Ultrasound-Guided Fascial Hydrorelease for Persistent Pain After Hamstring Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessment of Pain During Activity
2.3. Assessment of Tightness
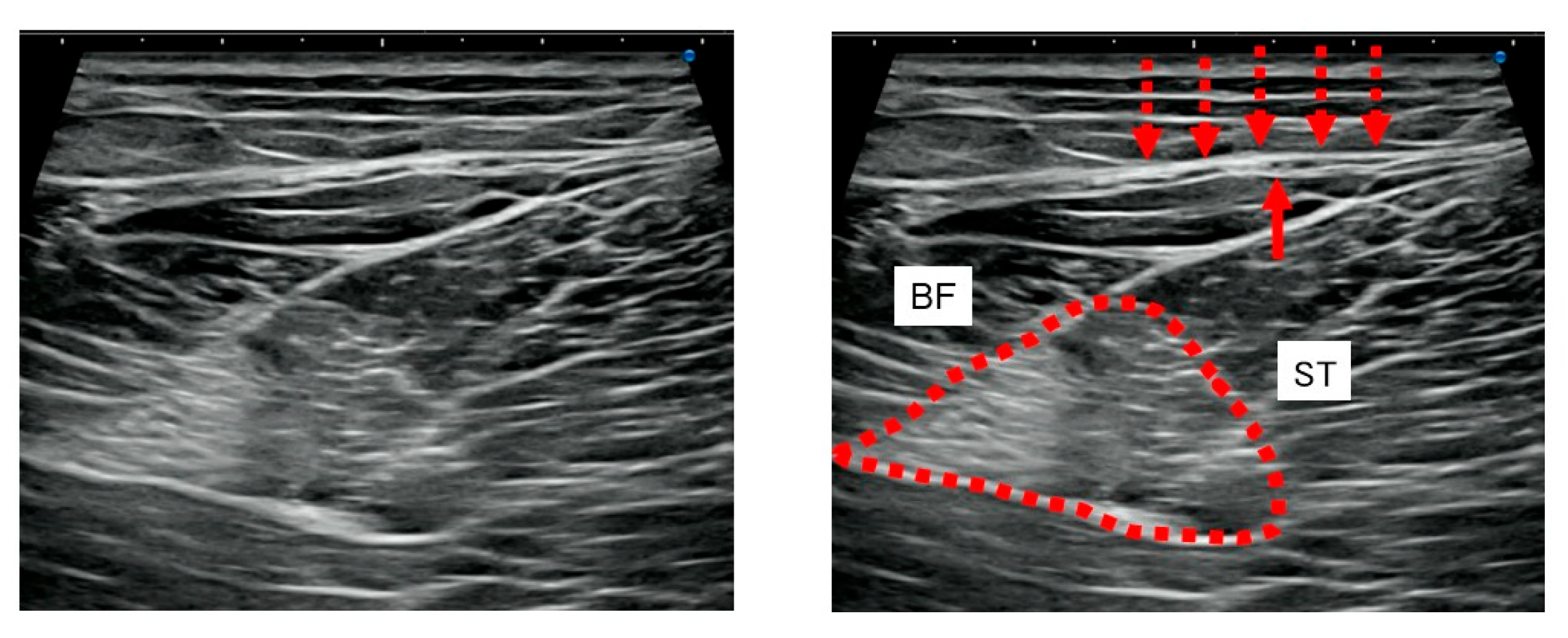
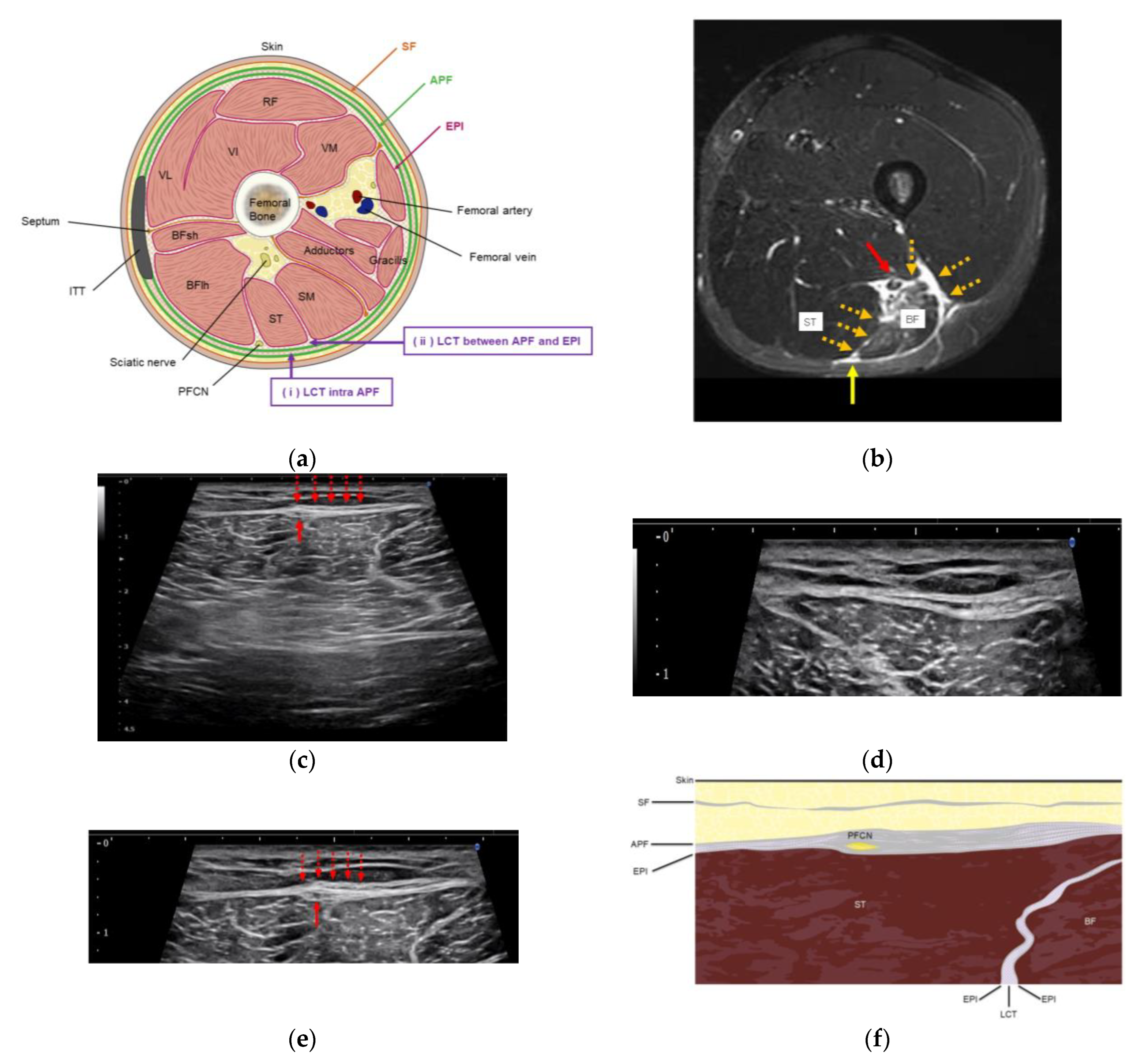
2.4. US Imaging and Sonopalpation
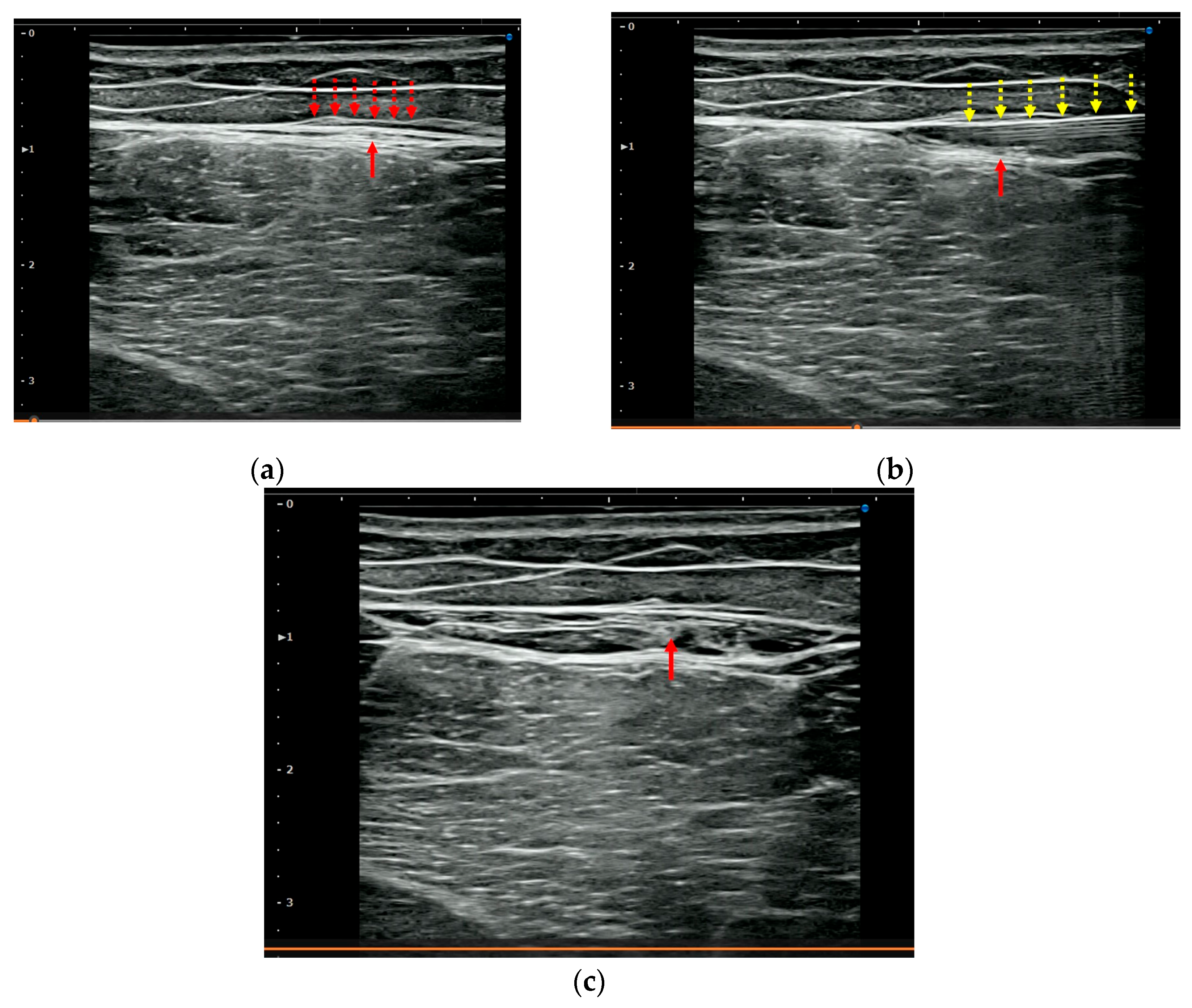
2.5. US-Guided Hydrorelease
2.6. Statistical Analysis
3. Results
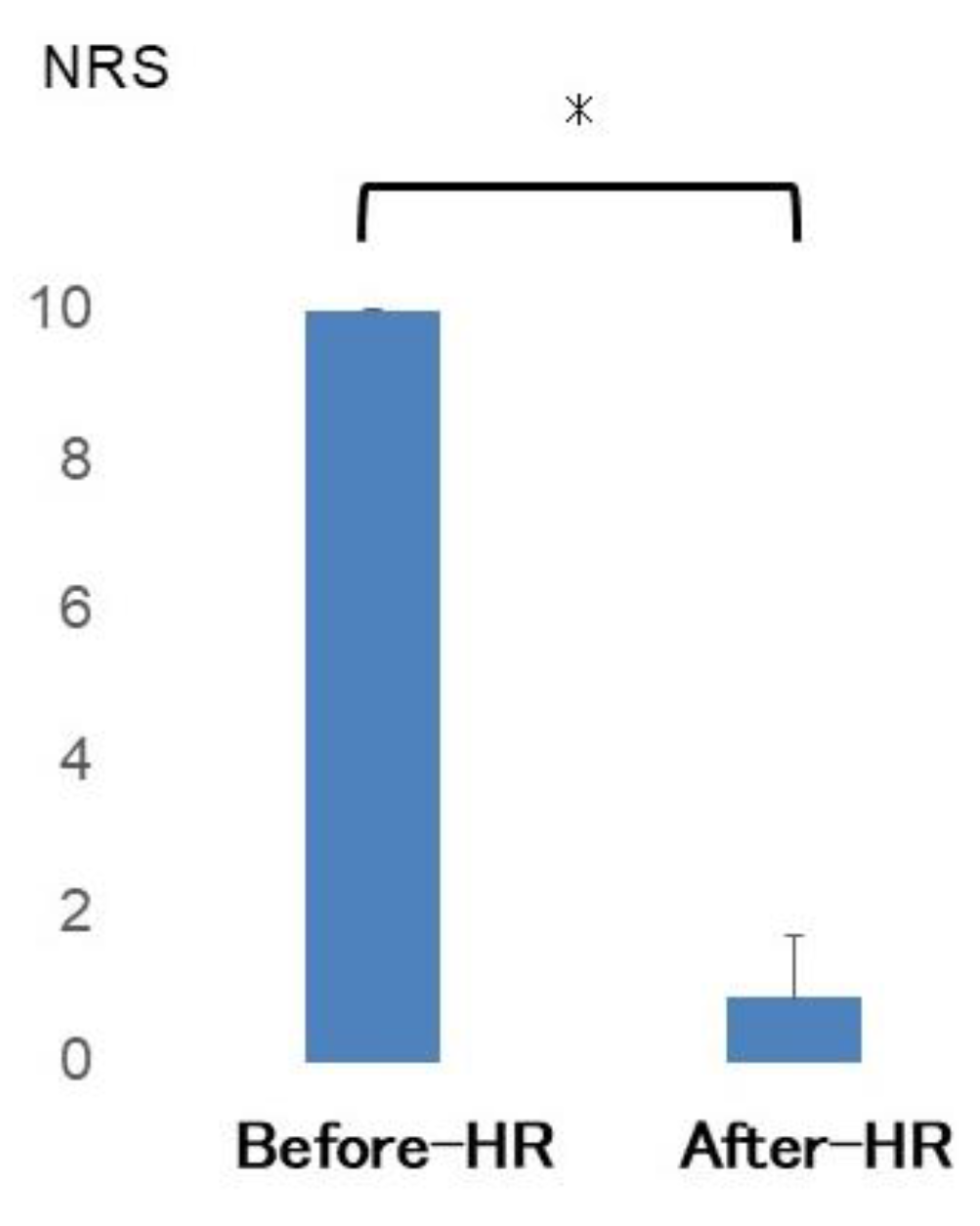
3.1. Assessment of Pain During Activity
3.2. Assessment of Tightness
3.3. US Imaging and Sonopalpation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APF | Aponeurotic Fascia |
| HD | Hydrodissection |
| HR | Hydrorelease |
| MPS | Myofascial Pain Syndrome |
| MRI | Magnetic Resonance Imaging |
| NRS | Numerical Rating Scale |
| PFCN | Posterior Femoral Cutaneous Nerve |
| PFP | Perineural Fascial Pain |
| SLR | Straight Leg Raise |
| STIR | Short Tau Inversion Recovery |
| US | Ultrasound |
References
- Orchard, J.; Seward, H. Epidemiology of injuries in the Australian Football League, seasons 1997–2000. Br. J. Sports Med. 2002, 36, 39–44. [Google Scholar] [CrossRef]
- Erickson, L.N.; Sherry, M.A. Rehabilitation and return to sport after hamstring strain injury. J. Sport Health Sci. 2017, 6, 262–270. [Google Scholar] [CrossRef]
- Copland, S.T.; Tipton, J.S.; Fields, K.B. Evidence-based treatment of hamstring tears. Curr. Sports Med. Rep. 2009, 8, 308–314. [Google Scholar] [CrossRef]
- Courseault, J.; Kessler, E.; Moran, A.; Labbe, A. Fascial hydrodissection for chronic hamstring injury. Curr. Sports Med. Rep. 2019, 18, 416–420. [Google Scholar] [CrossRef]
- Evers, S.; Thoreson, A.R.; Smith, J.; Zhao, C.; Geske, J.R.; Amadio, P.C. Ultrasound-guided hydrodissection decreases gliding resistance of the median nerve within the carpal tunnel. Muscle Nerve 2018, 57, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, N.; Galacho, J.; Ferreira-Dos-Santos, G.; Clendenen, S.R.; Hurdle, M.F.B. Ultrasound-guided hydrodissection of the superficial peroneal nerve for chronic neuropathic pain: A war veteran’s story. Pain Manag. 2022, 12, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ramos, C.; Gonzalez-Suarez, C.; Gomez, I.N.; Gonzalez, M.K.; Co, P.H.; Llamas, J.A. Effectiveness of ultrasound guided interfascial hydrodissection with the use of saline anesthetic solution for myofascial pain syndrome of the upper trapezius: A single blind randomized controlled trial. Front. Rehabil. Sci. 2023, 4, 1281813. [Google Scholar] [CrossRef]
- Kanamoto, H.; Orita, S.; Inage, K.; Shiga, Y.; Abe, K.; Eguchi, Y.; Ohtori, S. Effect of ultrasound-guided hydrorelease of the multifidus muscle on acute low back pain. J. Ultrasound Med. 2021, 40, 981–987. [Google Scholar] [CrossRef]
- Kimura, H.; Suda, M.; Kobayashi, T.; Suzuki, S.; Fukui, S.; Obata, H. Effectiveness of ultrasound-guided fascia hydrorelease on the coracohumeral ligament in patients with global limitation of the shoulder range of motion: A pilot study. Sci. Rep. 2022, 12, 19782. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, K.; Otsubo, H.; Suzuki, D.; Pirri, C.; Kodesyo, T.; Kamiya, T.; Taniguchi, K.; Ohnishi, H.; Teramoto, A.; Stecco, C. Biomechanical effects of fascial hydrorelease: A cadaveric study. BMC Musculoskelet. Disord. 2025, 26, 306. [Google Scholar] [CrossRef]
- Shiwaku, K.; Pirri, C.; Otsubo, H.; Kamiya, T.; Porzionato, A.; Nakao, G.; Teramoto, A.; Stecco, C. Fascial ultrasound-guided injection: Where do we really inject? Cureus 2025, 17, e78867. [Google Scholar] [CrossRef]
- Kongsagul, S.; Vitoonpong, T.; Kitisomprayoonkul, W.; Tantisiriwat, N. Ultrasound-guided physiological saline injection for patients with myofascial pain. J. Med. Ultrasound 2020, 28, 99–103. [Google Scholar] [CrossRef]
- Simons, D.G. New aspects of myofascial trigger points: Etiological and clinical. J. Musculoskelet. Pain 2004, 12, 15–21. [Google Scholar] [CrossRef]
- Fukui, S.; Rokutanda, R.; Kawaai, S.; Suda, M.; Iwata, F.; Okada, M.; Kishimoto, M. Current evidence and practical knowledge for ultrasound-guided procedures in rheumatology: Joint aspiration, injection, and other applications. Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101832. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Tafuri, E.; Savini, A.; Fabrizio, A.; Affaitati, G.; Lerza, R.; Di Ianni, L.; Lapenna, D.; Mezzetti, A. Contribution of myofascial trigger points to migraine symptoms. J. Pain 2007, 8, 869–878. [Google Scholar] [CrossRef]
- Hu, Y.E.; Ho, G.W.K.; Tortland, P.D. Deep gluteal syndrome: A pain in the buttock. Curr. Sports Med. Rep. 2021, 20, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Jiamjunyasiri, A.; Tsutsumi, M.; Muro, S.; Akita, K. Origin, course, and distribution of the posterior femoral cutaneous nerve and the spatial relationship among its branches. Anat. Sci. Int. 2023, 98, 540–547. [Google Scholar] [CrossRef]
- Gerard, N.O., 3rd; Mortell, T.M.; Kingry, C.; Couture, C.; Courseault, J. Hydrodissection of an ulnar nerve fascial adhesion in a baseball pitcher. JSES Rev. Rep. Tech. 2023, 3, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Vahdatinia, R.; Humbert, S.; Stecco, A. Myofascial injection using fascial layer-specific hydromanipulation technique (FLuSH) and the delineation of multifactorial myofascial pain. Medicina 2020, 56, 717. [Google Scholar] [CrossRef]
- Satkunskiene, D.; Khair, R.M.; Muanjai, P.; Mickevicius, M.; Kamandulis, S. Immediate effects of neurodynamic nerve gliding versus static stretching on hamstring neuromechanical properties. Eur. J. Appl. Physiol. 2020, 120, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Castellote-Caballero, Y.; Valenza, M.C.; Puentedura, E.J.; Fernández-de-Las-Peñas, C.; Alburquerque-Sendín, F. Immediate Effects of Neurodynamic Sliding versus Muscle Stretching on Hamstring Flexibility in Subjects with Short Hamstring Syndrome. J. Sports Med. 2014, 2014, 127471. [Google Scholar] [CrossRef] [PubMed]
- Warneke, K.; Lohmann, L.H.; Behm, D.G.; Wirth, K.; Keiner, M.; Schiemann, S.; Wilke, J. Effects of Chronic Static Stretching on Maximal Strength and Muscle Hypertrophy: A Systematic Review and Meta-Analysis with Meta-Regression. Sports Med. Open 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Devantéry, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering 2023, 10, 332. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiwaku, K.; Otsubo, H.; Nishikawa, D.; Itagaki, R.; Takashima, H.; Nakao, G.; Kamiya, T.; Suzuki, D.; Emori, M.; Stecco, C.; et al. Ultrasound-Guided Fascial Hydrorelease for Persistent Pain After Hamstring Injury. J. Funct. Morphol. Kinesiol. 2025, 10, 318. https://doi.org/10.3390/jfmk10030318
Shiwaku K, Otsubo H, Nishikawa D, Itagaki R, Takashima H, Nakao G, Kamiya T, Suzuki D, Emori M, Stecco C, et al. Ultrasound-Guided Fascial Hydrorelease for Persistent Pain After Hamstring Injury. Journal of Functional Morphology and Kinesiology. 2025; 10(3):318. https://doi.org/10.3390/jfmk10030318
Chicago/Turabian StyleShiwaku, Kousuke, Hidenori Otsubo, Daiki Nishikawa, Rikiya Itagaki, Hiroyuki Takashima, Gakuto Nakao, Tomoaki Kamiya, Daisuke Suzuki, Makoto Emori, Carla Stecco, and et al. 2025. "Ultrasound-Guided Fascial Hydrorelease for Persistent Pain After Hamstring Injury" Journal of Functional Morphology and Kinesiology 10, no. 3: 318. https://doi.org/10.3390/jfmk10030318
APA StyleShiwaku, K., Otsubo, H., Nishikawa, D., Itagaki, R., Takashima, H., Nakao, G., Kamiya, T., Suzuki, D., Emori, M., Stecco, C., & Teramoto, A. (2025). Ultrasound-Guided Fascial Hydrorelease for Persistent Pain After Hamstring Injury. Journal of Functional Morphology and Kinesiology, 10(3), 318. https://doi.org/10.3390/jfmk10030318






