Effects of Compression Garments on Muscle Oxygen Saturation Recovery in the Upper Limbs Using Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
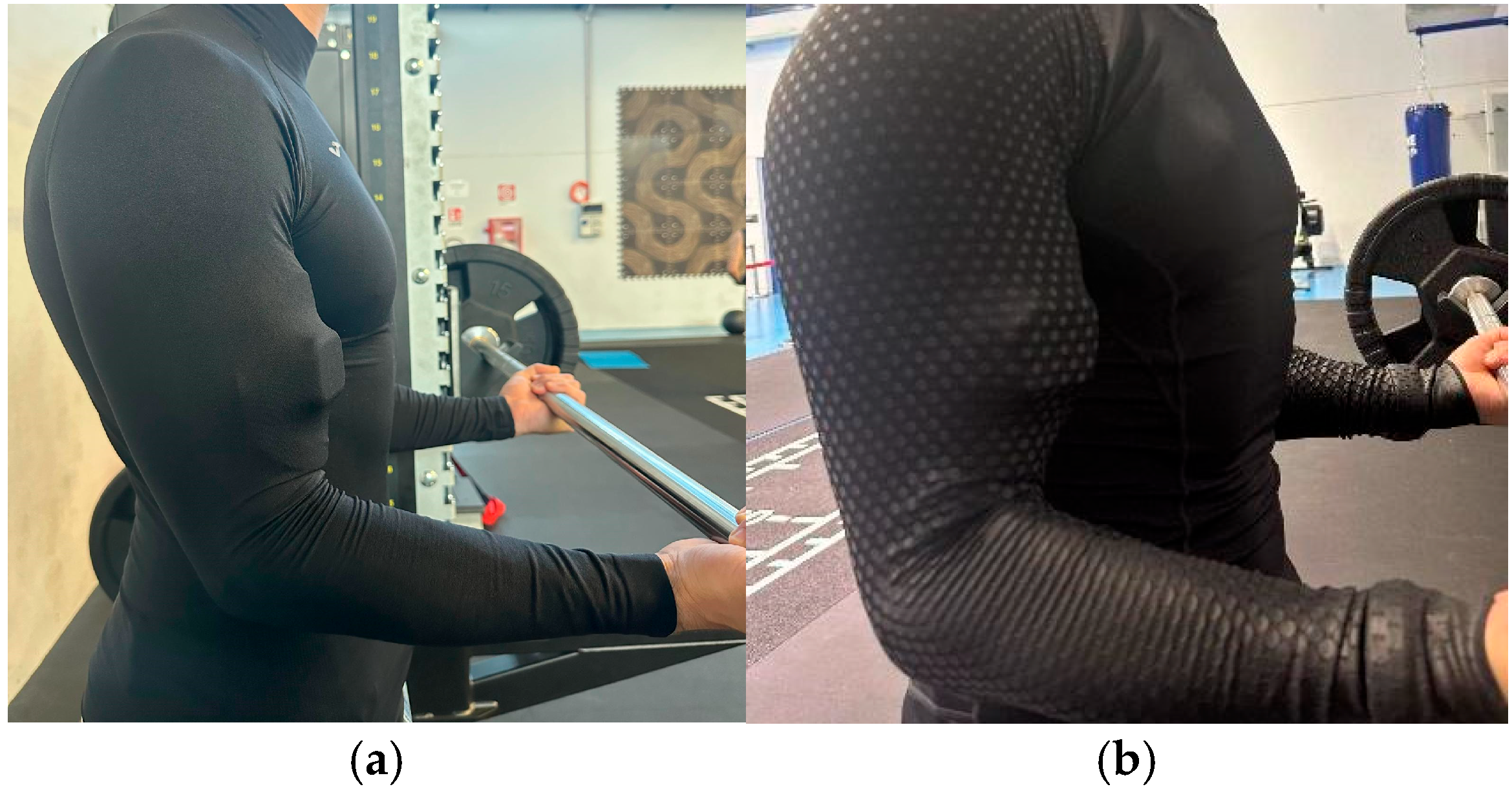
2.2. Procedures
2.3. Materials
2.4. Data Analysis
3. Results
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacRae, B.; Cotter, J.; Laing, R. Compression Garments and Exercise. Sports Med. 2011, 41, 815–843. [Google Scholar] [CrossRef]
- Galanaud, J.P.; Laroche, J.P.; Righini, M. The history and historical treatments of deep vein thrombosis. J. Thromb. Haemost. 2013, 11, 402–411. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.A.; Nelson, E.A.; Dumvillle, J.C. Compression for venous leg ulcers (Cochrane review). Cochrane Database Syst. Rev. 2012, 11, 1465–1858. [Google Scholar] [CrossRef]
- Qaseem, A.; Chou, R.; Humphrey, L.L.; Starkey, M.; Shekelle, P.; Physicians, C.G. Venous thromboembolism prophylaxis in hospitalized patients: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2011, 155, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.L.; Carati, C.J.; Piller, N.B. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 639–646. [Google Scholar] [CrossRef]
- Partsch, H.; Winiger, J.; Lun, B. Compression stockings reduce occupational leg swelling. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004, 30, 737–743. [Google Scholar] [CrossRef]
- Ibegbuna, V.; Delis, K.T.; Nicolaides, A.N.; Aina, O. Effect of elastic compression stockings on venous hemodynamics during walking. J. Vasc. Surg. 2003, 37, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Scurr, J.H.; Machin, S.J.; Bailey-King, S.; Mackie, I.J.; McDonald, S.; Smith, P.D. Frequency and prevention of symptomless deep-vein thrombosis in long-haul flights: A randomised trial. Lancet 2001, 357, 1485–1489. [Google Scholar] [CrossRef]
- Asano, H.; Matsubara, M.; Suzuki, K.; Morita, S.; Shinomiya, K. Prevention of pulmonary embolism by a foot sole pump. J. Bone Jt. Surg. Br. Vol. 2001, 83, 1130–1132. [Google Scholar] [CrossRef][Green Version]
- Amaragiri, S.V.; Lees, T.A. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst. Rev. 2000, 3, CD001484. [Google Scholar] [CrossRef]
- Millour, G.; Lepers, R.; Coste, A.; Hausswirth, C. Effects of combining cold exposure and compression on muscle recovery: A randomized crossover study. Front. Physiol. 2025, 16, 1598075. [Google Scholar] [CrossRef] [PubMed]
- Broatch, J.R.; Bishop, D.J.; Halson, S. Lower Limb Sports Compression Garments Improve Muscle Blood Flow and Exercise Performance During Repeated-Sprint Cycling. Int. J. Sports Physiol. Perform. 2018, 19, 882–890. [Google Scholar] [CrossRef]
- Wannop, J.W.; Worobets, J.T.; Madden, R.; Stefanyshyn, D.J. Influence of Compression and Stiffness Apparel on Vertical Jump Performance. J. Strength Cond. Res. 2016, 30, 1093–1101. [Google Scholar] [CrossRef]
- Stickford, A.S.; Chapman, R.F.; Johnston, J.D.; Stager, J.M. Lower-leg compression, running mechanics, and economy in trained distance runners. Int. J. Sports Physiol. Perform. 2015, 10, 76–83. [Google Scholar] [CrossRef]
- Gupta, A.; Bryers, J.J.; Clothier, P.J. The effect of leg compression garments on the mechanical characteristics and performance of single-leg hopping in healthy male volunteers. BMC Sports Sci. Med. Rehabil. 2015, 7, 10. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Y.; Fang, Y. Research Advancements in Humanoid Compression Garments in Sports. Int. J. Adv. Robot. Syst. 2013, 10, 66. [Google Scholar] [CrossRef]
- Driller, M.W.; Halson, S.L. The effects of wearing lower body compression garments during a cycling performance test. Int. J. Sports Physiol. Perform. 2013, 8, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Troynikov, O.; Wardiningsih, W.; Koptug, A.; Watson, C.; Oggiano, L. Influence of material properties and garment composition on pressure generated by sport compression garments. Procedia Eng. 2013, 60, 157–162. [Google Scholar] [CrossRef]
- Ali, A.; Creasy, R.H.; Edge, J.A. The effect of graduated compression stockings on running performance. J. Strength Cond. Res. 2011, 25, 1385–1392. [Google Scholar] [CrossRef]
- Dascombe, B.J.; Hoare, T.K.; Sear, J.A.; Reaburn, P.R.; Scanlan, A.T. The effects of wearing undersized lower-body compression garments on endurance running performance. Int. J. Sports Physiol. Perform. 2011, 6, 160–173. [Google Scholar] [CrossRef]
- Doan, B.; Kwon, Y.H.; Newton, R.; Shim, J.; Popper, E.V.A.; Rogers, R.; Bolt, L.; Robertson, M.; Kraemer, W. Evaluation of a lower-body compression garment. J. Sports Sci. 2003, 21, 601–610. [Google Scholar] [CrossRef]
- Serra, E.; Albano, D.; Benincasa, M.T.; Vastola, R. Influence of compression garments on perceived exertion during maximal isometric exercises. J. Hum. Sport Exerc. 2024, 19, 930–940. [Google Scholar] [CrossRef]
- Leabeater, A.; James, L.; Driller, M. Tight Margins: Compression Garment Use during Exercise and Recovery—A Systematic Review. Textiles 2022, 2, 395–421. [Google Scholar] [CrossRef]
- Araujo, A.M.; Cardoso, R.K.; Rombaldi, A.J. Post-exercise effects of graduated compression garment use on skeletal muscle recovery and delayed onset muscle soreness: A systematic review. Motricidade 2018, 14, 129–137. [Google Scholar] [CrossRef]
- Brown, F.; Gissane, C.; Howatson, G.; Van Someren, K.; Pedlar, C.; Hill, J. Compression Garments and Recovery from Exercise: A Meta-Analysis. Sports Med. 2017, 47, 2245–2267. [Google Scholar] [CrossRef] [PubMed]
- Marqués-Jiménez, D.; Calleja-González, J.; Arratibel, I.; Delextrat, A.; Terrados, N. Are compression garments effective for the recovery of exercise-induced muscle damage? A systematic review with meta-analysis. Physiol. Behav. 2016, 153, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Howatson, G.; Van Someren, K.; Leeder, J.; Pedlar, C. Compression garments and recovery from exercise-induced muscle damage: A meta-analysis. Br. J. Sports Med. 2014, 48, 1340–1346. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Flanagan, S.D.; Comstock, B.A.; Fragala, M.S.; Earp, J.E.; Dunn-Lewis, C.; Ho, J.Y.; Thomas, G.A.; Solomon-Hill, G.; Penwell, Z.R.; et al. Effects of a whole body compression garment on markers of recovery after a heavy resistance workout in men and women. J. Strength Cond. Res. 2010, 24, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.; Stade, M.; Morse, B.; Schick, E. Graded Compression Garments Worn During Resistance Exercise: Impact on Muscle Damage, Fatigue, and Oxygenation in Untrained Individuals. Int. J. Kinesiol. Sports Sci. 2022, 10, 51–59. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Helal, L.; Da Silva, R.P.; Belli, K.C.; Umpierre, D.; Stein, R. Association of Lower Limb Compression Garments During High-Intensity Exercise with Performance and Physiological Responses: A Systematic Review and Meta-analysis. Sports Med. 2018, 48, 1859–1873. [Google Scholar] [CrossRef]
- Dascombe, B.; Laursen, P.; Nosaka, K.; Polglaze, T. No effect of upper body compression garments in elite flat-water kayakers. Eur. J. Sport Sci. 2013, 13, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.I. A brief review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports Med. 2007, 37, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S. Muscle oxygenation unlocks the secrets of physiological responses to exercise: Time to exploit it in the training monitoring. Front. Sports Act. Living 2022, 4, 864825. [Google Scholar] [CrossRef]
- Perrey, S.; Betik, A.; Candau, R.; Rouillon, J.D.; Hughson, R.L. Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J. Appl. Physiol. 2001, 91, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Bringard, A.; Perrey, S. Influence of repeated isometric contractions on muscle deoxygenation and pulmonary oxygen uptake kinetics in humans. Clin. Physiol. Funct. Imaging 2004, 24, 229–236. [Google Scholar] [CrossRef]
- Stöggl, T.; Born, D.P. Near Infrared Spectroscopy for Muscle Specific Analysis of Intensity and Fatigue during Cross-Country Skiing Competition-A Case Report. Sensors 2021, 21, 2535. [Google Scholar] [CrossRef]
- Born, D.P.; Stöggl, T.; Swarén, M.; Björklund, G. Near-infrared spectroscopy: More accurate than heart rate for monitoring intensity in running in hilly terrain. Int. J. Sports Physiol. Perform. 2017, 12, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Tandirerung, F.J.; Jamieson, A.; Hendrick, E.; Hughes, A.D.; Jones, S. Near-infrared spectroscopy (NIRS) in vivo assessment of skeletal muscle oxidative capacity: A comparison of results from short versus long exercise protocols and reproducibility in non-athletic adults. Front. Physiol. 2024, 15, 1429673. [Google Scholar] [CrossRef]
- Motobe, M.; Murase, N.; Osada, T.; Homma, T.; Ueda, C.; Nagasawa, T.; Kitahara, A.; Ichimura, S.; Kurosawa, Y.; Katsumura, T.; et al. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn. Med. DM 2004, 3, 2. [Google Scholar] [CrossRef]
- Ryan, T.E.; Southern, W.M.; Reynolds, M.A.; McCully, K.K. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J. Appl. Physiol. 2013, 115, 1757–1766. [Google Scholar] [CrossRef]
- Dunst, A.; Manunzio, C.; Feldmann, A.; Hesse, C. Applications of near-infrared spectroscopy in “anaerobic” diagnostics–SmO2 kinetics reflect PCr dephosphorylation and correlate with maximal lactate accumulation and maximal pedalling rate. Biol. Sport 2023, 40, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.-H.; Lo, S.-F.; Wu, H.-C.; Chiu, M.-C. Effects of compression garment on muscular efficacy, proprioception, and recovery after exercise-induced muscle fatigue onset for people who exercise regularly. PLoS ONE 2022, 17, e0264569. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-sprint ability—Part I: Factors contributing to fatigue. Sports Med. 2011, 41, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Bishop, D.; Dawson, B.; Goodman, C. Physiological and metabolic responses of repeated-sprint activities: Specific to field-based team sports. Sports Med. 2005, 35, 1025–1044. [Google Scholar] [CrossRef]
- Perrey, S.; Ferrari, M. Muscle oximetry in sports science: A systematic review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Ufland, P.; Lapole, T.; Ahmaidi, S.; Buchheit, M. Muscle force recovery in relation to muscle oxygenation. Clin. Physiol. Funct. Imaging 2012, 32, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.N.; Somani, Y.B.; Al-Qahtani, A.M.; Proctor, D.N.; Murias, J.M. Near-infrared spectroscopy detects transient decrements and recovery of microvascular responsiveness following prolonged forearm ischemia. Microvasc. Res. 2019, 125, 103879. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, R.P. External compression increases forearm perfusion. J. Appl. Physiol. 2005, 99, 2337–2344. [Google Scholar] [CrossRef]
- Li, X.; Su, H.; Du, L.; Li, G.; Lv, Y.; Liu, X.; Feng, L.; Yu, L. Effects of Compression Garments on Muscle Strength and Power Recovery Post-Exercise: A Systematic Review and Meta-Analysis. Life 2025, 15, 438. [Google Scholar] [CrossRef]
- Carati, C.; Gannon, B.; Piller, N. Anatomy and physiology in relation to compression of the upper limb and thorax. J. Lymphoedema 2010, 5, 58–67. [Google Scholar]
- Newcomer, S.C.; Leuenberger, U.A.; Hogeman, C.S.; Handly, B.D.; Proctor, D.N. Different vasodilator responses of human arms and legs. J. Physiol. 2004, 556 Pt 3, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- DiFrancisco-Donoghue, J.; Rothstein, A.; Jung, M.K.; Zwibel, H.; Werner, W.G. Upper body compression wear improves muscle oxygenation following intense video game training: A randomized cross-over study among competitive gamers. BMC Sports Sci. Med. Rehabil. 2023, 15, 108. [Google Scholar] [CrossRef]
- Limmer, M.; de Marées, M.; Roth, R. Effects of Forearm Compression Sleeves on Muscle Hemodynamics and Muscular Strength and Endurance Parameters in Sports Climbing: A Randomized, Controlled Crossover Trial. Front. Physiol. 2022, 13, 888860. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.T.; Serra, E.; Albano, D.; Vastola, R. Comparing muscle oxygen saturation patterns in swimmers of different competitive levels. J. Phys. Educ. Sport 2024, 24, 1920–1926. [Google Scholar] [CrossRef]
- Dalamitros, A.A.; Semaltianou, E.; Toubekis, A.G.; Kabasakalis, A. Muscle oxygenation, heart rate, and blood lactate concentration during submaximal and maximal interval swimming. Front. Sports Act. Living 2021, 3, 759925. [Google Scholar] [CrossRef]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef]
- Paquette, M.; Bieuzen, F.; Billaut, F. Muscle Oxygenation Rather Than VO2max as a Strong Predictor of Performance in Sprint Canoe–Kayak. Int. J. Sports Physiol. Perform. 2018, 13, 1299–1307. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Van Beekvelt, M.C.; van Engelen, B.G.; Wevers, R.A.; Colier, W.N. In vivo quantitative near-infrared spectroscopy in skeletal muscle during incremental isometric handgrip exercise. Clin. Physiol. Funct. Imaging 2002, 22, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Muthalib, M.; Millet, G.Y.; Quaresima, V.; Nosaka, K. Reliability of near-infrared spectroscopy for measuring biceps brachii oxygenation during sustained and repeated isometric contractions. J. Biomed. Opt. 2010, 15, 017008. [Google Scholar] [CrossRef]
- Norton, K.; Eston, R. (Eds.) Kinanthropometry and Exercise Physiology; Routledge: London, UK, 2019; pp. 107–108. [Google Scholar]
- Zhao, H.; Nishioka, T.; Okada, J. Validity of using perceived exertion to assess muscle fatigue during resistance exercises. PeerJ 2022, 10, e13019. [Google Scholar] [CrossRef]
- Bayles, M.P. ACSM’s Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Oranchuk, D.J.; Switaj, Z.J.; Zuleger, B.M. The addition of a “rapid response” neuromuscular activation to a standard dynamic warm-up improves isometric force and rate of force development. J. Aust. Strength Cond. 2017, 25, 19–24. [Google Scholar]
- Peixoto, L.R.; da Rocha, A.F.; de Carvalho, J.L.; Goncalves, C.A. Electromyographic evaluation of muscle recovery after isometric fatigue. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annu. Int. Conf. 2010, 2010, 4922–4925. [Google Scholar] [CrossRef]
- Reinpõld, K.; Rannama, I. Oxygen Uptake and Bilaterally Measured Vastus Lateralis Muscle Oxygen Desaturation Kinetics in Well-Trained Endurance Cyclists. J. Funct. Morphol. Kinesiol. 2023, 8, 64. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hagg, G. SENIAM Raccomandazioni Europee per L’elettromiografia di Superficie; CLUT Edition: Torino, Italy, 2000. [Google Scholar]
- Barstow, T. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Design, F. Introduction to muscle oxygen monitor. Muscle Oxygen. 2015. Available online: https://cdn2.hubspot.net/hub/188620/file-433442739-pdf/docs/moxy-ebook-intro-to-muscle-oxygen.pdf?t=1488816603832 (accessed on 27 November 2024).
- Kirby, B.S.; Clark, D.A.; Bradley, E.M.; Wilkins, B.W. The balance of muscle oxygen supply and demand reveals critical metabolic rate and predicts time to exhaustion. J. Appl. Physiol. 2021, 130, 1915–1927. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Batterson, P.; Kirby, B.; Hasselmann, G.; Feldman, A. Muscle oxygen saturation rates coincide with lactate-based exercise thresholds. Eur. J. Appl. Physiol. 2023, 123, 2249–2258. [Google Scholar] [CrossRef]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 015007. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Reilly, T. (Eds.) Kinanthropometry and Exercise Physiology Laboratory Manual; Routledge: London, UK, 2001; Volume 1. [Google Scholar]
- Design, F. Using Moxy for Multimodal Fitness. Muscle Oxygen. 2023. Available online: https://188620.fs1.hubspotusercontent-na1.net/hubfs/188620/documents/Multimodal%20Fitness.pdf?utm_campaign=muscle-oxygen-ebook&utm_medium=email&_hsenc=p2ANqtz--kMYYcgwH8tyAOJcbOcRH53cV383xswPzG4rOzGLgHLX-tMVJiO6Hn4yn_-Xfn2A6ejA8Tpx3A_f4AYVoy0EfN0TlP4wiH-LjXgbLBaJV598dSays&_hsmi=248580871&utm_content=248580871&utm_source=hs_automation (accessed on 27 November 2024).
- Spencer, M.D.; Murias, J.M.; Paterson, D.H. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur. J. Appl. Physiol. 2012, 112, 3349–3360. [Google Scholar] [CrossRef]
- Porter, M.; Langley, J. The relationship between muscle oxygen saturation kinetics and maximal blood lactate accumulation rate across varying sprint cycle durations. Eur. J. Sport Sci. 2025, 25, e12242. [Google Scholar] [CrossRef] [PubMed]
- Yogev, A.; Arnold, J.; Nelson, H.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M.S. The effect of severe intensity bouts on muscle oxygen saturation responses in trained cyclists. Front. Sports Act. Living 2023, 5, 1086227. [Google Scholar] [CrossRef]
- Arnold, J.I.; Yogev, A.; Nelson, H.; van Hooff, M.; Koehle, M.S. Muscle reoxygenation is slower after higher cycling intensity, and is faster and more reliable in locomotor than in accessory muscle sites. Front. Physiol. 2024, 15, 1449384. [Google Scholar] [CrossRef]
- Rębiś, K.; Sadowska, D.; Starczewski, M.; Klusiewicz, A. Usefulness of Portable Device to Establish Differences in Muscle Oxygenation Between the Wingate Test and Graded Exercise Test: Effect of Gender on Anaerobic and Aerobic Capacity in Speed Skaters. Front. Physiol. 2022, 13, 809864. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; White, A.E.M.; Nixon, D.G.D.; Gould, D.W.; Watson, E.L.; Smith, A.C. Characterising skeletal muscle haemoglobin saturation during exercise using near-infrared spectroscopy in chronic kidney disease. Clin. Exp. Nephrol. 2019, 23, 32–42. [Google Scholar] [CrossRef]
- Crenshaw, A.G.; Elcadi, G.H.; Hellstrom, F.; Mathiassen, S.E. Reliability of near-infrared spectroscopy for measuring forearm and shoulder oxygenation in healthy males and females. Eur. J. Appl. Physiol. 2012, 112, 2703–2715. [Google Scholar] [CrossRef] [PubMed]
- Usher, A.; Babraj, J. Impact of sprint interval training on post-fatigue mitochondrial rate in professional boxers. Eur. J. Appl. Physiol. 2025, 125, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Niemeijer, V.M.; Jansen, J.P.; Spraakman, L.; Stam, H.J.; Praet, S.F.E. Oxygen delivery is not a limiting factor during post-exercise recovery in healthy young adults. J. Exerc. Sci. Fit. 2017, 15, 43–47. [Google Scholar] [CrossRef]
- Ryan, T.E.; Southern, W.M.; Brizendine, J.T.; McCully, K.K. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med. Sci. Sports Exerc. 2013, 45, 10–1249. [Google Scholar] [CrossRef]
- Mavridis, K.; Petridou, A.; Chatzinikolaou, A.; Mougios, V. Oxygenation Kinetics of Three Quadriceps Muscles During Squatting Exercise in Trained Men. Sports 2024, 12, 283. [Google Scholar] [CrossRef]
- O’Riordan, S.F.; Bishop, D.J.; Halson, S.L.; Broatch, J.R. Compression-induced improvements in post-exercise recovery are associated with enhanced blood flow and are not due to the placebo effect. Sci. Rep. 2022, 12, 16762. [Google Scholar] [CrossRef]
- Liu, R.; Lao, T.T.; Kwok, Y.L.; Li, Y.; Ying, M.T.C. Effects of graduated compression stockings with different pressure profiles on lower-limb venous structures and haemodynamics. Adv. Therapy 2008, 25, 465–478. [Google Scholar] [CrossRef]
- Kime, R.; Karlsen, T.; Nioka, S.; Lech, G.; Madsen, Ø.; Sæterdal, R.; Im, J.; Chance, B.; Stray-Gundersen, J. Discrepancy between cardiorespiratory system and skeletal muscle in elite cyclists after hypoxic training. Dyn. Med. 2003, 2, 1–9. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Tena, T.; Trascasa, C.; Pérez-Parra, J.; Godoy, R.; García, J.M.; Stringer, W.W. Training improves muscle oxidative capacity and oxygenation recovery kinetics in patients with chronic obstructive pulmonary disease. Eur. J. Appl. Physiol. 2003, 88, 580–587. [Google Scholar] [CrossRef]
- Rennerfelt, K.; Lindorsson, S.; Brisby, H.; Baranto, A.; Zhang, Q. Effects of Exercise Compression Stockings on Anterior Muscle Compartment Pressure and Oxygenation During Running: A Randomized Crossover Trial Conducted in Healthy Recreational Runners. Sports Med. 2019, 49, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Book, J.; Prince, C.N.; Villar, R.; Hughson, R.L.; Peterson, S.D. Investigating the impact of passive external lower limb compression on central and peripheral hemodynamics during exercise. Eur. J. Appl. Physiol. 2016, 116, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ménétrier, A.; Mourot, L.; Bouhaddi, M.; Regnard, J.; Tordi, N. Compression sleeves increase tissue oxygen saturation but not running performance. Int. J. Sports Med. 2011, 32, 864–868. [Google Scholar] [CrossRef]
- Sperlich, B.; Haegele, M.; Achtzehn, S.; Linville, J.; Holmberg, H.C.; Mester, J. Different types of compression clothing do not increase sub-maximal and maximal endurance performance in well-trained athletes. J. Sports Sci. 2010, 28, 609–614. [Google Scholar] [CrossRef]
- Brophy-Williams, N.; Fell, J.; Halson, S.; Kitic, C.; Driller, M. Pressure gradient differences between medical grade and sports compression socks. J. Text. Inst. 2021, 112, 187–191. [Google Scholar] [CrossRef]
- Hill, J.; Howatson, G.; van Someren, K.; Davidson, S.; Pedlar, C. The variation in pressures exerted by commercially available compression garments. Sports Eng. 2015, 18, 115–121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benincasa, M.T.; Coiro, F.; Coppola, S.; Serra, E.; Celentano, E.; Costa, C.; Albano, D.; Vastola, R. Effects of Compression Garments on Muscle Oxygen Saturation Recovery in the Upper Limbs Using Near-Infrared Spectroscopy. J. Funct. Morphol. Kinesiol. 2025, 10, 317. https://doi.org/10.3390/jfmk10030317
Benincasa MT, Coiro F, Coppola S, Serra E, Celentano E, Costa C, Albano D, Vastola R. Effects of Compression Garments on Muscle Oxygen Saturation Recovery in the Upper Limbs Using Near-Infrared Spectroscopy. Journal of Functional Morphology and Kinesiology. 2025; 10(3):317. https://doi.org/10.3390/jfmk10030317
Chicago/Turabian StyleBenincasa, Maria Teresa, Francesco Coiro, Silvia Coppola, Enrico Serra, Ester Celentano, Claudia Costa, Daniele Albano, and Rodolfo Vastola. 2025. "Effects of Compression Garments on Muscle Oxygen Saturation Recovery in the Upper Limbs Using Near-Infrared Spectroscopy" Journal of Functional Morphology and Kinesiology 10, no. 3: 317. https://doi.org/10.3390/jfmk10030317
APA StyleBenincasa, M. T., Coiro, F., Coppola, S., Serra, E., Celentano, E., Costa, C., Albano, D., & Vastola, R. (2025). Effects of Compression Garments on Muscle Oxygen Saturation Recovery in the Upper Limbs Using Near-Infrared Spectroscopy. Journal of Functional Morphology and Kinesiology, 10(3), 317. https://doi.org/10.3390/jfmk10030317




