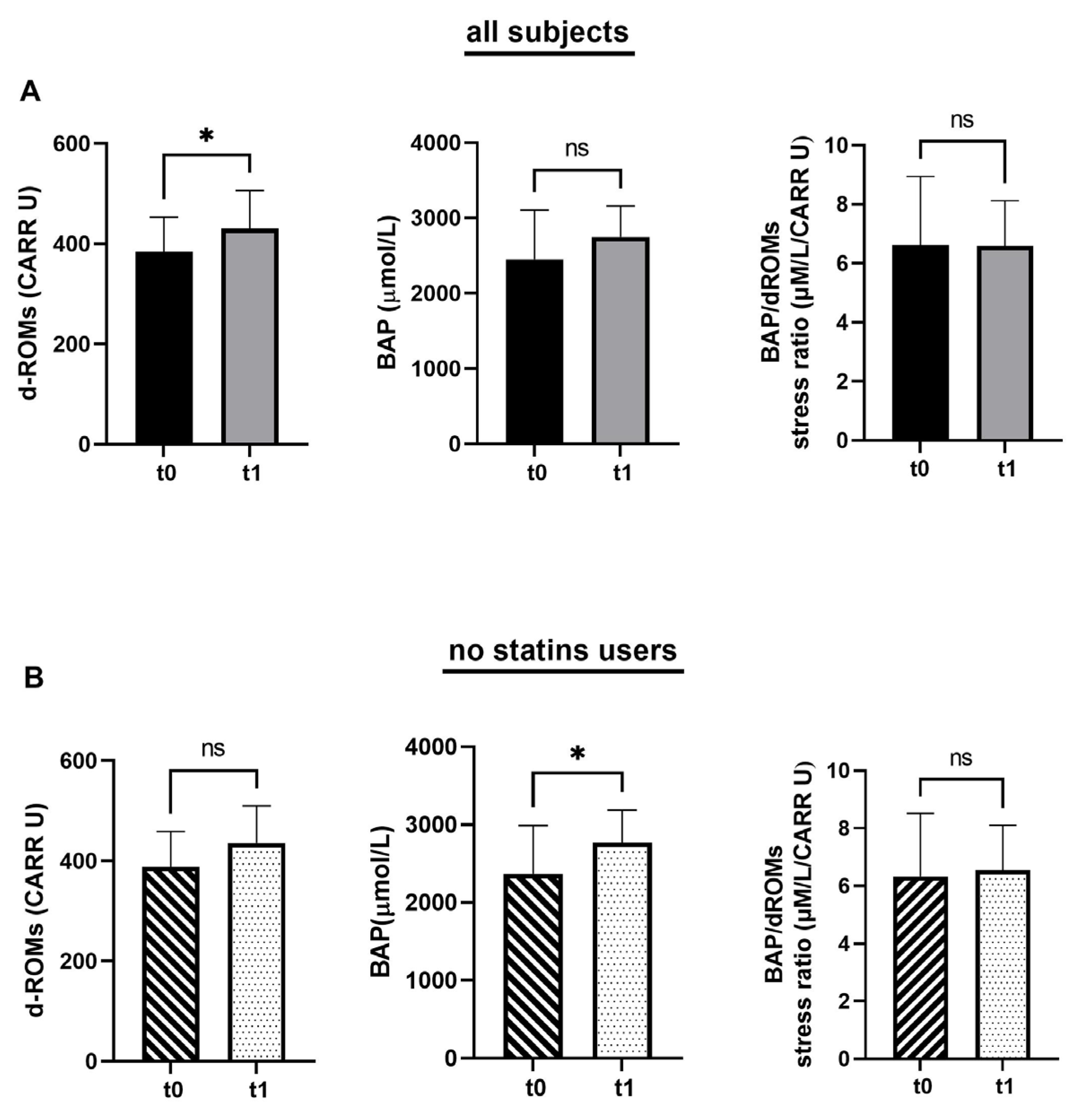
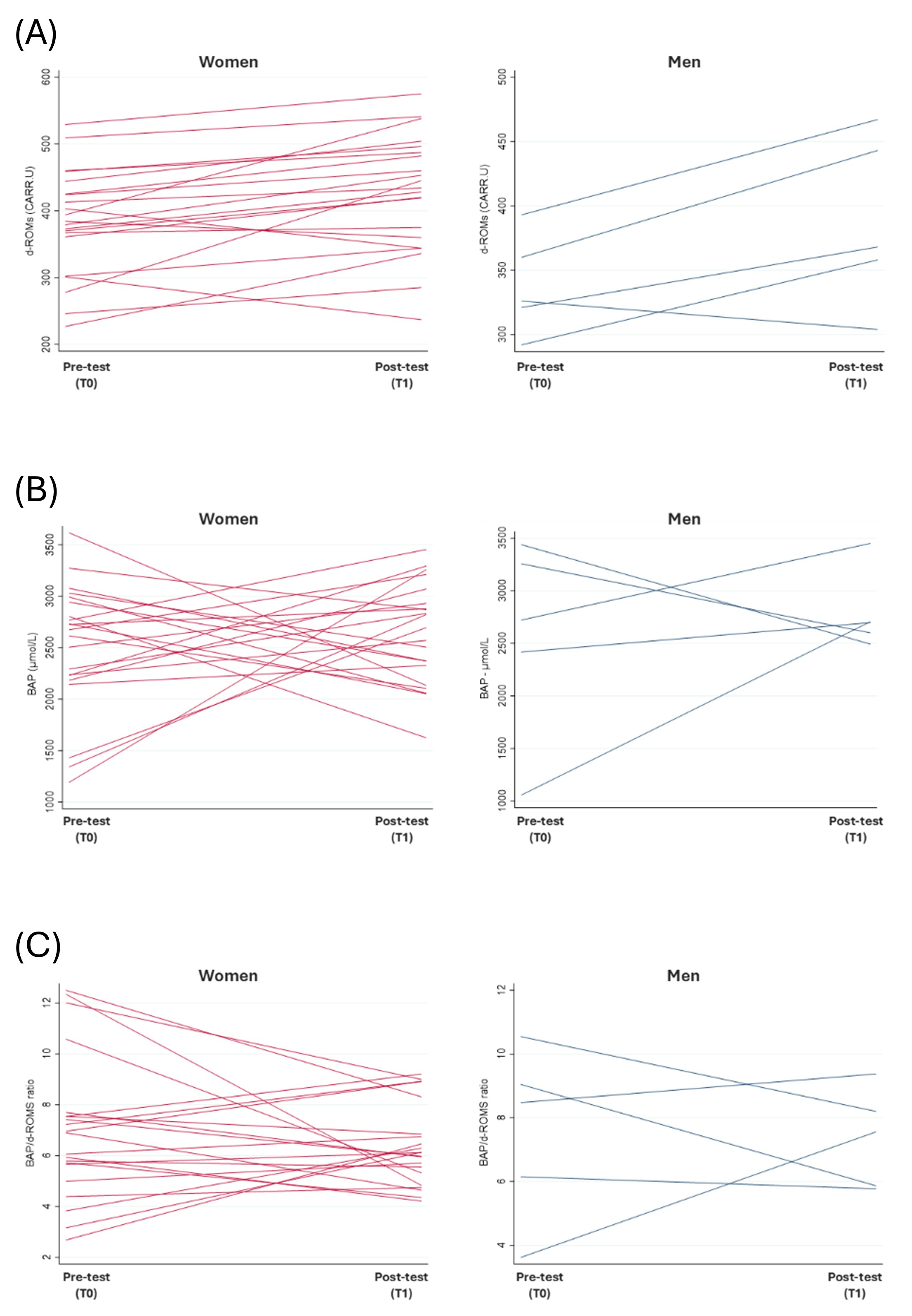
3.3. Oxidative Stress
3.3.1. Status of Employees over Time
3.3.2. Association of Oxidative Stress with Metabolic and Other Health Correlates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WPAP | Workplace physical activity program |
| PF | Physical fitness |
| 2MST | 2-minute step test |
| d-ROMs | Derived reactive oxygen metabolites |
| BAP | Biological antioxidant potential |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| PA | Physical activity |
| QoL | Quality of life |
| NCDs | Non-communicable diseases |
| ROS | Reactive oxygen species |
| U.CARR | Carratelli units |
| BMI | Body mass index |
| CRF | Cardiorespiratory fitness |
| GAPPA | Global action plan for physical activity |
| ISPAH | International society for physical activity and health |
References
- Scatigna, M.; D’Eugenio, S.; Cesarini, V.; Coppola, L.; Lemma, P.; Fabiani, L.; Romano Spica, V. Physical activity as a key issue for promoting human health on a local and global scale: Evidences and perspectives. Ann. Ig. Med. Prev. E Comunità 2019, 31, 595–613. [Google Scholar] [CrossRef]
- Szychowska, A.; Drygas, W. Physical activity as a determinant of successful aging: A narrative review article. Aging Clin. Exp. Res. 2022, 34, 1209–1214. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef]
- Clemes, S.A.; Houdmont, J.; Munir, F.; Wilson, K.; Kerr, R.; Addley, K. Descriptive epidemiology of domain-specific sitting in working adults: The Stormont Study. J. Public Health 2016, 38, 53–60. [Google Scholar] [CrossRef]
- Waters, C.N.; Ling, E.P.; Chu, A.H.; Ng, S.H.; Chia, A.; Lim, Y.W.; Müller-Riemenschneider, F. Assessing and understanding sedentary behaviour in office-based working adults: A mixed-method approach. BMC Public Health 2016, 16, 360. [Google Scholar] [CrossRef]
- Edge, C.E.; Cooper, A.M.; Coffey, M. Barriers and facilitators to extended working lives in Europe: A gender focus. Public Health Rev. 2017, 38, 2. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Woo, S.; Webster-Dekker, K.; Chen, W.; Veliz, P.; Larson, J.L. Sedentary behaviors and physical activity of the working population measured by accelerometry: A systematic review and meta-analysis. BMC Public Health 2024, 24, 2123. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; De Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Freak-Poli, R.; Wolfe, R.; Peeters, A. Risk of Cardiovascular Disease and Diabetes in a Working Population With Sedentary Occupations. J. Occup. Environ. Med. 2010, 52, 1132–1137. [Google Scholar] [CrossRef]
- Totaro Garcia, L.M.; da Silva, K.S.; Duca, G.F.D.; da Costa, F.F.; Nahas, M.V. Sedentary Behaviors, Leisure-Time Physical Inactivity, and Chronic Diseases in Brazilian Workers: A Cross Sectional Study. J. Phys. Act. Health 2014, 11, 1622–1634. [Google Scholar] [CrossRef]
- Adams, E.J.; Chalkley, A.E.; Esliger, D.W.; Sherar, L.B. Evaluation of the implementation of a whole-workplace walking programme using the RE-AIM framework. BMC Public Health 2017, 17, 466. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Ohta, M.; Takigami, C.; Ikeda, M. Effect of lifestyle modification program implemented in the community on workers’ job satisfaction. Ind. Health 2007, 45, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pronk, N.P.; Malan, D.; Christie, G.; Hajat, C.; Yach, D. Health and well-being metrics in business: The value of integrated reporting. Med. J. Occup. Environ. Med. 2018, 60, 19–22. [Google Scholar] [CrossRef]
- Adams, J.M. The value of worker well-being. Public Health Rep. 2019, 134, 583–586. [Google Scholar] [CrossRef]
- Marin-Farrona, M.; Wipfli, B.; Thosar, S.S.; Colino, E.; Garcia-Unanue, J.; Gallardo, L.; Felipe, J.L.; López-Fernández, J. Effectiveness of worksite wellness programs based on physical activity to improve workers’ health and productivity: A systematic review. Syst. Rev. 2023, 12, 87. [Google Scholar] [CrossRef]
- Bonatesta, L.; Palermi, S.; Sirico, F.; Mancinelli, M.; Torelli, P.; Russo, E.; Annarumma, G.; Vecchiato, M.; Fernando, F.; Gregori, G. Short-term economic evaluation of physical activity-based corporate health programs: A systematic review. J. Occup. Health 2024, 66, uiae002. [Google Scholar] [CrossRef]
- Pugliese, L.; Tuccella, C.; Maisto, G.; D’Angelo, E.; Delle Monache, S.; Scatigna, M.; Rodrigues Moreira, M.H.; Bonavolontà, V.; Vinciguerra, M.G. The Effects of a 24-Week Combined Circuit Training and Mobility Program on the Physical Fitness and Body Composition of an Adult Academic Community. Sports 2025, 13, 79. [Google Scholar] [CrossRef]
- Ohta, M.; Nanri, H.; Matsushima, Y.; Sato, Y.; Ikeda, M. Blood pressure-lowering effects of lifestyle modification: Possible involvement of nitric oxide bioavailability. Hypertens. Res. 2005, 28, 779–786. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of different exercise modalities on oxidative stress: A systematic review. Biomed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Eguchi, Y.; Inoue, T.; Honda, T.; Morita, Y.; Konno, Y.; Yamato, H.; Kumashiro, M. Effects of bench step exercise intervention on work ability in terms of cardiovascular risk factors and oxidative stress: A randomized controlled study. Int. J. Occup. Saf. Ergon. 2015, 21, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Skogstad, M.; Lunde, L.K.; Skare, Ø.; Mamen, A.; Alfonso, J.H.; Øvstebø, R.; Ulvestad, B. Physical activity initiated by employer and its health effects; an eight week follow-up study. BMC Public Health 2016, 16, 377. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, N.B.; Shearman, J.P.; Cooper, C.E. Exercise-induced oxidative stress: Myths, realities and physiological relevance. Sports Med. 2005, 35, 1045–1062. [Google Scholar] [CrossRef]
- Dillard, C.J.; Litov, R.E.; Tappel, A.L. Effects of dietary vitamin E, selenium, and polyunsaturated fats on in vivo lipid peroxidation in the rat as measured by pentane production. Lipids 1978, 13, 396–402. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef]
- Oh-Ishi, S.; Kizaki, T.; Nagasawa, J.; Izawa, T.; Komabayashi, T.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin. Exp. Pharmacol. Physiol. 1997, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, C.-H. The impact of physical exercise on oxidative and nitrosative stress: Balancing the benefits and risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2011, 2, 1143–1211. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rojo, F.I.; Enríquez-del Castillo, L.A.; González-Chávez, S.A.; Flores-Olivares, L.A.; Quintana-Mendias, E.; Carrasco-Legleu, C.E. Effect of exercise intensity on redox biomarkers in healthy adults: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2025, 20, e0330185. [Google Scholar] [CrossRef]
- Schaller, A.; Stassen, G.; Baulig, L.; Lange, M. Physical activity interventions in workplace health promotion: Objectives, related outcomes, and consideration of the setting-a scoping review of reviews. Front. Public Health 2024, 12, 1353119. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. A systematic review and meta-analysis of the effect of statins on glutathione peroxidase, superoxide dismutase, and catalase. Antioxidants 2021, 10, 1841. [Google Scholar] [CrossRef]
- Kaminsky, L.; Bryant, C.; Mahler, D.; Durstine, J.; Humphrey, R. ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2009. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Iorio, E.; Balestrieri, M.L. Lo stress ossidativo. In Diagnostica Molecolare Nella Medicina di Laboratorio; Piccin Nuova Libraria: Padova, Italy, 2009; pp. 533–549. [Google Scholar]
- Klarod, K.; Philippe, M.; Gatterer, H.; Burtscher, M. Different training responses to eccentric endurance exercise at low and moderate altitudes in pre-diabetic men: A pilot study. Sport Sci. Health 2017, 13, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Morucci, G.; Ryskalin, L.; Pratesi, S.; Branca, J.J.V.; Modesti, A.; Modesti, P.A.; Gulisano, M.; Gesi, M. Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects. Medicina 2022, 58, 1341. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hashimoto, J.; Suzuki, T.; Satoh, A. The effects of exercise load during development on oxidative stress levels and antioxidant potential in adulthood. Free Radic. Res. 2017, 51, 179–186. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Yuba, T.; Koyama, Y.; Ootaki, C.; Fujino, Y.; Shimada, S. Effect of blood sample storage period on d-ROMs and BAP test data. Heliyon 2024, 10, e34573. [Google Scholar] [CrossRef]
- Suni, J.; Husu, P.; Rinne, M. Fitness for Health: The ALPHA-FIT Test Battery for Adults Aged 18–69; Tester’s Manual; European Union: Tampere, Finland, 2009. [Google Scholar]
- Bohannon, R.W.; Crouch, R.H. Two-Minute Step Test of Exercise Capacity: Systematic Review of Procedures, Performance, and Clinimetric Properties. J. Geriatr. Phys. Ther. 2019, 42, 105–112. [Google Scholar] [CrossRef]
- World Health Organization. 2018 Global reference list of 100 core health indicators (plus health-related SDGs). In 2018 Global Reference List of 100 Core Health Indicators (Plus Health-Related SDGs); World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Tofas, T.; Draganidis, D.; Deli, C.K.; Georgakouli, K.; Fatouros, I.G.; Jamurtas, A.Z. Exercise-Induced Regulation of Redox Status in Cardiovascular Diseases: The Role of Exercise Training and Detraining. Antioxidants 2019, 9, 13. [Google Scholar] [CrossRef]
- Sutkowy, P.; Woźniak, A.; Mila-Kierzenkowska, C.; Szewczyk-Golec, K.; Wesołowski, R.; Pawłowska, M.; Nuszkiewicz, J. Physical Activity vs. Redox Balance in the Brain: Brain Health, Aging and Diseases. Antioxidants 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.; Allen, J.A. Effect of short term workplace exercise intervention on lipid profile, depression, work ability and selected physical parameters of university employees in Saudi Arabia: A randomized controlled trail. Indian J. Sci. Technol. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Juhas, I.; Skof, B.; Popović, D.; Matić, M.; Janković, N. Effects of an eight-week exercise program on parameters of the lipid profile of female students. J. Med. Biochem. 2020, 39, 40. [Google Scholar] [CrossRef]
- Scapellato, M.L.; Comiati, V.; Buja, A.; Buttignol, G.; Valentini, R.; Burati, V.; La Serra, L.; Maccà, I.; Mason, P.; Scopa, P.; et al. Combined Before-and-After Workplace Intervention to Promote Healthy Lifestyles in Healthcare Workers (STI-VI Study): Short-Term Assessment. Int. J. Environ. Res. Public Health 2018, 15, 2053. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- International Society for Physical Activity and Health (ISPAH). ISPAH’s Eight Investments that Work for Physical Activity; ISPAH: London, UK, 2020. [Google Scholar]
- Iborra, R.T.; Ribeiro, I.C.; Neves, M.Q.; Charf, A.M.; Lottenberg, S.A.; Negrão, C.E.; Nakandakare, E.R.; Passarelli, M. Aerobic exercise training improves the role of high-density lipoprotein antioxidant and reduces plasma lipid peroxidation in type 2 diabetes mellitus. Scand. J. Med. Sci. Sports 2008, 18, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.E.; Branco, L.G. Recent advances in molecular hydrogen research reducing exercise-induced oxidative stress and inflammation. Curr. Pharm. Des. 2021, 27, 731–736. [Google Scholar] [CrossRef]
- Dimauro, I.; Grazioli, E.; Lisi, V.; Guidotti, F.; Fantini, C.; Antinozzi, C.; Sgrò, P.; Antonioni, A.; Di Luigi, L.; Capranica, L.; et al. Systemic Response of Antioxidants, Heat Shock Proteins, and Inflammatory Biomarkers to Short-Lasting Exercise Training in Healthy Male Subjects. Oxid. Med. Cell. Longev. 2021, 2021, 1938492. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Physiological and Pathological Role of ROS: Benefits and Limitations of Antioxidant Treatment. Int. J. Mol. Sci. 2019, 20, 4810. [Google Scholar] [CrossRef]
- Mani, S.; Tyagi, S.; Pal, K.V.; Jaiswal, H.; Jain, A.; Gulati, A.; Singh, M. Drug-induced oxidative stress and cellular toxicity. In Free Radical Biology and Environmental Toxicity; Springer: Singapore, 2022; pp. 73–113. [Google Scholar]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Baretti, M.; Le, D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; McKenzie, M.J.; Bloomer, R.J. Gender comparisons of exercise-induced oxidative stress: Influence of antioxidant supplementation. Appl. Physiol. Nutr. Metab. 2007, 32, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kurano, M.; Fukumura, K.; Yasuda, T.; Iida, H.; Morita, T.; Yamamoto, Y.; Takano, N.; Komuro, I.; Nakajima, T. Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress. Korean Circ. J. 2013, 43, 481–487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of high intensity exercise on oxidative stress and antioxidant status in untrained humans: A systematic review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress and cell senescence as drivers of ageing: Chicken and egg. Ageing Res. Rev. 2024, 102, 102558. [Google Scholar] [CrossRef]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of human coenzyme Q10 metabolism: An overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Capili, B.; Anastasi, J.K. An Introduction to Types of Quasi-Experimental Designs. Am. J. Nurs. 2024, 124, 50–52. [Google Scholar] [CrossRef]
| Women (21) | Men (5) | Total (26) | Sign. | |
|---|---|---|---|---|
| Regular Physical Activity | 19 (90.5%) | 5 (100.0% | 24 (92.3%) | n.s. (a) |
| No. of times/week—mean (range) | 2.9 (1–7) | 3.2 (2.4) | 3.0 (1–7) | n.s. (b) |
| Total min/week—mean (range) | 195.8 (60–420) | 228.0 (180–360) | 202.5 (60–420) | n.s. (b) |
| Use of medicine | 9 (42.9%) | 5 (100.0%) | 14 (53.8%) | p < 0.05 (a) |
| No. of medications—mean (range) | 0.8 (0–5) | 1 (1–1) | 0.9 (0–5) | n.s. (b) |
| Use of supplements | 14 (66.7%) | 2 (40.0%) | 16 (61.5%) | n.s. (a) |
| Women (21) | Men (5) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | Sign. | T0 | T1 | Sign. | |
| Body Mass Index (kg/m2) | 24.9 ± 4.4 | 24.8 ± 4.2 | n.s. (a) | 25.3 ± 3.6 | 25.2 ± 3.3 | n.s. (a) |
| Chol TOT (≥200 mg/dL) | 12 (57.1%) | 6 (28.6%) | p ≤ 0.05 (b) | 1 (20.0%) | 0 (0.0%) | n.s. (b) |
| Chol HDL (<35 or >75 mg/dL) | 1 (4.8%) | 1 (4.8%) | n.s. (b) | 0 (0.0%) | 0 (0.0%) | n.s. (b) |
| Chol LDL (≥130 mg/dL) | 12 (57.1%) | 5 (23.8%) | p < 0.01 (b) | 1 (20.0%) | 0 (0.0%) | n.s. (b) |
| Triglycerides (≥130 mg/dL) | 2 (9.5%) | 1 (4.8%) | n.s. (b) | 1 (20.0%) | 2 (40.0%) | n.s. (b) |
| Fasting Glucose (≥110 mg/dL) | 2 (9.5%) | 0 (0.0%) | n.s. (b) | 0 (0.0%) | 0 (0.0%) | n.s. (b) |
| Women (15) | Men (5) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | Sign. | T0 | T1 | Sign. | |
| One Leg Stand | ||||||
| High | 8 (53.3%) | 8 (53.3%) | n.s. (a) | 3 (60.0%) | 5 (100.0%) | n.s. (a) |
| Low/Mid | 7 (46.7%) | 7 (46.7%) | 2 (40.0%) | 0 (0.0%) | ||
| Shoulder/Neck Mobility | ||||||
| None or mild restriction (4–5) | 6 (40.0%) | 8 (53.3%) | n.s. (a) | 2 (40.0%) | 2 (40.0%) | n.s. (a) |
| Moderate or high restriction (1–3) | 9 (60.0%) | 7 (46.7%) | 3 (60.0%) | 3 (60.0%) | ||
| Handgrip | ||||||
| kg (mean ± standard deviation) | 28.7 ± 6.1 | 30.0 ± 6.6 | n.s. (b) | 48.0 ± 5.9 | 50.6 ± 11.0 | n.s. (b) |
| Jump and Reach | ||||||
| High score (3–4° quartile) | 9 (60.0%) | 8 (53.3%) | n.s. (a) | 2 (40.0%) | 4 (80.0%) | n.s. (a) |
| Low score (1–2° quartile) | 6 (40.0%) | 7 (46.7%) | 3 (60.0%) | 1 (20.0%) | ||
| Dynamic Sit-up | ||||||
| High | 10 (66.7%) | 12 (80.0%) | n.s. (a) | 5 (100.0%) | 5 (100.0%) | N/A (a) |
| Low/Mid | 5 (33.3%) | 3 (20.0%) | 0 (0.0%) | (0.0%) | ||
| 2-Minute Step Test | ||||||
| % at risk (<65 steps) | 1 (6.7%) | 2 (13.3%) | n.s. (a) | 0 (0.0%) | 0 (0.0%) | N/A (a) |
| No. steps (mean ± standard dev.) | 82.7 ± 16.8 | 85.5 ± 19.0 | n.s. (b) | 87.6 ± 16.2 | 100.0 ± 21.8 | n.s. (b) p = 0.0796 |
| Women (21) | Men (5) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | Sign. (a) | T0 | T1 | Sign. (a) | |
| d-ROMs (U.CARR) | 383.2 ± 79.3 | 426.8 ± 87.3 | p < 0.01 (a) | 338.4 ± 38.9 | 388.0 ± 66.4 | n.s. (a) p = 0.0796 |
| BAP (µmol/L) | 2513.8 ± 624.6 | 2640.4 ± 488.2 | n.s. (a) | 2578.2 ± 943.6 | 2789.2 ± 379.6 | n.s. (a) |
| BAP/d-ROM ratio | 7.00 ± 2.8 | 6.40 ± 1.6 | n.s. (a) | 7.6 ± 2.7 | 7.4 ± 1.5 | n.s. (a) |
| Women (21) | Men (5) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | Sign. | T0 | T1 | Sign. | |
| d-ROMs | ||||||
| Not high (<300 U.CARR) | 3 (14.3%) | 2 (9.5%) | n.s. (a) | 1 (20.0%) | 0 (0.0%) | n.s. (a) |
| High (≥300 U.CARR) | 18 (85.7%) | 19 (90.5%) | 4 (80.0%) | 5 (100.0%) | ||
| BAP | ||||||
| Optimal (≥2200 µmol/L) | 16 (76.2%) | 16 (76.2%) | n.s. (a) | 4 (80.0%) | 5 (100.0%) | n.s. (a) |
| Not optimal (<2200 µmol/L) | 5 (23.8%) | 5 (23.8%) | 1 (20.0%) | 0 (0.0%) | ||
| Oxidative risk | ||||||
| Optimal ox balance [d-ROMs ~ BAP ~] | 1 (4.8%) | 1 (4.8%) | n.s. (b) | 0 (0.0%) | 0 (0.0%) | n.s. (b) |
| Relative hyporesponsiveness [d-ROMs ↓ BAP ~] | 2 (9.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Absolute hypo-reactivity [d-ROMs ↓ BAP ↓] | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | ||
| Relative stress ox [d-ROMs ~ BAP ↓] | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | ||
| Stress ox potential [d-ROMs ↑ BAP ~] | 13 (61.9%) | 15 (71.4%) | 4 (80.0%) | 5 (100.0%) | ||
| Absolute stress ox [d-ROMs ↑ BAP↓] | 5 (23.8%) | 4 (19.1%) | 0 (0.0%) | 0 (0.0%) | ||
| Metabolic Risk and Lifestyle Factors Whole Sample at T0 (26) | BAP/d-ROM Ratio Mean ± St Dev. | Sign. | Absolute Oxidative Stress % | Sign. | |
|---|---|---|---|---|---|
| Weight | Normal | 8.1 ± 2.7 | p < 0.05 (a) | 23.1% | n.s. (b) |
| Overweight or Obesity | 6.1 ± 2.4 | 15.4% | |||
| Total Cholesterol | <200 mg/dL | 8.7 ± 2.7 | p < 0.01 (a) | 7.7% | n.s. (b) |
| ≥200 mg/dL | 5.5 ± 1.5 | 30.8% | |||
| LDL Cholesterol | <130 mg/dL | 8.7 ± 2.7 | p < 0.01 (a) | 7.7% | n.s. (b) |
| ≥130 mg/dL | 5.5 ± 1.5 | 30.8% | |||
| Triglycerides | <60 mg/dL | 8.5 ± 3.1 | p = 0.0719 (c) | 16.7% | n.s. (b) |
| 60–130 mg/dL | 7.0 ± 2.6 | 17.6% | |||
| ≥130 mg/dL | 4.8 ± 1.1 | 33.3% | |||
| Fasting Blood Glucose | <110 mg/dL | 7.2 ± 2.8 | n.s. (a) | 20.8% | n.s. (b) |
| ≥110 mg/dL | 6.0 ± 1.3 | 0.0% | |||
| Smoking | Yes | 5.0 ± 3.3 | n.s. (c) | 50.0% | n.s. (b) |
| Ex | 6.4 ± 2.3 | 12.5% | |||
| No | 7.7 ± 2.8 | 18.8% | |||
| Alcohol (occasional) | Yes | 6.6 ± 2.0 | n.s. (a) | 14.3% | n.s. (b) |
| No | 7.7 ± 3.3 | 25.0% | |||
| Regular Physical Activity | Yes | 7.3 ± 2.7 | n.s. (a) | 17.7% | n.s. (b) |
| No | 4.4 ± 0.8 | 50.0% | |||
| Medicine Use | Yes | 6.3 ± 2.6 | p < 0.05 (a) | 14.3% | n.s. (b) |
| No | 8.1 ± 2.6 | 25.0% | |||
| Supplement Use | Yes | 7.6 ± 2.8 | n.s. (a) | 14.3% | n.s. (b) |
| No | 6.5 ± 2.6 | 25.0% | |||
| Performances Levels at Motor Tests Whole Sample at T0 (26) | BAP/d-ROM Ratio Mean ± St Dev. | Sign. | Absolute Oxidative Stress % | Sign. |
|---|---|---|---|---|
| One leg stand | ||||
| High | 7.3 ± 2.4 | n.s. (a) | 16.7% | n.s. (b) |
| Low/Mid | 7.0 ± 3.0 | 21.4% | ||
| Shoulder/neck mobility | ||||
| No or light limitation (score 4–5) | 6.8 ± 3.0 | n.s. (a) | 25.0% | n.s. (b) |
| Moderate or severe limitation (score 1–3) | 7.4 ± 2.5 | 14.3% | ||
| Handgrip | ||||
| Spearman’s Rho | 0.242 | n.s (d) | - | |
| 1° tertile | 6.8 ± 3.1 | n.s. (c) | 36.3% | n.s. (b) |
| 2° tertile | 6.1 ± 2.0 | 16.7% | ||
| 3° tertile | 8.2 ± 2.6 | 0.0% | ||
| Jump and reach | ||||
| 3–4° quartile | 7.04 ± 2.7 | n.s. (a) | 14.3% | n.s. (b) |
| 1–2° quartile | 7.2 ± 2.9 | 25.0% | ||
| Dynamic sit-up | ||||
| High | 7.7 ± 2.5 | p = 0.0668 (a) | 11.1% | n.s. (b) |
| Low/Mid | 5.8 ± 3.0 | 37.5% | ||
| 2-Minute Step Test | ||||
| Risk (<65 elevations) | 3.9 ± 1.3 | p < 0.01 (a) | 100.0% | p < 0.001 (b) |
| No risk (≥65 elevations) | 7.7 ± 2.5 | 4.5% | ||
| Spearman’s Rho | 0.404 | p < 0.05 (d) | - | |
| 1° tertile | 6.1 ± 2.7 | p = 0.0519 (c) | 30.8% | n.s. (b) |
| 2° tertile | 8.1 ± 2.9 | 0.0% | ||
| 3° tertile | 8.1 ± 2.2 | 16.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisto, G.; Scatigna, M.; Delle Monache, S.; Coppolino, M.F.; Pugliese, L.; Sponta, A.M.; Tobia, L.; Tolli, E.; Zito, P.; Bonavolontà, V.; et al. Impact of a 24-Week Workplace Physical Activity Program on Oxidative Stress Markers, Metabolic Health, and Physical Fitness: A Pilot Study in a Real-World Academic Setting. J. Funct. Morphol. Kinesiol. 2025, 10, 348. https://doi.org/10.3390/jfmk10030348
Maisto G, Scatigna M, Delle Monache S, Coppolino MF, Pugliese L, Sponta AM, Tobia L, Tolli E, Zito P, Bonavolontà V, et al. Impact of a 24-Week Workplace Physical Activity Program on Oxidative Stress Markers, Metabolic Health, and Physical Fitness: A Pilot Study in a Real-World Academic Setting. Journal of Functional Morphology and Kinesiology. 2025; 10(3):348. https://doi.org/10.3390/jfmk10030348
Chicago/Turabian StyleMaisto, Gabriele, Maria Scatigna, Simona Delle Monache, Maria Francesca Coppolino, Lorenzo Pugliese, Anna Maria Sponta, Loreta Tobia, Elio Tolli, Pierfrancesco Zito, Valerio Bonavolontà, and et al. 2025. "Impact of a 24-Week Workplace Physical Activity Program on Oxidative Stress Markers, Metabolic Health, and Physical Fitness: A Pilot Study in a Real-World Academic Setting" Journal of Functional Morphology and Kinesiology 10, no. 3: 348. https://doi.org/10.3390/jfmk10030348
APA StyleMaisto, G., Scatigna, M., Delle Monache, S., Coppolino, M. F., Pugliese, L., Sponta, A. M., Tobia, L., Tolli, E., Zito, P., Bonavolontà, V., Fabiani, L., Tuccella, C., & Vinciguerra, M. G. (2025). Impact of a 24-Week Workplace Physical Activity Program on Oxidative Stress Markers, Metabolic Health, and Physical Fitness: A Pilot Study in a Real-World Academic Setting. Journal of Functional Morphology and Kinesiology, 10(3), 348. https://doi.org/10.3390/jfmk10030348










