Short-Term, Significant Gains from a 10-Day Field-Based Multi-Modal Outdoor Activity Camp with Time-Restricted Feeding Dissipate at Three-Month Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Procedures
2.2. Participants
2.3. Intervention Protocol and Internal Load Monitoring
2.4. Dietary Intake and Meal Timing
2.5. Measurements
2.6. Statistical Analyses
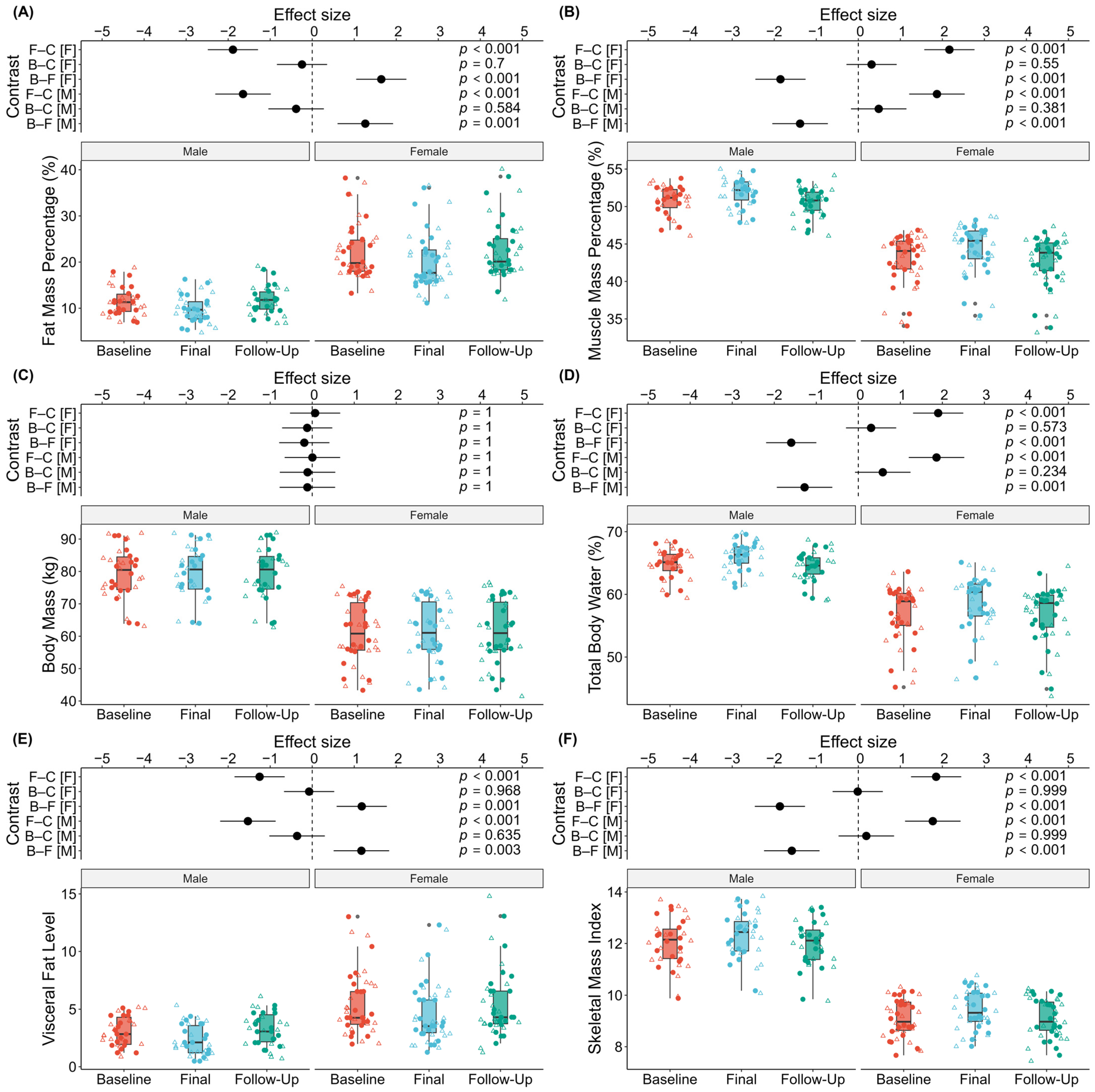
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical Impedance Analyzer |
| BFM | Body Fat Mass |
| BM | Body Mass |
| SMM | Skeletal Muscle Mass |
| %BFM | Body Fat Percentage |
| %SMM | Skeletal Muscle Mass Percentage |
| %TBW | Total Body Water Percentage |
| SMI | Skeletal Muscle Index |
| TBW | Total Body Water |
| DXA | Dual-energy X-ray Absorptiometry |
| MRI | Magnetic Resonance Imaging |
| PAL | Physical Activity Level |
| REE | Resting Energy Expenditure |
| RPE | Rate of Perceived Exertion |
| RDA | Recommended Daily Allowance |
References
- Rehman, R.; ullah Shaikh, S.; Syed, S.; Shakeel, N. Relationship of Life Style Choices on Body Fat Mass in Young Adults. J. Ayub Med. Coll. Abbottabad JAMC 2010, 22, 146–149. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, X.; Li, J.; Zhong, H.; Pan, C.-W. Associations of Outdoor Activity and Screen Time with Adiposity: Findings from Rural Chinese Adolescents with Relatively Low Adiposity Risks. BMC Public Health 2020, 20, 1769. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, Y. Early Time-Restricted Eating Reduces Weight and Improves Glycemic Response in Young Adults: A Pre-Post Single-Arm Intervention Study. Obes. Facts 2023, 16, 69–81. [Google Scholar] [CrossRef]
- Park, S.-J.; Yang, J.-W.; Song, Y.-J. The Effect of Four Weeks Dietary Intervention with 8-Hour Time-Restricted Eating on Body Composition and Cardiometabolic Risk Factors in Young Adults. Nutrients 2021, 13, 2164. [Google Scholar] [CrossRef]
- Cui, T.; Sun, Y.; Ye, W.; Liu, Y.; Korivi, M. Efficacy of Time Restricted Eating and Resistance Training on Body Composition and Mood Profiles among Young Adults with Overweight/Obesity: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2025, 22, 2481127. [Google Scholar] [CrossRef]
- Grigoletto, A.; Mauro, M.; Oppio, A.; Greco, G.; Fischetti, F.; Cataldi, S.; Toselli, S. Effects of Nordic Walking Training on Anthropometric, Body Composition and Functional Parameters in the Middle-Aged Population. Int. J. Environ. Res. Public Health 2022, 19, 7433. [Google Scholar] [CrossRef] [PubMed]
- Poli, L.; Greco, G.; Gabriele, M.; Pepe, I.; Centrone, C.; Cataldi, S.; Fischetti, F. Effect of Outdoor Cycling, Virtual and Enhanced Reality Indoor Cycling on Heart Rate, Motivation, Enjoyment and Intention to Perform Green Exercise in Healthy Adults. J. Funct. Morphol. Kinesiol. 2024, 9, 183. [Google Scholar] [CrossRef]
- Leadbetter, B.; Sénéchal, M.; Seaman, K.; Bouchard, D.R. Resistance Training on an Outdoor Exercise Structure Improves Lower-Body Relative Strength in Older Adults. Gerontol. Geriatr. Med. 2024, 10, 23337214241232552. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Espeso-García, A.; Vaquero-Cristóbal, R.; Abelleira-Lamela, T.; González-Gálvez, N. The Effect of Resistance Training with Outdoor Fitness Equipment on the Body Composition, Physical Fitness, and Physical Health of Middle-Aged and Older Adults: A Randomized Controlled Trial. Healthcare 2024, 12, 726. [Google Scholar] [CrossRef]
- Stanaszek, M.; Fugiel, J.; Kozieł, S.; Sebastjan, A.; Suder, A.; Ignasiak, Z. Effect of Winter Outdoor Physical Activity on Body Composition and Motor Performance of Polish Adult Men. Healthcare 2023, 11, 2348. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Kelly, E.; Louise, B. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Kryst, J.; Ziemann, E.; Borkowska, A.; Reczkowicz, J.; Dzidek, A.; Rydzik, Ł.; Pałka, T.; Żychowska, M.; Kupczak, W.; et al. The Beneficial Effects of Nordic Walking Training Combined with Time-Restricted Eating 14/24 in Women with Abnormal Body Composition Depend on the Application Period. Nutrients 2024, 16, 1413. [Google Scholar] [CrossRef] [PubMed]
- Siedzik, K.; Góral, K.; Rodziewicz-Flis, E.; Olek, R.A.; Ziółkowski, W. Impact of a Fish-Based Restrictive Ketogenic Diet on Body Composition and Strength Capacity: A Pre-Post Study. Nutrients 2025, 17, 1297. [Google Scholar] [CrossRef]
- Boughman, J.K.; Masters, M.A.; Morgan, C.A.; Ruden, T.M.; Rochelle, S.G. Assessing the Validity of Bioelectrical Impedance and Skinfold Calipers for Measuring Body Composition in NOLS Backcountry Hikers. Wilderness Environ. Med. 2019, 30, 369–377. [Google Scholar] [CrossRef]
- Izzicupo, P.; Di Blasio, A.; Di Credico, A.; Ghinassi, B.; Capranica, L.; Napolitano, G.; Di Baldassarre, A.; Modestini, E.; Di Pietro, M. Objectively Measured Physical Activity Increases Only in Males During a Summer Camp for Obese Children. Front. Sports Act. Living 2021, 3, 624449. [Google Scholar] [CrossRef] [PubMed]
- Laja García, A.I.; Moráis-Moreno, C.; Samaniego-Vaesken, M.D.L.; Puga, A.M.; Partearroyo, T.; Varela-Moreiras, G. Influence of Water Intake and Balance on Body Composition in Healthy Young Adults from Spain. Nutrients 2019, 11, 1923. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Mifflin, M.; St Jeor, S.; Hill, L.; Scott, B.; Daugherty, S.; Koh, Y. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Panel on Macronutrients; Panel on the Definition of Dietary Fiber; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board. Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Sun, G.; French, C.R.; Martin, G.R.; Younghusband, B.; Green, R.C.; Xie, Y.; Mathews, M.; Barron, J.R.; Fitzpatrick, D.G.; Gulliver, W.; et al. Comparison of Multifrequency Bioelectrical Impedance Analysis with Dual-Energy X-Ray Absorptiometry for Assessment of Percentage Body Fat in a Large, Healthy Population. Am. J. Clin. Nutr. 2005, 81, 74–78. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Lacome, M.; Rodriguez, C.; Tinsley, G.M. Tracking Body Composition Over a Competitive Season in Elite Soccer Players Using Laboratory- and Field-Based Assessment Methods. J. Strength Cond. Res. 2024, 38, e104–e115. [Google Scholar] [CrossRef]
- Czartoryski, P.; Garcia, J.; Manimaleth, R.; Napolitano, P.; Watters, H.; Weber, C.; Alvarez-Beaton, A.; Nieto, A.C.; Patel, A.; Peacock, C. Body Composition Assessment: A Comparison of the DXA, Inbody 270, and Omron. J. Exerc. Nutr. 2020, 3, 1–6. Available online: https://journalofexerciseandnutrition.com/index.php/JEN/article/view/57 (accessed on 17 May 2025).
- Gao, B.; Liu, Y.; Ding, C.; Liu, S.; Chen, X.; Bian, X. Comparison of Visceral Fat Area Measured by CT and Bioelectrical Impedance Analysis in Chinese Patients with Gastric Cancer: A Cross-Sectional Study. BMJ Open 2020, 10, e036335. [Google Scholar] [CrossRef] [PubMed]
- InBody Canada. InBody 270 Body Composition Analyzer. Available online: https://inbodycanada.ca/products/inbody-270-body-composition-analyzer-2/ (accessed on 2 June 2025).
- U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data (510(k) Number K123228) for InBody S10 Body Composition Analyzer; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf12/K123228.pdf (accessed on 4 June 2025).
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.7, 2024. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 17 May 2025).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Russell, L. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.11.1, 2025. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 17 May 2025).
- Convertino, V.A. Blood Volume: Its Adaptation to Endurance Training. Med. Sci. Sports Exerc. 1991, 23, 1338–1348. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1689–1697. [Google Scholar] [CrossRef]
- Phillips, S.M.; Moore, D.R.; Tang, J.E. A Critical Examination of Dietary Protein Requirements, Benefits, and Excesses in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S58–S76. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical Activity and Exercise in the Regulation of Human Adipose Tissue Physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I: Short Term Insufficient Training Stimulus. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Neiva, H.P.; Izquierdo, M.; Cadore, E.L.; Alves, A.R.; Marinho, D.A. Concurrent Training and Detraining: Brief Review on the Effect of Exercise Intensities. Int. J. Sports Med. 2019, 40, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Phelan, S. Long-Term Weight Loss Maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef]
- Prochaska, J.O.; DiClemente, C.C. Stages and Processes of Self-Change of Smoking: Toward an Integrative Model of Change. J. Consult. Clin. Psychol. 1983, 51, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Fatima, S.; Zaman, M.H.; Khan, S.A.; Safdar, M.; Alam, I.; Feng, Q. Time-Restricted Feeding Regulates Molecular Mechanisms with Involvement of Circadian Rhythm to Prevent Metabolic Diseases. Nutrition 2021, 89, 111244. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef]
- Barton, J.; Pretty, J. What Is the Best Dose of Nature and Green Exercise for Improving Mental Health? A Multi-Study Analysis. Environ. Sci. Technol. 2010, 44, 3947–3955. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]

| Day | Session 1 (90 min) | Session 2 (90 min) | Session 3 (90 min) |
|---|---|---|---|
| Day 1 | Rock Climbing and Bouldering | Nautical Operations | Environmental Stewardship |
| Day 2 | Open-Water Swimming | Rock Climbing and Bouldering | Nautical Operations |
| Day 3 | Windsurfing | Open-Water Swimming | Rock Climbing and Bouldering |
| Day 4 | Applied Paddling | Windsurfing | Open-Water Swimming |
| Day 5 | Hiking and Navigation | Applied Paddling | Windsurfing |
| Day 6 | Obstacle Course Movement | Hiking and Navigation | Applied Paddling |
| Day 7 | Campcraft Fundamentals | Obstacle Course Movement | Hiking and Navigation |
| Day 8 | Survival Skills | Campcraft Fundamentals | Obstacle Course Movement |
| Day 9 | Rafting Expedition | Survival Skills | Campcraft Fundamentals |
| Day 10 | Environmental Stewardship | Rafting Expedition | Survival Skills |
| Time | Activity | Duration (h) | REE × | Weighted REE (h) |
|---|---|---|---|---|
| 23:00–07:00 | Sleep/night duty | 8.0 | 1.0 | 8.0 |
| 07:00–07:30 | Wake-up and toilet | 0.5 | 1.0 | 0.5 |
| 07:30–08:00 | Morning gymnastics | 0.5 | 5.0 | 2.5 |
| 08:00–09:00 | Camp cleanup and breakfast | 1.0 | 2.5 | 2.5 |
| 09:00–10:30 | Class 1 (practical PA) | 1.5 | 5.0 | 7.5 |
| 10:30–11:00 | Break (moving around) | 0.5 | 2.5 | 1.25 |
| 11:00–12:30 | Class 2 (practical PA) | 1.5 | 5.0 | 7.5 |
| 12:30–14:00 | Free time (light activities) | 1.5 | 2.5 | 3.75 |
| 14:00–16:00 | Lunch and light free time | 2.0 | 2.5 | 5.0 |
| 16:00–17:30 | Class 3 (vigorous PA, RPE > 13) | 1.5 | 7.0 | 10.5 |
| 17:30–19:00 | Lecture/presentations (sitting) | 1.5 | 1.0 | 1.5 |
| 19:00–21:00 | Dinner and prep (light) | 2.0 | 2.5 | 5.0 |
| 21:00–23:00 | Evening program (light) | 2.0 | 2.5 | 5.0 |
| TOTAL | 24.0 | 60.5 | ||
| PAL = | 2.52 |
| Variable | Sex | Baseline | Final | Follow-Up | ΔF–B (%) | ΔC–B (%) | ΔF–C (%) |
|---|---|---|---|---|---|---|---|
| BFM (kg) | male | 9.06 ± 2.59 | 7.79 ± 2.57 | 9.56 ± 3.12 | −13.9% | 5.7% | −17.0% |
| female | 13.99 ± 5.49 | 12.72 ± 5.40 | 14.28 ± 6.03 | −9.6% | 2.0% | −10.6% | |
| SMI | male | 12.02 ± 0.98 | 12.31 ± 0.98 | 11.98 ± 0.89 | 2.5% | −0.2% | 2.8% |
| female | 9.09 ± 0.74 | 9.44 ± 0.80 | 9.09 ± 0.79 | 3.8% | 0.0% | 3.9% | |
| SMM (kg) | male | 40.36 ± 4.88 | 41.22 ± 4.83 | 40.09 ± 4.46 | 2.2% | −0.5% | 2.8% |
| female | 26.03 ± 3.42 | 26.98 ± 3.61 | 25.93 ± 3.43 | 3.6% | −0.4% | 4.1% | |
| TBW (kg) | male | 51.53 ± 5.84 | 52.56 ± 5.84 | 51.13 ± 5.39 | 2.0% | −0.7% | 2.8% |
| female | 34.47 ± 4.21 | 35.53 ± 4.44 | 34.34 ± 4.21 | 3.1% | −0.4% | 3.5% | |
| BM (kg) | male | 79.35 ± 8.21 | 79.49 ± 8.31 | 79.49 ± 8.29 | 0.2% | 0.2% | 0.0% |
| female | 61.04 ± 9.45 | 61.28 ± 9.34 | 61.19 ± 9.82 | 0.4% | 0.2% | 0.3% | |
| VFL | male | 3.00 ± 1.33 | 2.28 ± 1.36 | 3.22 ± 1.48 | −24.9% | 9.1% | −27.2% |
| female | 5.36 ± 2.66 | 4.64 ± 2.61 | 5.41 ± 3.17 | −14.5% | −0.7% | −9.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanović, K.; Stojanović, N.; Miletić, V.; Rajković, Ž.; Stojanović, D.; Ilić, V.; Filipović, M.; Durlević, S.; Orlić, A.; Ilić, I. Short-Term, Significant Gains from a 10-Day Field-Based Multi-Modal Outdoor Activity Camp with Time-Restricted Feeding Dissipate at Three-Month Follow-Up. J. Funct. Morphol. Kinesiol. 2025, 10, 229. https://doi.org/10.3390/jfmk10020229
Milanović K, Stojanović N, Miletić V, Rajković Ž, Stojanović D, Ilić V, Filipović M, Durlević S, Orlić A, Ilić I. Short-Term, Significant Gains from a 10-Day Field-Based Multi-Modal Outdoor Activity Camp with Time-Restricted Feeding Dissipate at Three-Month Follow-Up. Journal of Functional Morphology and Kinesiology. 2025; 10(2):229. https://doi.org/10.3390/jfmk10020229
Chicago/Turabian StyleMilanović, Katarina, Nikola Stojanović, Vladimir Miletić, Željko Rajković, Darko Stojanović, Vladimir Ilić, Milica Filipović, Slavka Durlević, Ana Orlić, and Igor Ilić. 2025. "Short-Term, Significant Gains from a 10-Day Field-Based Multi-Modal Outdoor Activity Camp with Time-Restricted Feeding Dissipate at Three-Month Follow-Up" Journal of Functional Morphology and Kinesiology 10, no. 2: 229. https://doi.org/10.3390/jfmk10020229
APA StyleMilanović, K., Stojanović, N., Miletić, V., Rajković, Ž., Stojanović, D., Ilić, V., Filipović, M., Durlević, S., Orlić, A., & Ilić, I. (2025). Short-Term, Significant Gains from a 10-Day Field-Based Multi-Modal Outdoor Activity Camp with Time-Restricted Feeding Dissipate at Three-Month Follow-Up. Journal of Functional Morphology and Kinesiology, 10(2), 229. https://doi.org/10.3390/jfmk10020229










