Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation, and Eccentric Exercise for Shoulder Pain and Functionality in Supraspinatus Tendinopathy: A Single-Blind Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Subjects, Randomization, and Blinding
2.4. Outcomes Measurements
2.5. Interventions
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DASH | Disabilities of the Arm, Shoulder, and Hand |
| EE | Eccentric Exercise |
| NPRS | Numerical Pain Rate Scale |
| PE | Percutaneous Electrolysis |
| PNS | Percutaneous peripheral Nerve Stimulation |
| PPT | Pressure Pain Threshold |
| ROM | Range of Motion |
| SPADI | Shoulder Pain and Disability Index |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| US | Therapeutic Ultrasound |
References
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator Cuff Calcific Tendinopathy: From Diagnosis to Treatment. Acta Biomed. 2018, 89, 186–196. [Google Scholar] [CrossRef]
- Doiron-Cadrin, P.; Lafrance, S.; Saulnier, M.; Cournoyer, É.; Roy, J.S.; Dyer, J.O.; Frémont, P.; Dionne, C.; MacDermid, J.C.; Tousignant, M.; et al. Shoulder Rotator Cuff Disorders: A Systematic Review of Clinical Practice Guidelines and Semantic Analyses of Recommendations. Arch. Phys. Med. Rehabil. 2020, 101, 1233–1242. [Google Scholar] [CrossRef]
- Alcántara-Martos, T.; Delgado-Martínez, A.; Aznar-Zafra, S.; Fernández-Rodríguez, J.; Fernández-Jaén, T. Tendinopatías. Trauma Fund MAPFRE 2011, 22, 12–21. [Google Scholar]
- Cook, J.L.; Purdam, C.R. Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load-Induced Tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Maffulli, N.; Kannus, P. Where Is the Pain Coming from in Tendinopathy? It May Be Biochemical, Not Only Structural, in Origin. Br. J. Sports Med. 2000, 34, 81–83. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Taunton, J.E.; Bonar, F. Overuse Tendinosis, Not Tendinitis Part 1: A New Paradigm for a Difficult Clinical Problem. Phys. Sportsmed. 2000, 28, 38–48. [Google Scholar] [CrossRef][Green Version]
- Cook, J.L.; Khan, K.M.; Maffulli, N.; Purdam, C. Overuse Tendinosis, Not Tendinitis Part 2: Applying the New Approach to Patellar Tendinopathy. Phys. Sportsmed. 2000, 28, 31–46. [Google Scholar] [CrossRef]
- Docking, S.I.; Cook, J. How Do Tendons Adapt? Going beyond Tissue Responses to Understand Positive Adaptation and Pathology Development: A Narrative Review. J. Musculoskelet. Neuronal Interact. 2019, 19, 300–310. [Google Scholar]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the Continuum Model of Tendon Pathology: What Is Its Merit in Clinical Practice and Research? Br. J. Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef]
- Walz, D.M.; Newman, J.S.; Konin, G.P.; Ross, G. Epicondylitis: Pathogenesis, Imaging, and Treatment. Radiographics 2010, 30, 167–184. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, K. Advances in Musculoskeletal Ultrasound for Assistive Diagnosis in Pain Clinics. Pain. Ther. 2025, 14, 791–801. [Google Scholar] [CrossRef]
- Santilli, G.; Ciccarelli, A.; Martino, M.; Pacini, P.; Agostini, F.; Bernetti, A.; Giuliani, L.; Del Gaudio, G.; Mangone, M.; Colonna, V.; et al. Pain, Function, and Elastosonographic Assessment After Shockwave Therapy in Non-Calcific Supraspinatus Tendinopathy: A Retrospective Observational Study. J. Funct. Morphol. Kinesiol. 2025, 10, 39. [Google Scholar] [CrossRef]
- Vincenten, S.C.C.; Voermans, N.C.; Cameron, D.; van Engelen, B.G.M.; van Alfen, N.; Mul, K. The Complementary Use of Muscle Ultrasound and MRI in FSHD: Early versus Later Disease Stage Follow-Up. Clin. Neurophysiol. 2024. [Google Scholar] [CrossRef]
- Girgis, B.; Duarte, J.A. Physical Therapy for Tendinopathy: An Umbrella Review of Systematic Reviews and Meta-Analyses. Phys. Ther. Sport 2020, 46, 30–46. [Google Scholar] [CrossRef]
- Cardoso, T.B.; Pizzari, T.; Kinsella, R.; Hope, D.; Cook, J.L. Current Trends in Tendinopathy Management. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 122–140. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Ferreira, M.H.L.; Araujo, G.A.S.; De-La-Cruz-Torrez, B. Effectiveness of Percutaneous Needle Electrolysis to Reduce Pain in Tendinopathies: A Systematic Review With Meta-Analysis. J. Sport. Rehabil. 2024, 33, 307–316. [Google Scholar] [CrossRef]
- Ragone, F.; Pérez-Guillén, S.; Carrasco-Uribarren, A.; Cabanillas-Barea, S.; Ceballos-Laita, L.; Ramón Rodríguez-Rubio, P.; Cabanas-Valdés, R. The Effects of Soft-Tissue Techniques and Exercise in the Treatment of Patellar Tendinopathy-Systematic Review and Meta-Analysis. Healthcare 2024, 12, 427. [Google Scholar] [CrossRef]
- Yildizgoren, M.T.; Bagcier, F. A Modern Interpretation of Traditional Galvanic Current: Percutaneous Needle Electrolysis Therapy. Acupunct. Med. 2024, 42, 56–57. [Google Scholar] [CrossRef]
- Minaya-Muñoz, F.; Valera-Garrido, F. Neuromodulación Percutánea Ecoguiada. In Fisioterapia invasiva; Valera-Garrido, F., Minaya-Muñoz, F., Eds.; Elsevier: Barcelona, Spain, 2017; pp. 283–294. [Google Scholar]
- Dejaco, B.; Habets, B.; van Loon, C.; van Grinsven, S.; van Cingel, R. Eccentric versus Conventional Exercise Therapy in Patients with Rotator Cuff Tendinopathy: A Randomized, Single Blinded, Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2051–2059. [Google Scholar] [CrossRef]
- Heron, S.; Woby, S.; Thompson, D. Comparison of Three Types of Exercise in the Treatment of Rotator Cuff Tendinopathy/Shoulder Impingement Syndrome: A Randomized Controlled Trial. Physiotherapy 2017, 103, 167–173. [Google Scholar] [CrossRef]
- Viti, A.; Panconi, G.; Guarducci, S.; Garfagnini, S.; Mondonico, M.; Bravi, R.; Minciacchi, D. Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study. Int. J. Environ. Res. Public Health 2023, 20, 3934. [Google Scholar] [CrossRef]
- Rodríguez-Huguet, M.; Góngora-Rodríguez, J.; Rodríguez-Huguet, P.; Ibañez-Vera, A.J.; Rodríguez-Almagro, D.; Martín-Valero, R.; Díaz-Fernández, Á.; Lomas-Vega, R. Effectiveness of Percutaneous Electrolysis in Supraspinatus Tendinopathy: A Single-Blinded Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1837. [Google Scholar] [CrossRef]
- Littlewood, C.; Bateman, M.; Connor, C.; Gibson, J.; Horsley, I.; Jaggi, A.; Jones, V.; Meakins, A.; Scott, M. Physiotherapists’ Recommendations for Examination and Treatment of Rotator Cuff Related Shoulder Pain: A Consensus Exercise. Physiother. Pract. Res. 2019, 40, 87–94. [Google Scholar] [CrossRef]
- Kinsella, R.; Cowan, S.M.; Watson, L.; Pizzari, T. A Comparison of Isometric, Isotonic Concentric and Isotonic Eccentric Exercises in the Physiotherapy Management of Subacromial Pain Syndrome/Rotator Cuff Tendinopathy: Study Protocol for a Pilot Randomised Controlled Trial. Pilot. Feasibility Stud. 2017, 3, 45. [Google Scholar] [CrossRef]
- Góngora-Rodríguez, J.; Rosety-Rodríguez, M.Á.; Rodríguez-Almagro, D.; Martín-Valero, R.; Góngora-Rodríguez, P.; Rodríguez-Huguet, M. Structural and Functional Changes in Supraspinatus Tendinopathy through Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation and Eccentric Exercise Combined Therapy: A Single-Blinded Randomized Clinical Trial. Biomedicines 2024, 12, 771. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test-Retest Reliability, Validity, and Minimum Detectable Change of Visual Analog, Numerical Rating, and Verbal Rating Scales for Measurement of Osteoarthritic Knee Pain. J. Pain. Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The Validity of Pain Intensity Measures: What Do the NRS, VAS, VRS, and FPS-R Measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Hervás, M.T.; Navarro Collado, M.J.; Peiró, S.; Rodrigo Pérez, J.L.; López Matéu, P.; Martínez Tello, I. Versión Española Del Cuestionario DASH. Adaptación Transcultural, Fiabilidad, Validez y Sensibilidad a Los Cambios. Med. Clin. 2006, 127, 441–447. [Google Scholar] [CrossRef]
- Engebretsen, K.; Grotle, M.; Bautz-Holter, E.; Ekeberg, O.M.; Brox, J.I. Determinants of the Shoulder Pain and Disability Index in Patients with Subacromial Shoulder Pain. J. Rehabil. Med. 2010, 42, 499–505. [Google Scholar] [CrossRef]
- Torres-Lacomba, M.; Sánchez-Sánchez, B.; Prieto-Gómez, V.; Pacheco-da-Costa, S.; Yuste-Sánchez, M.J.; Navarro-Brazález, B.; Gutiérrez-Ortega, C. Spanish Cultural Adaptation and Validation of the Shoulder Pain and Disability Index, and the Oxford Shoulder Score after Breast Cancer Surgery. Health Qual. Life Outcomes 2015, 13, 63. [Google Scholar] [CrossRef]
- Kolber, M.J.; Hanney, W.J. The Reliability and Concurrent Validity of Shoulder Mobility Measurements Using a Digital Inclinometer and Goniometer: A Technical Report. Int. J. Sports Phys. Ther. 2012, 7, 306–313. [Google Scholar]
- Kinser, A.M.; Sands, W.A.; Stone, M.H. Realiability and Validity of a Pressure Algometer. J. Strength Cond. Res. 2009, 23, 312–314. [Google Scholar] [CrossRef]
- Lozano, A.; Morales, M.; Lorenzo, C.; Sánchez, A. Dolor y Estrés En Fisioterapia: Algometría de Presión. Rev. Iberoam. Fisioter. Kinesiol. 2006, 9, 3–10. [Google Scholar] [CrossRef]
- Chesterton, L.; Barlas, P.; Foster, N.; Baxter, G.; Wright, C. Gender Differences in Pressure Pain Threshold in Healthy Humans. Pain 2003, 101, 259–266. [Google Scholar] [CrossRef]
- Mäkelä, S.; Pöntinen, P. Reliability of Pressure Threshold Meter in Location of Latent Trigger Points in Healthy Subjects. Scand. J. Acupunct. Electrother 1988, 3, 45–50. [Google Scholar]
- Maya-Martín, J.; Albornoz-Cabello, M. Estimulación Eléctrica Transcutánea. In Electroestimulación Transcutánea y Neuromuscular, y Neuromodulación; Albornoz-Cabello, M., Maya-Martín, J., Eds.; Elsevier: Barcelona, Spain, 2021; pp. 1–76. ISBN 978-84-9113-606-4. [Google Scholar]
- Gunay Ucurum, S.; Kaya, D.O.; Kayali, Y.; Askin, A.; Tekindal, M.A. Comparison of Different Electrotherapy Methods and Exercise Therapy in Shoulder Impingement Syndrome: A Prospective Randomized Controlled Trial. Acta Orthop. Traumatol. Turc. 2018, 52, 249–255. [Google Scholar] [CrossRef]
- Balci, T.O.; Turk, A.C.; Sahin, F.; Kotevoglu, N.; Kuran, B. Efficacy of Therapeutic Ultrasound in Treatment of Adhesive Capsulitis: A Prospective Double Blind Placebo-Controlled Randomized Trial. J. Back Musculoskelet. Rehabil. 2018, 31, 955–961. [Google Scholar] [CrossRef]
- Desmeules, F.; Boudreault, J.; Roy, J.S.; Dionne, C.; Frémont, P.; MacDermid, J.C. The Efficacy of Therapeutic Ultrasound for Rotator Cuff Tendinopathy: A Systematic Review and Meta-Analysis. Phys. Ther. Sport 2015, 16, 276–284. [Google Scholar] [CrossRef]
- Page, M.J.; Green, S.; Mrocki, M.A.; Surace, S.J.; Deitch, J.; Mcbain, B.; Lyttle, N.; Buchbinder, R. Electrotherapy Modalities for Rotator Cuff Disease. Cochrane Database Syst. Rev. 2016, 2016, CD012225. [Google Scholar] [CrossRef]
- Valera-Garrido, F.; Minaya-Muñoz, F. Electrolisis Percutánea En Tendón y Bursa. Metodología de Aplicación. In Electrolisis Percutánea Musculoesquelética. Tendón y Bursa; Valera-Garrido, F., Minaya-Muñoz, F., Eds.; Elsevier: Barcelona, Spain, 2021; pp. 45–70. ISBN 978-84-9113-016-1. [Google Scholar]
- Arias-Buría, J.L.; Truyols-Domínguez, S.; Valero-Alcaide, R.; Salom-Moreno, J.; Atín-Arratibel, M.A.; Fernández-De-Las-Peñas, C. Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 315219. [Google Scholar] [CrossRef]
- De-Miguel-Valtierra, L.; Salom-Moreno, J.; Fernández-de-las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L. Ultrasound-Guided Application of Percutaneous Electrolysis as an Adjunct to Exercise and Manual Therapy for Subacromial Pain Syndrome: A Randomized Clinical Trial. J. Pain 2018, 19, 1201–1210. [Google Scholar] [CrossRef]
- Valera-Garrido, F.; Minaya-Muñoz, F.; Pereira-Barbosa, M. Electrolisis Percutánea Ecoguiada En El Manguito Rotador. In Electrolisis Percutánea Musculoesquelética. Tendón y Bursa; Valera-Garrido, F., Minaya-Muñoz, F., Eds.; Elsevier: Barcelona, Spain, 2021; pp. 107–130. [Google Scholar]
- Vallés-Carrascosa, E.; Gallego-Izquierdo, T.; Jiménez-Rejano, J.J.; Plaza-Manzano, G.; Pecos-Martín, D.; Hita-Contreras, F.; Achalandabaso Ochoa, A. Pain, Motion and Function Comparison of Two Exercise Protocols for the Rotator Cuff and Scapular Stabilizers in Patients with Subacromial Syndrome. J. Hand Ther. 2018, 31, 227–237. [Google Scholar] [CrossRef]
- Macías-Hernández, S.I.; Pérez-Ramírez, L.E. Fortalecimiento Excéntrico En Tendinopatías Del Manguito de Los Rotadores Asociadas a Pinzamiento Subacromial. Evidencia Actual. Cirugía Cir. 2015, 83, 74–80. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Asensio-Olea, L.; Leirós-Rodríguez, R.; Marqués-Sánchez, M.P.; de Carvalho, F.O.; Maciel, L.Y.S. Efficacy of Percutaneous Electrolysis for the Treatment of Tendinopathies: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2022, 37, 747–759. [Google Scholar] [CrossRef]
- Gómez-Chiguano, G.F.; Navarro-Santana, M.J.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effectiveness of Ultrasound-Guided Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1055–1071. [Google Scholar] [CrossRef]
- Osborne, J.D.; Gowda, A.L.; Wiater, B.; Wiater, J.M. Rotator Cuff Rehabilitation: Current Theories and Practice. Physician Sportsmed. 2016, 44, 85–92. [Google Scholar] [CrossRef]
- Lewis, J.S. Rotator Cuff Tendinopathy. Br. J. Sports Med. 2009, 43, 236–241. [Google Scholar] [CrossRef]
- Dubé, M.; Desmeules, F.; Lewis, J.; Roy, J. Rotator Cuff-Related Shoulder Pain: Does the Type of Exercise Influence the Outcomes? Protocol of a Randomised Controlled Trial. BMJ Open 2020, 10, e039976. [Google Scholar] [CrossRef]
- Martínez-Silván, D.; Santomé-Martínez, F.; Champón-Chekroun, A.M.; Velázquez-Saornil, J.; Gómez-Merino, S.; Cos-Morera, M.A.; Morral-Fernández, A.; Mascaró-Vilella, A.; Ricis-Guerra, M.; García-Bol, F.; et al. Clinical Use of Percutaneous Needle Electrolysis in Musculoskeletal Injuries: A Critical and Systematic Review of the Literature. Apunt. Sports Med. 2022, 57, 100396. [Google Scholar] [CrossRef]
- Augustyn, D.; Paez, A. The Effectiveness of Intratissue Percutaneous Electrolysis for the Treatment of Tendinopathy: A Systematic Review. South Afr. J. Sports Med. 2022, 34, v34i1a12754. [Google Scholar] [CrossRef]
- Fakontis, C.; Iakovidis, P.; Lytras, D.; Kasimis, K.; Koutras, G.; Ntinou, S.R.; Kottaras, A.; Chatziprodromidou, I.P.; Chatzikonstantinou, P.; Apostolou, T. Efficacy of Percutaneous Needle Electrolysis versus Dry Needling in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J. Back Musculoskelet. Rehabil. 2023, 36, 1033–1046. [Google Scholar] [CrossRef]
- Peñín-Franch, A.; García-Vidal, J.A.; Gómez, A.I.; Escolar-Reina, P.; Medina-Mirapeix, F.; Pelegrín, P. The Total Electric Charge and Time of Application of Galvanic Currents to Macrophages Can Optimize the Release of IL-1β with Low Cell Death. Sci. Rep. 2024, 14, 30871. [Google Scholar] [CrossRef]
- Margalef, R.; Minaya-Muñoz, F.; Valera-Garrido, F.; Bosque, M.; Santafé, M.M. Changes in PH as a Result of Galvanic Currents Used in Percutaneous Needle Electrolysis. Rev. Fisioter. Invasiva J. Invasive Tech. Phys. Ther. 2020, 03, 006. [Google Scholar] [CrossRef]
- Varela-Rodríguez, S.; Sánchez-Sánchez, J.L.; Velasco, E.; Delicado-Miralles, M.; Sánchez-González, J.L. Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 2889. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Mayoral-Martín, A.; Varol, U. Short-Term Effectiveness of High- and Low-Intensity Percutaneous Electrolysis in Patients with Patellofemoral Pain Syndrome: A Pilot Study. World J. Orthop. 2021, 12, 781–790. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Navarro-López, V.; Cañada-Sánchez, P.; Juárez-Vela, R.; Viñaspre-Hernández, R.R.d.; Varela-Rodríguez, S. Efficacy of Different Intensities of Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 66. [Google Scholar] [CrossRef]
- García-Vidal, J.; Pelegrín-Vivancos, P.; Escolar-Reina, P.; Medina-Mirapeix, F. Inflammatory Response of Two Invasive Techniques in the Mouse with Collagenase Induced Tendinopathy. Rev. Fisioter. Invasiva J. Invasive Tech. Phys. Ther. 2019, 02, 80. [Google Scholar] [CrossRef]
- Wilson, R.D.; Harris, M.A.; Gunzler, D.D.; Bennett, M.E.; Chae, J. Percutaneous Peripheral Nerve Stimulation for Chronic Pain in Subacromial Impingement Syndrome: A Case Series. Neuromodulation 2014, 17, 771–776. [Google Scholar] [CrossRef]
- Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Sánchez-Ibáñez, J.M.; Belón-Pérez, P. Percutaneous Electrolysis (EPI®), a Promising Technology in the Treatment of Insertional Patellar Tendinopathy in Soccer Players. In Ambient Intelligence—Software and Applications—13th International Symposium on Ambient Intelligence; Springer: Cham, Switzerland, 2023; pp. 24–31. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.L.; Calderón-Díez, L.; Herrero-Turrión, J.; Méndez-Sánchez, R.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C. Changes in Gene Expression Associated with Collagen Regeneration and Remodeling of Extracellular Matrix after Percutaneous Electrolysis on Collagenase-Induced Achilles Tendinopathy in an Experimental Animal Model: A Pilot Study. J. Clin. Med. 2020, 9, 3316. [Google Scholar] [CrossRef]
- Peñín-Franch, A.; García-Vidal, J.A.; Martínez, C.M.; Escolar-Reina, P.; Martínez-Ojeda, R.M.; Gómez, A.I.; Bueno, J.M.; Minaya-Muñoz, F.; Valera-Garrido, F.; Medina-Mirapeix, F.; et al. Galvanic Current Activates the NLRP3 Inflammasome to Promote Type I Collagen Production in Tendon. Elife 2022, 11, e73675. [Google Scholar] [CrossRef]
- Peñín-Franch, A.; García-Vidal, J.A.; Escolar-Reina, P.; Medina-Mirapeix, F.; Pelegrín-Vivancos, P. Electrolisis e Inflamación. Bases Biológicas de La Electrolisis Percutánea. In Electrolisis Percutánea Musculoesquelética. Tendón y Bursa; Valera-Garrido, F., Minaya-Muñoz, F., Eds.; Elsevier: Barcelona, Spain, 2021; pp. 25–32. ISBN 978-84-9113-016-1. [Google Scholar]
- Benito-de-Pedro, A.I.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Benito-de-Pedro, M. Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial. Life 2023, 13, 939. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Castro-Sánchez, A.M. Neuromodulación Periférica Transcutánea. In Electroestimulación Transcutánea y Neuromuscular, y Neuromodulación; Albornoz-Cabello, M., Maya-Martín, J., Eds.; Elsevier: Barcelona, Spain, 2021; pp. 171–196. ISBN 978-84-9113-606-4. [Google Scholar]
- Shi, L.L.; Freehill, M.T.; Yannopoulos, P.; Warner, J.J.P. Suprascapular Nerve: Is It Important in Cuff Pathology? Adv. Orthop. 2012, 2012, 516985. [Google Scholar] [CrossRef]
- Wu, W.T.; Chen, L.R.; Chang, H.C.; Chang, K.V.; Özçakar, L. Quantitative Ultrasonographic Analysis of Changes of the Suprascapular Nerve in the Aging Population With Shoulder Pain. Front. Bioeng. Biotechnol. 2021, 9, 640747. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Kwak, J.M.; Jung, H.-W.; Kholinne, E.; Jeon, I.H. Suprascapular Nerve Neuropathy Leads to Supraspinatus Tendon Degeneration. J. Orthop. Sci. 2020, 25, 588–594. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric Exercise Induces Analgesia and Reduces Inhibition in Patellar Tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ko, J.B.; Farthing, J.P.; Butcher, S.J. Investigation of Supraspinatus Muscle Architecture Following Concentric and Eccentric Training. J. Sci. Med. Sport 2015, 18, 378–382. [Google Scholar] [CrossRef]
- Saylor-Pavkovich, E. Strength Exercises Combined With Dry Needling With Electrical Stimulation Improve Pain and Function in Patients With Chronic Rotator Cuff Tendinopathy: A Retrospective Case Series. Int. J. Sports Phys. Ther. 2016, 11, 409–422. [Google Scholar]
- Ortega-Castillo, M.; Medina-Porqueres, I. Effectiveness of the Eccentric Exercise Therapy in Physically Active Adults with Symptomatic Shoulder Impingement or Lateral Epicondylar Tendinopathy: A Systematic Review. J. Sci. Med. Sport 2016, 19, 438–453. [Google Scholar] [CrossRef]
- Stasinopoulos, D.; Stasinopoulos, I. Comparison of Effects of Eccentric Training, Eccentric-Concentric Training, and Eccentric-Concentric Training Combined with Isometric Contraction in the Treatment of Lateral Elbow Tendinopathy. J. Hand Ther. 2017, 30, 13–19. [Google Scholar] [CrossRef]
- Habets, B.; van Cingel, R.; Backx, F.; Huisstede, B. Alfredson versus Silbernagel Exercise Therapy in Chronic Midportion Achilles Tendinopathy: Study Protocol for a Randomized Controlled Trial. BMC Musculoskelet Disord 2017, 18, 296. [Google Scholar] [CrossRef]
- Habets, B.; van Cingel, R.E.H.; Backx, F.J.G.; van Elten, H.J.; Zuithoff, P.; Huisstede, B.M.A. No Difference in Clinical Effects When Comparing Alfredson Eccentric and Silbernagel Combined Concentric-Eccentric Loading in Achilles Tendinopathy: A Randomized Controlled Trial. Orthop J. Sports Med. 2021, 9, 23259671211031254. [Google Scholar] [CrossRef]
- Littlewood, C.; Ashton, J.; Chance-Larsen, K.; May, S.; Sturrock, B. Exercise for Rotator Cuff Tendinopathy: A Systematic Review. Physiotherapy 2012, 98, 101–109. [Google Scholar] [CrossRef]
- Maenhout, A.G.; Mahieu, N.N.; De Muynck, M.; De Wilde, L.F.; Cools, A.M. Does Adding Heavy Load Eccentric Training to Rehabilitation of Patients with Unilateral Subacromial Impingement Result in Better Outcome? A Randomized, Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1158–1167. [Google Scholar] [CrossRef]
- Santilli, G.; Martino, M.; Pacini, P.; Agostini, F.; Bernetti, A.; Giuliani, L.; Gaudio, G.D.; Mangone, M.; Colonna, V.; Vetrano, M.; et al. Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study. J. Funct. Morphol. Kinesiol. 2024, 10, 2. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y. The Therapeutic Effect of Extracorporeal Shock Wave Therapy Combined with Kinesio Tape on Plantar Fasciitis. J. Back Musculoskelet. Rehabil. 2023, 36, 1203–1211. [Google Scholar] [CrossRef]
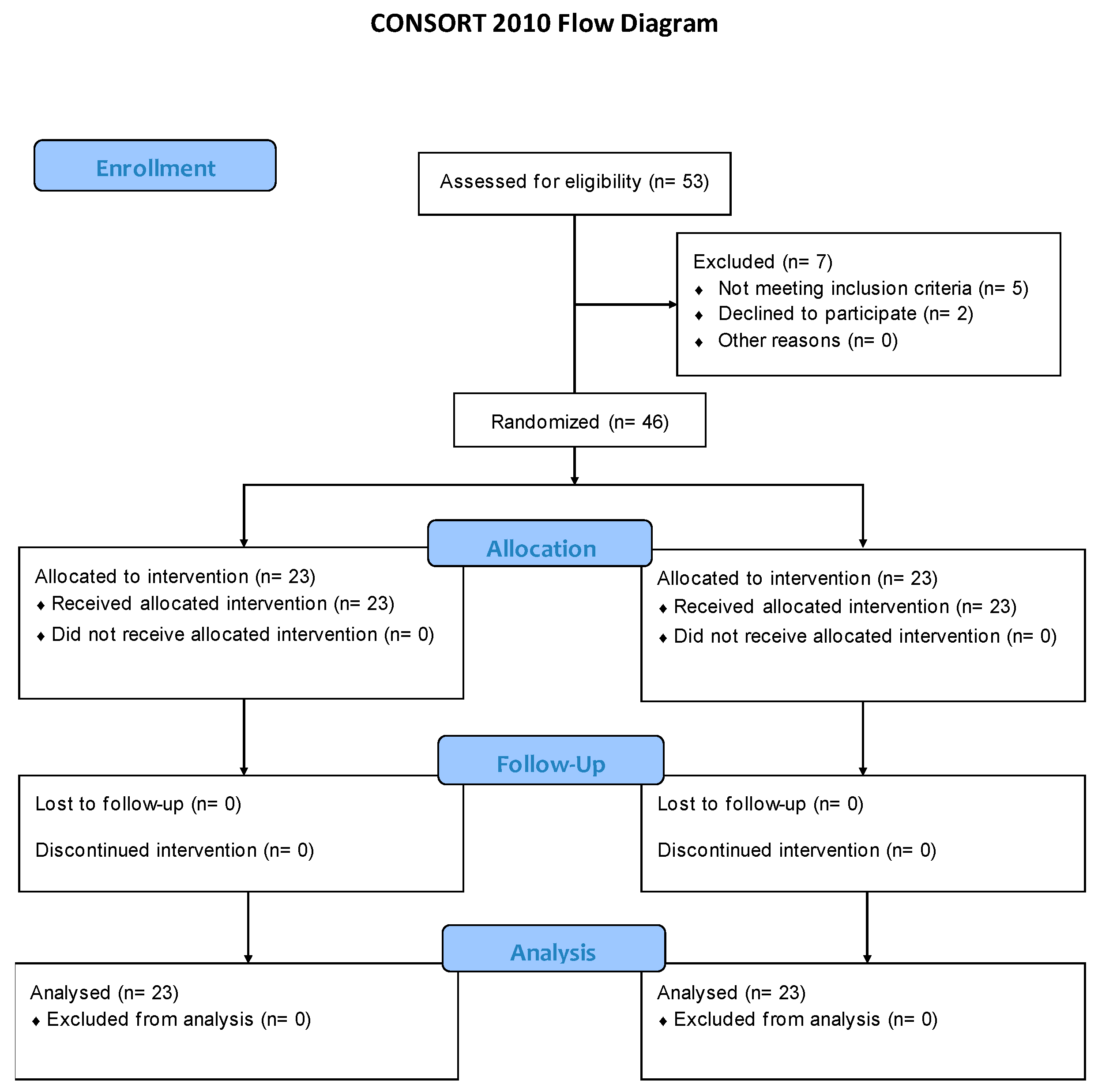

| ALL (46) | PE+PNS+EE GROUP (23) | TENS+US+EE GROUP (23) | ||||||
|---|---|---|---|---|---|---|---|---|
| CATEGORICAL | Frequency | % | Frequency | % | Frequency | % | p | |
| Sex | Male | 32 | 69.60 | 17 | 73.90 | 15 | 65.20 | 0.522 |
| Female | 14 | 30.40 | 6 | 26.10 | 8 | 34.80 | ||
| Affected Hand | Right | 31 | 67.40 | 15 | 65.20 | 16 | 69.60 | 0.753 |
| Left | 15 | 32.60 | 8 | 34.80 | 7 | 30.40 | ||
| Dominant Hand | Right | 43 | 93.50 | 22 | 95.70 | 21 | 91.30 | 0.550 |
| Left | 3 | 6.50 | 1 | 4.30 | 2 | 8.70 | ||
| CONTINUOUS | Mean | SD | Mean | SD | Mean | SD | p | |
| Age | 44.15 | 12.26 | 44.39 | 13.90 | 43.91 | 10.69 | 0.897 | |
| Weight | 82.70 | 11.47 | 84.04 | 7.60 | 81.35 | 14.41 | 0.433 | |
| Height | 1.74 | 0.07 | 1.74 | 0.07 | 1.74 | 0.08 | 0.984 | |
| BMI | 27.44 | 4.15 | 27.87 | 2.83 | 27.01 | 5.18 | 0.488 | |
| NPRS | 7.24 | 1.37 | 7.52 | 1.31 | 6.96 | 1.40 | 0.164 | |
| PPT Proximal | 2.63 | 0.97 | 2.62 | 0.92 | 2.65 | 1.04 | 0.905 | |
| PPT Medium | 2.78 | 1.01 | 2.75 | 1.09 | 2.81 | 0.95 | 0.853 | |
| PPT Distal | 2.62 | 0.98 | 2.60 | 1.09 | 2.63 | 0.89 | 0.918 | |
| Disability (DASH) | 54.72 | 19.86 | 62.00 | 16.46 | 47.43 | 20.61 | 0.011 | |
| Disability (DASH) % | 45.60 | 16.55 | 51.67 | 13.72 | 39.53 | 17.17 | 0.011 | |
| Disability (SPADI) | 69.26 | 19.46 | 70.83 | 18.17 | 67.70 | 20.96 | 0.591 | |
| Disability (SPADI) % | 53.28 | 14.97 | 54.48 | 13.98 | 52.07 | 16.12 | 0.591 | |
| Flexion ROM | 118.09 | 22.44 | 115.00 | 21.94 | 121.17 | 22.99 | 0.357 | |
| Extension ROM | 25.20 | 7.26 | 26.87 | 8.00 | 23.52 | 6.16 | 0.119 | |
| Abduction ROM | 99.98 | 20.86 | 104.52 | 22.80 | 95.43 | 18.09 | 0.142 | |
| Adduction ROM | 26.80 | 8.22 | 27.83 | 9.79 | 25.78 | 6.32 | 0.406 | |
| Internal Rotation ROM | 56.54 | 19.29 | 57.43 | 22.18 | 55.65 | 16.36 | 0.758 | |
| External Rotation ROM | 60.76 | 21.41 | 60.96 | 24.83 | 60.57 | 17.92 | 0.951 | |
| VARIABLE | F | p | η2 | POWER |
|---|---|---|---|---|
| NPRS | 45.787 | <0.001 ** | 0.510 | 1.000 |
| PPT Proximal | 22.232 | <0.001 ** | 0.336 | 1.000 |
| PPT Medium | 22.464 | <0.001 ** | 0.338 | 1.000 |
| PPT Distal | 17.883 | <0.001 ** | 0.289 | 1.000 |
| Disability (DASH) | 78.393 | <0.001 ** | 0.641 | 1.000 |
| Disability (DASH) % | 78.393 | <0.001 ** | 0.641 | 1.000 |
| Disability (SPADI) | 69.063 | <0.001 ** | 0.611 | 1.000 |
| Disability (SPADI) % | 69.063 | <0.001 ** | 0.611 | 1.000 |
| Flexion ROM | 28.185 | <0.001 ** | 0.390 | 1.000 |
| Extension ROM | 4.840 | 0.017 * | 0.099 | 0.715 |
| Abduction ROM | 7.751 | 0.002 * | 0.150 | 0.894 |
| Adduction ROM | 1.746 | 0.193 | 0.038 | 0.273 |
| Internal Rotation ROM | 11.042 | 0.001 * | 0.201 | 0.927 |
| External Rotation ROM | 7.504 | 0.006 * | 0.146 | 0.806 |
| VARIABLE | Post-Treatment | Within-Group Change Score | Effect Size | Between-Groups Change Score | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean Difference | p | d | Mean Difference | p | d | ||
| NPRS | PE+PNS+EE | 1.57 | 1.67 | −5.96 | <0.001 | −2.996 | −3.61 | <0.001 ** | −2.323 |
| TENS+US+EE | 4.61 | 1.62 | −2.35 | <0.001 | −2.512 | ||||
| PPT Proximal | PE+PNS+EE | 4.09 | 0.99 | 1.47 | <0.001 | 1.947 | 0.88 | <0.001 ** | 1.323 |
| TENS+US+EE | 3.24 | 1.01 | 0.59 | <0.001 | 1.050 | ||||
| PPT Medium | PE+PNS+EE | 4.11 | 0.93 | 1.36 | <0.001 | 1.505 | 0.91 | <0.001 ** | 1.261 |
| TENS+US+EE | 3.25 | 1.02 | 0.44 | <0.001 | 0.914 | ||||
| PPT Distal | PE+PNS+EE | 4.06 | 1.03 | 1.46 | <0.001 | 1.442 | 0.89 | 0.001 * | 1.033 |
| TENS+US+EE | 3.20 | 0.90 | 0.57 | <0.001 | 0.825 | ||||
| Disability (DASH) | PE+PNS+EE | 11.26 | 10.99 | −50.74 | <0.001 | −3.007 | −36.83 | <0.001 ** | −2.531 |
| TENS+US+EE | 33.52 | 24.12 | −13.91 | <0.001 | −1.182 | ||||
| Disability (DASH) % | PE+PNS+EE | 9.38 | 9.16 | −42.28 | <0.001 | −3.007 | −30.69 | <0.001 ** | −2.531 |
| TENS+US+EE | 27.93 | 20.10 | −11.59 | <0.001 | −1.182 | ||||
| Disability (SPADI) | PE+PNS+EE | 15.17 | 12.42 | −55.65 | <0.001 | −3.353 | −35.35 | <0.001 ** | −2.601 |
| TENS+US+EE | 47.39 | 20.66 | −20.30 | <0.001 | −2.094 | ||||
| Disability (SPADI) % | PE+PNS+EE | 11.67 | 9.56 | −42.81 | <0.001 | −3.353 | −27.19 | <0.001 ** | −2.601 |
| TENS+US+EE | 36.45 | 15.89 | −15.62 | <0.001 | −2.094 | ||||
| Flexion ROM | PE+PNS+EE | 155.04 | 13.49 | 40.04 | <0.001 | 2.057 | 22.70 | <0.001 ** | 1.443 |
| TENS+US+EE | 138.52 | 23.70 | 17.35 | <0.001 | 1.612 | ||||
| Extension ROM | PE+PNS+EE | 36.65 | 5.99 | 9.78 | <0.001 | 1.547 | 4.04 | 0.015 * | 0.749 |
| TENS+US+EE | 29.26 | 5.50 | 5.74 | <0.001 | 1.342 | ||||
| Abduction ROM | PE+PNS+EE | 143.39 | 17.50 | 38.87 | <0.001 | 2.232 | 14.26 | 0.004 * | 0.903 |
| TENS+US+EE | 120.04 | 23.44 | 24.61 | <0.001 | 1.760 | ||||
| Adduction ROM | PE+PNS+EE | 32.91 | 5.40 | 5.09 | 0.002 | 0.736 | 1.52 | 0.349 | 0.279 |
| TENS+US+EE | 29.35 | 4.82 | 3.57 | <0.001 | 1.046 | ||||
| Internal Rotation ROM | PE+PNS+EE | 85.70 | 8.04 | 28.26 | <0.001 | 1.372 | 16.83 | <0.001 ** | 1.084 |
| TENS+US+EE | 67.09 | 15.17 | 11.43 | <0.001 | 1.503 | ||||
| External Rotation ROM | PE+PNS+EE | 84.04 | 10.77 | 23.09 | <0.001 | 1.202 | 12.70 | 0.006 * | 0.853 |
| TENS+US+EE | 70.96 | 17.14 | 10.39 | <0.001 | 1.202 | ||||
| VARIABLE | 12 Weeks Follow-Up | Within-Group Change Score | Effect Size | Between-Groups Change Score | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean Difference | p | d | Mean Difference | p | d | ||
| NPRS | PE+PNS+EE | 0.80 | 1.23 | −6.72 | <0.001 | −4.054 | −3.41 | <0.001 ** | −2.206 |
| TENS+US+EE | 3.65 | 2.31 | −3.30 | <0.001 | −2.314 | ||||
| PPT Proximal | PE+PNS+EE | 4.50 | 0.78 | 1.88 | <0.001 | 2.011 | 1.20 | <0.001 ** | 1.530 |
| TENS+US+EE | 3.34 | 0.93 | 0.69 | <0.001 | 1.170 | ||||
| PPT Medium | PE+PNS+EE | 4.54 | 0.72 | 1.79 | <0.001 | 1.723 | 1.26 | <0.001 ** | 1.528 |
| TENS+US+EE | 3.34 | 0.98 | 0.53 | <0.001 | 1.008 | ||||
| PPT Distal | PE+PNS+EE | 4.49 | 0.81 | 1.89 | <0.001 | 1.766 | 1.21 | <0.001 ** | 1.362 |
| TENS+US+EE | 3.31 | 0.91 | 0.68 | <0.001 | 1.031 | ||||
| Disability (DASH) | PE+PNS+EE | 4.09 | 5.25 | −57.91 | <0.001 | −3.589 | −40.43 | <0.001 ** | −2.866 |
| TENS+US+EE | 29.96 | 23.39 | −17.48 | <0.001 | −1.489 | ||||
| Disability (DASH) % | PE+PNS+EE | 3.41 | 4.37 | −48.26 | <0.001 | −3.589 | −33.70 | <0.001 ** | −2.866 |
| TENS+US+EE | 24.96 | 19.50 | −14.57 | <0.001 | −1.489 | ||||
| Disability (SPADI) | PE+PNS+EE | 7.00 | 7.29 | −63.83 | <0.001 | −3.670 | −38.35 | <0.001 ** | −2.571 |
| TENS+US+EE | 42.22 | 22.28 | −25.48 | <0.001 | −2.135 | ||||
| Disability (SPADI) % | PE+PNS+EE | 5.38 | 5.60 | −49.10 | <0.001 | −3.670 | −29.50 | <0.001 ** | −2.571 |
| TENS+US+EE | 32.47 | 17.14 | −19.60 | <0.001 | −2.135 | ||||
| Flexion ROM | PE+PNS+EE | 157.17 | 12.67 | 42.17 | <0.001 | 2.287 | 24.96 | <0.001 ** | 1.711 |
| TENS+US+EE | 138.39 | 24.02 | 17.22 | <0.001 | 1.863 | ||||
| Extension ROM | PE+PNS+EE | 37.09 | 5.21 | 10.22 | <0.001 | 1.448 | 2.87 | 0.119 | 0.469 |
| TENS+US+EE | 30.87 | 5.07 | 7.35 | <0.001 | 1.471 | ||||
| Abduction ROM | PE+PNS+EE | 145.43 | 16.57 | 40.91 | <0.001 | 2.253 | 12.35 | 0.015 * | 0.748 |
| TENS+US+EE | 124.00 | 24.14 | 28.57 | <0.001 | 1.947 | ||||
| Adduction ROM | PE+PNS+EE | 32.83 | 5.37 | 5.00 | 0.002 | 0.720 | 1.87 | 0.258 | 0.338 |
| TENS+US+EE | 28.91 | 4.78 | 3.13 | <0.001 | 0.867 | ||||
| Internal Rotation ROM | PE+PNS+EE | 86.26 | 7.93 | 28.83 | <0.001 | 1.376 | 15.22 | 0.002 * | 0.958 |
| TENS+US+EE | 69.26 | 16.49 | 13.61 | <0.001 | 1.685 | ||||
| External Rotation ROM | PE+PNS+EE | 85.70 | 8.92 | 24.74 | <0.001 | 1.238 | 12.35 | 0.013 * | 0.763 |
| TENS+US+EE | 72.96 | 17.35 | 12.39 | <0.001 | 1.111 | ||||
| VARIABLE | 24 Weeks Follow-Up | Within-Group Change Score | Between-Groups Change Score | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean Difference | p | d | Mean Difference | p | d | ||
| NPRS | PE+PNS+EE | 0.57 | 0.90 | −6.96 | <0.001 | −4.571 | −3.61 | <0.001 ** | −2.579 |
| TENS+US+EE | 3.61 | 2.13 | −3.35 | <0.001 | −2.646 | ||||
| PPT Proximal | PE+PNS+EE | 4.75 | 0.55 | 2.13 | <0.001 | 2.539 | 1.34 | <0.001 ** | 1.846 |
| TENS+US+EE | 3.44 | 0.94 | 0.79 | <0.001 | 1.319 | ||||
| PPT Medium | PE+PNS+EE | 4.75 | 0.49 | 2.00 | <0.001 | 1.989 | 1.39 | <0.001 ** | 1.72 |
| TENS+US+EE | 3.42 | 0.99 | 0.61 | <0.001 | 1.138 | ||||
| PPT Distal | PE+PNS+EE | 4.73 | 0.60 | 2.12 | <0.001 | 2.176 | 1.40 | <0.001 ** | 1.635 |
| TENS+US+EE | 3.36 | 0.88 | 0.72 | <0.001 | 1.004 | ||||
| Disability (DASH) | PE+PNS+EE | 1.87 | 2.58 | −60.13 | <0.001 | −3.694 | −41.30 | <0.001 ** | −2.89 |
| TENS+US+EE | 28.61 | 23.31 | −18.83 | <0.001 | −1.571 | ||||
| Disability (DASH) % | PE+PNS+EE | 1.56 | 2.15 | −50.11 | <0.001 | −3.694 | −34.42 | <0.001 ** | −2.89 |
| TENS+US+EE | 23.84 | 19.43 | −15.69 | <0.001 | −1.571 | ||||
| Disability (SPADI) | PE+PNS+EE | 4.52 | 5.84 | −66.30 | <0.001 | −3.758 | −39.61 | <0.001 ** | −2.582 |
| TENS+US+EE | 41.00 | 22.01 | −26.70 | <0.001 | −2.115 | ||||
| Disability (SPADI) % | PE+PNS+EE | 3.48 | 4.49 | −51.00 | <0.001 | −3.758 | −30.47 | <0.001 ** | −2.582 |
| TENS+US+EE | 31.54 | 16.93 | −20.54 | <0.001 | −2.115 | ||||
| Flexion ROM | PE+PNS+EE | 156.91 | 12.18 | 41.91 | <0.001 | 2.208 | 26.96 | <0.001 ** | 1.772 |
| TENS+US+EE | 136.13 | 22.27 | 14.96 | <0.001 | 1.476 | ||||
| Extension ROM | PE+PNS+EE | 37.87 | 4.75 | 11.00 | <0.001 | 1.398 | 4.96 | 0.012 * | 0.768 |
| TENS+US+EE | 29.57 | 5.42 | 6.04 | <0.001 | 1.309 | ||||
| Abduction ROM | PE+PNS+EE | 145.04 | 14.10 | 40.52 | <0.001 | 2.096 | 16.70 | 0.002 * | 0.964 |
| TENS+US+EE | 119.26 | 22.50 | 23.83 | <0.001 | 1.584 | ||||
| Adduction ROM | PE+PNS+EE | 33.30 | 5.23 | 5.48 | 0.001 | 0.773 | 2.65 | 0.116 | 0.473 |
| TENS+US+EE | 28.61 | 4.47 | 2.83 | <0.001 | 0.799 | ||||
| Internal Rotation ROM | PE+PNS+EE | 85.87 | 8.21 | 28.43 | <0.001 | 1.374 | 15.26 | 0.002 * | 0.961 |
| TENS+US+EE | 68.83 | 15.85 | 13.17 | <0.001 | 1.509 | ||||
| External Rotation ROM | PE+PNS+EE | 85.22 | 9.71 | 24.26 | <0.001 | 1.220 | 13.74 | 0.005 * | 0.875 |
| TENS+US+EE | 71.09 | 17.45 | 10.52 | <0.001 | 1.066 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góngora-Rodríguez, J.; Rodríguez-Huguet, M.; Rodríguez-Almagro, D.; Martín-Valero, R.; Góngora-Rodríguez, P.; Ayala-Martínez, C.; Rosety-Rodríguez, M.Á. Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation, and Eccentric Exercise for Shoulder Pain and Functionality in Supraspinatus Tendinopathy: A Single-Blind Randomized Clinical Trial. J. Funct. Morphol. Kinesiol. 2025, 10, 295. https://doi.org/10.3390/jfmk10030295
Góngora-Rodríguez J, Rodríguez-Huguet M, Rodríguez-Almagro D, Martín-Valero R, Góngora-Rodríguez P, Ayala-Martínez C, Rosety-Rodríguez MÁ. Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation, and Eccentric Exercise for Shoulder Pain and Functionality in Supraspinatus Tendinopathy: A Single-Blind Randomized Clinical Trial. Journal of Functional Morphology and Kinesiology. 2025; 10(3):295. https://doi.org/10.3390/jfmk10030295
Chicago/Turabian StyleGóngora-Rodríguez, Jorge, Manuel Rodríguez-Huguet, Daniel Rodríguez-Almagro, Rocío Martín-Valero, Pablo Góngora-Rodríguez, Carmen Ayala-Martínez, and Miguel Ángel Rosety-Rodríguez. 2025. "Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation, and Eccentric Exercise for Shoulder Pain and Functionality in Supraspinatus Tendinopathy: A Single-Blind Randomized Clinical Trial" Journal of Functional Morphology and Kinesiology 10, no. 3: 295. https://doi.org/10.3390/jfmk10030295
APA StyleGóngora-Rodríguez, J., Rodríguez-Huguet, M., Rodríguez-Almagro, D., Martín-Valero, R., Góngora-Rodríguez, P., Ayala-Martínez, C., & Rosety-Rodríguez, M. Á. (2025). Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation, and Eccentric Exercise for Shoulder Pain and Functionality in Supraspinatus Tendinopathy: A Single-Blind Randomized Clinical Trial. Journal of Functional Morphology and Kinesiology, 10(3), 295. https://doi.org/10.3390/jfmk10030295









