Cardiocirculatory and Metabolic Responses to Low- and High-Load Squat Exercise in Young and Middle-Aged Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.1.1. Ramp Incremental Test
2.1.2. One Repetition Maximum Estimation
- 3 min of mobility exercises for the hips, back, and shoulders;
- 3 min of unloaded cycling;
- 2 min core activation consisting in the exercises of front plant, lateral plank, and back bridge;
- 2 sets of squat, respectively, 12 repetitions at 50% of the hypothetical 1RM, and 6 repetitions at 70% of the hypothetical 1RM. The hypothetical 1RM was the last self-reported 1RM of the participants. Between sets, a recovery of 4 min was given.
2.1.3. Squat Training Sessions
- 3 sets × 12 repetitions at 55% 1RM (training load of 1980 arbitrary units);
- 5 sets × 5 repetitions at 80% 1RM (training load of 2000 arbitrary units).
2.2. Data Analysis
2.2.1. Ramp Incremental Test
2.2.2. Squat Training Sessions
2.3. Statistics
3. Results
4. Discussion
Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | Resistance Training |
| 1RM | One Repetition Maximum |
| O2 | Oxygen Consumption |
| O2max | Maximal Oxygen Consumption |
| PO | Power Output |
| HR | Heart Rate |
| E | Ventilation |
| Rf | Respiratory Frequency |
| [La−] | Blood Lactate Concentration |
| PAs | Systolic Pressure |
| PAd | Diastolic Pressure |
| DP | Double Product |
| RPE | Rating of Perceived Exertion |
| RM ANOVA | Repeated Measures Analysis of Variance |
| SD | Standard Deviation |
| O2max | Percentage of Maximal Oxygen Consumption |
| %HRmax | Percentage of Maximal Heart Rate |
References
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. J. Am. Med. Assoc. 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Flanagan, S.D.; Shurley, J.P.; Todd, J.S.; Todd, T.C. Understanding the Science of Resistance Training: An Evolutionary Perspective. Sports Med. 2017, 47, 2415–2435. [Google Scholar] [CrossRef] [PubMed]
- Brendt, A.; Evotech, T.; Housh, T.; Kibler, B.; Kraemer, W.; Triplett, T. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 3, 687–708. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’S Guidelines for Exercise Testing and Prescription, 11th ed.; Lippincott, W., Wilkins, Eds.; Kluwer, Wolters: Philadelphia, PA, USA, 2020. [Google Scholar]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological Adaptations to Low-Volume, High-Intensity Interval Training in Health and Disease. J. Physiol. 2012, 5, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.R.; De Oliveira, R.J.; Dutra, M.T.; Pardono, E.; Terra, D.F.; Lima, R.M.; Simoes, H.G.; Da Silva, F.M. Acute And Chronic Effects Of Resistive Exercise On Blood Pressure In Hypertensive Elderly Women. J. Strength Cond. Res. 2013, 27, 3475–3480. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.S.; Asano, R.Y.; Filho, I.G.; Lopes, N.L.; Panelli, P.; da C Nascimento, D.; Collier, S.R.; Prestes, J. Acute And Chronic Cardiovascular Response To 16 Weeks Of Combined Eccentric Or Traditional Resistance And Aerobic Training In Elderly Hypertensive Women: A Randomized Controlled Trial. J. Strength Cond. Res. 2014, 28, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H.V.; Johnson, B.T.; Huedo-Medina, T.B.; Livingston, J.; Forsyth, K.C.; Kraemer, W.J.; Farinatti, P.T.V.; Pescatello, L.S. Dynamic Resistance Training as Stand-Alone Antihypertensive Lifestyle Therapy: A Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e003231. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial Blood Pressure Response to Heavy Resistance Exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary Exercise Testing and Its Application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.L.; Neiva, H.P.; Faíl, L.B.; Gil, M.H.; Marques, M.C. Acute Effects of Low and High-Volume Resistance Training on Hemodynamic, Metabolic and Neuromuscular Parameters in Older Adults. Exp. Gerontol. 2019, 125, 110685. [Google Scholar] [CrossRef] [PubMed]
- Lässing, J.; Maudrich, T.; Kenville, R.; Uyar, Z.; Bischoff, C.; Fikenzer, S.; Busse, M.; Falz, R. Intensity-Dependent Cardiopulmonary Response during and after Strength Training. Sci. Rep. 2023, 13, 6632. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise. J. Funct. Morphol. Kinesiol. 2018, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Dello Iacono, A.; Martone, D.; Hayes, L. Acute Mechanical, Physiological and Perceptual Responses in Older Men to Traditional-Set or Different Cluster-Set Configuration Resistance Training Protocols. Eur. J. Appl. Physiol. 2020, 120, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Engelhardt, M. Strength and Muscle Mass Loss with Aging Process. Age and Strength Loss. Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef]
- Haff, G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- Colosio, A.L.; Caen, K.; Bourgois, J.G.; Boone, J.; Pogliaghi, S. Metabolic Instability vs Fibre Recruitment Contribution to the V˙O2 Slow Component in Different Exercise Intensity Domains. Pflug. Arch. 2021, 473, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Colosio, A.L.; Baldessari, E.; Basso, E.; Pogliaghi, S. Respiratory and Muscular Response to Acute Non-Metabolic Fatigue during Ramp Incremental Cycling. Respir. Physiol. Neurobiol. 2019, 270, 103281. [Google Scholar] [CrossRef] [PubMed]
- Brzycki, M. Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Rodiles-Guerrero, L.; Rendeiro-Pinho, G.; Prieto-Veloso, A.; Pareja-Blanco, F. Acute Mechanical and Metabolic Responses to Different Resistance Training Protocols With Equated Volume Load. Int. J. Sports Physiol. Perform. 2023, 18, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Borg, E.; Kaijser, L. A Comparison between Three Rating Scales for Perceived Exertion and Two Different Work Tests. Scand. J. Med. Sci. Sports 2006, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.; Jones, A.M. Oxygen Uptake Kinetics. Compr. Physiol. 2012, 2, 933–996. [Google Scholar] [CrossRef] [PubMed]
- Colosio, A.L.; Caen, K.; Bourgois, J.G.; Boone, J.; Pogliaghi, S. Bioenergetics of the VO2 Slow Component between Exercise Intensity Domains. Pflug. Arch. 2020, 472, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Pogliaghi, S.; Teso, M.; Ferrari, L.; Boone, J.; Murias, J.M.; Colosio, A.L. Easy Prediction of the Maximal Lactate Steady-State in Young and Older Men and Women. J. Sports Sci. Med. 2023, 22, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Mang, Z.A.; Ducharme, J.B.; Mermier, C.; Kravitz, L.; De Castro Magalhaes, F.; Amorim, F. Aerobic Adaptations to Resistance Training: The Role of Time under Tension. Int. J. Sports Med. 2022, 43, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Rosenberg, J.G.; Klei, S.; Dougherty, B.M.; Kang, J.; Smith, C.R.; Ross, R.E.; Faigenbaum, A.D. Acute Oxygen Uptake And Resistance Exercise Using Different Rest Interval: The Influence Of Maximal Aerobic And Exercise Sequence. J. Strength Cond. Res. 2014, 28, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, G.R.H.; Moran, J.; Cohen, D.D. Who Is Meeting the Strengthening Physical Activity Guidelines by Definition: A Cross-Sectional Study of 253 423 English Adults? PLoS ONE 2022, 17, e0267277. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.P.; James, M.G.; Phillip, J.W.; Kelly, L.P.; Mark, S.K. Acute and Session RPE Responses during Resistance Training: Bouts to Failure at 60% and 90% of 1RM. S. Afr. J. Sports Med. 2009, 21, 23–26. [Google Scholar]
- Weakley, J.; Schoenfeld, B.J.; Ljungberg, J.; Halson, S.L.; Phillips, S.M. Physiological Responses and Adaptations to Lower Load Resistance Training: Implications for Health and Performance. Sports Med. Open 2023, 9, 28. [Google Scholar] [CrossRef] [PubMed]
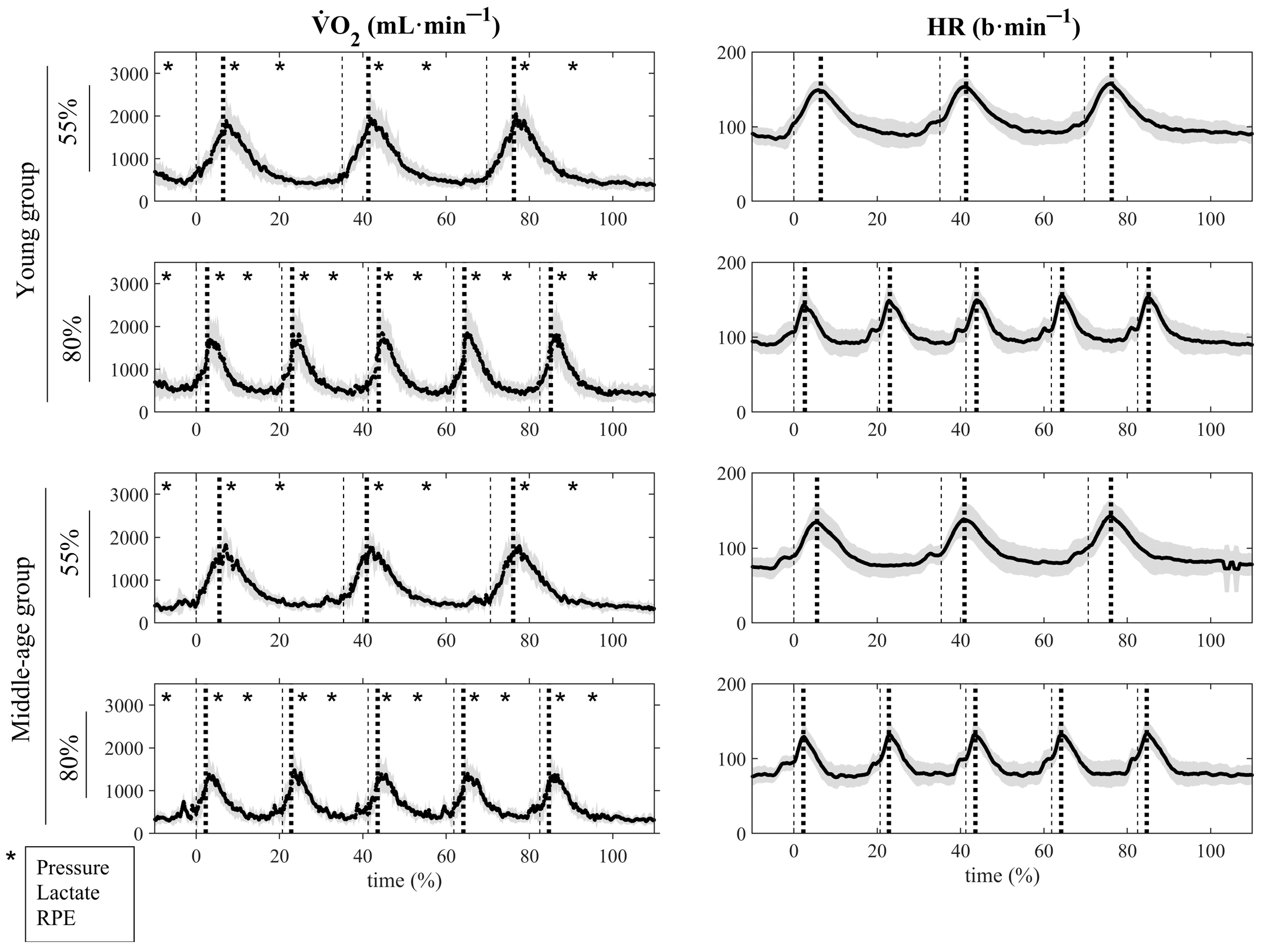
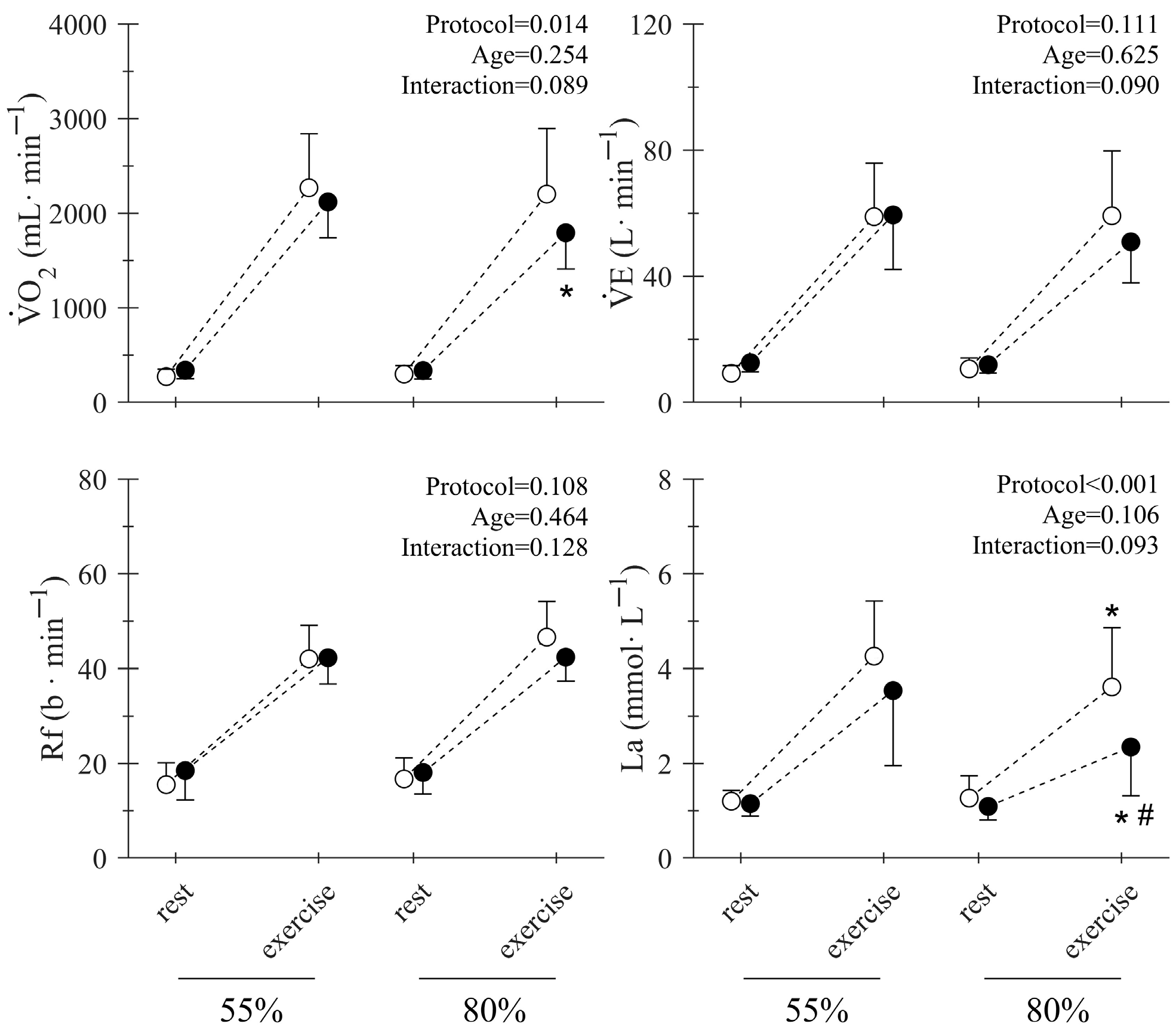
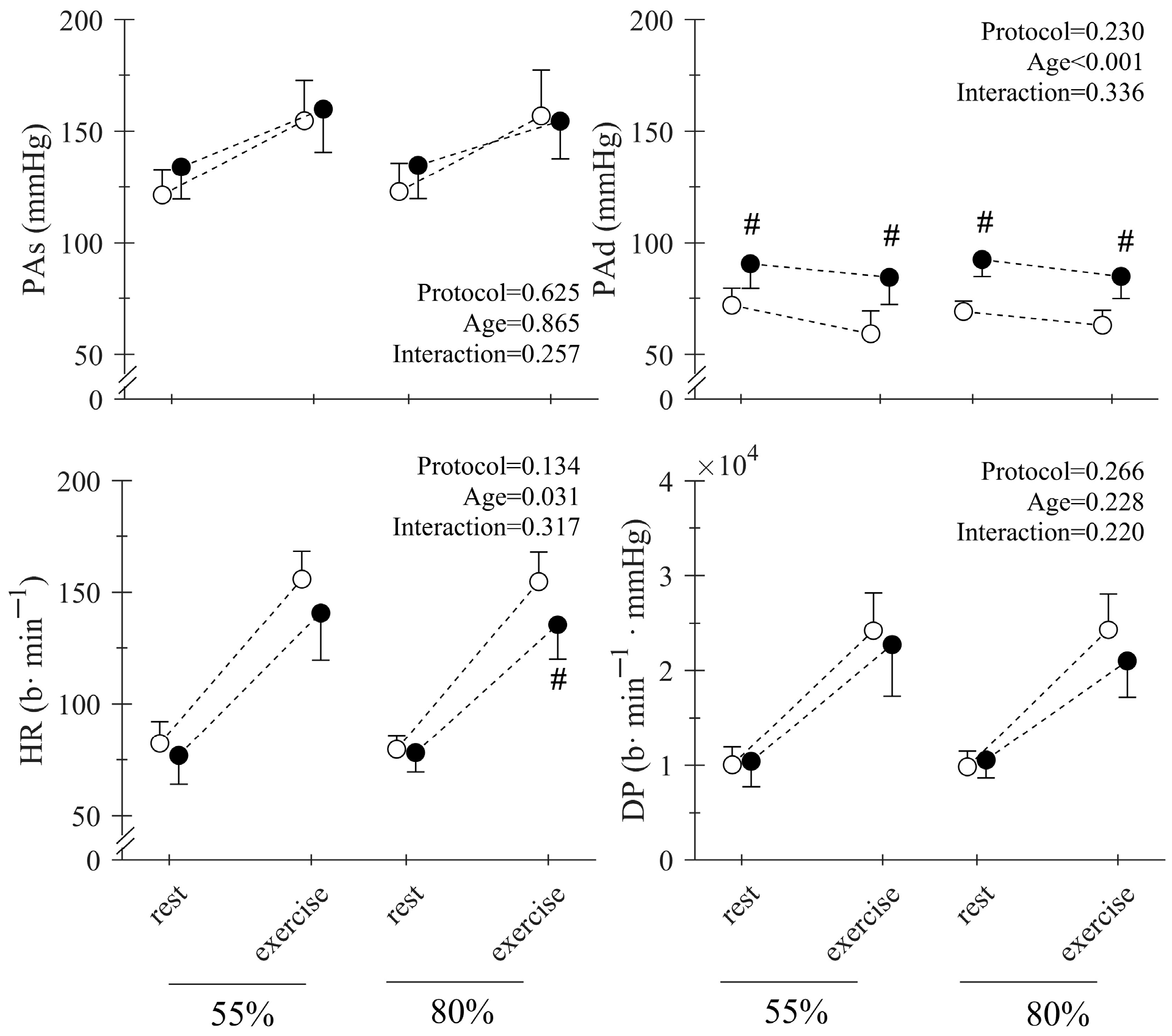

| Young | Middle-Aged | Total | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n° | 9 | 9 | 18 | |||||||
| Age (yrs) | 25.6 | ± | 2.2 | 50.1 | ± | 5.4 | 37.8 | ± | 13.2 | 0.000 * |
| Weight (kg) | 66.7 | ± | 13.0 | 76.3 | ± | 14.4 | 71.5 | ± | 14.2 | 0.160 |
| Height (cm) | 1.73 | ± | 0.09 | 1.74 | ± | 0.07 | 1.74 | ± | 0.08 | 0.724 |
| BMI (kg/m2) | 22.1 | ± | 2.4 | 24.9 | ± | 3.3 | 23.5 | ± | 3.2 | 0.054 |
| O2max (mL·kg−1·min−1) | 42.7 | ± | 6.6 | 34.0 | ± | 6.4 | 38.4 | ± | 7.7 | 0.012 * |
| HR_max (b·min−1) | 192.3 | ± | 4.4 | 176.6 | ± | 7.4 | 184.5 | ± | 10.1 | 0.000 * |
| PAs (mmHg) | 122.4 | ± | 9.9 | 131.0 | ± | 8.5 | 126.7 | ± | 10.0 | 0.067 |
| PAd (mmHg) | 67.8 | ± | 6.8 | 90.5 | ± | 7.0 | 79.1 | ± | 13.5 | 0.000 * |
| 1RM squat (kg) | 88.9 | ± | 28.4 | 78.6 | ± | 25.2 | 83.7 | ± | 26.6 | 0.427 |
| 1RM norm squat (kg/kg) | 1.3 | ± | 0.2 | 1.0 | ± | 0.3 | 1.2 | ± | 0.3 | 0.028 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosio, A.L.; Teso, M.; Bottari, A.; Ferrari, L.; Bochicchio, G.; Boone, J.; Pogliaghi, S. Cardiocirculatory and Metabolic Responses to Low- and High-Load Squat Exercise in Young and Middle-Aged Individuals. J. Funct. Morphol. Kinesiol. 2025, 10, 287. https://doi.org/10.3390/jfmk10030287
Colosio AL, Teso M, Bottari A, Ferrari L, Bochicchio G, Boone J, Pogliaghi S. Cardiocirculatory and Metabolic Responses to Low- and High-Load Squat Exercise in Young and Middle-Aged Individuals. Journal of Functional Morphology and Kinesiology. 2025; 10(3):287. https://doi.org/10.3390/jfmk10030287
Chicago/Turabian StyleColosio, Alessandro L., Massimo Teso, Alberto Bottari, Luca Ferrari, Gianluca Bochicchio, Jan Boone, and Silvia Pogliaghi. 2025. "Cardiocirculatory and Metabolic Responses to Low- and High-Load Squat Exercise in Young and Middle-Aged Individuals" Journal of Functional Morphology and Kinesiology 10, no. 3: 287. https://doi.org/10.3390/jfmk10030287
APA StyleColosio, A. L., Teso, M., Bottari, A., Ferrari, L., Bochicchio, G., Boone, J., & Pogliaghi, S. (2025). Cardiocirculatory and Metabolic Responses to Low- and High-Load Squat Exercise in Young and Middle-Aged Individuals. Journal of Functional Morphology and Kinesiology, 10(3), 287. https://doi.org/10.3390/jfmk10030287






