Abstract
Background: The term tendinopathy commonly describes a series of alterations in the tendon, leading in functional impairment and pain, treated with several approaches, including exercises, physical agents, and injection therapies. Among the latter, autologous tenocyte injection (ATI) involves harvesting tenocytes from a healthy tendon of the patients and then isolating the tenocytes and culturing for 4–5 weeks. To date, there is still a lack of consensus about the efficacy of ATI in improving pain and function; therefore, the present review aimed to assess the role of ATI in the treatment of chronic tendinopathies. Methods: Two authors conducted a comprehensive search across PubMed Medline, Web of Science, Scopus, Cochrane Library, and Google Scholar (PROSPERO: CRD42024565211). From 174 articles, we finally included 5 articles. Results: The main effect obtained was the pain relief and, consequently, the improvement of patients’ quality of life. The clinical improvement is also evident at MRI in which it is possible to see a progressive reduction with a general disappearance of the T2 signal hyperintensity between 4 months and 1 year. All the articles agree on the safety of ATI in chronic tendinopathies. Conclusions: ATI might represent a safe and valuable option in the management of chronic tendinopathies as a second line treatment in the case of resistant tendinopathies, with a minimal risk of side effects.
1. Introduction
The term “tendinopathy” commonly describes a series of alterations in the tendon, resulting in reduced functionality and pain. Tendons play an essential role in contributing strength, storing force, and allowing daily activities. During sports, where the load significantly increases, mechanical forces on the tendon also rise, demanding greater effort from the tendon [1,2].
In an “altered” tendon, collagen bundles become highly disorganized, microvascularization increases, and neoinnervation occurs [3,4].
Since 2000, the prevalence of tendinopathies has risen globally, impacting both athletes and people of all generations [5,6,7,8]. The prevalence and incidence of tendinopathies vary across different body parts, influenced by factors such as age, gender, sports type, occupation, and comorbidities. Among lower limbs, the tendinopathies at Achilles tendons (2.4%) and patellar tendons (1.6%) are predominantly affected [9,10]. Moreover, recent studies highlight an increasing incidence of gluteal tendinopathies (4.2%). On the other hand, among upper limb tendinopathies, the rotator cuff tendons are commonly affected (5.5%). Lateral epicondylitis is the prevalent elbow tendinopathy with an incidence of 0.7% [2,5,11,12].
Tendinopathy development involves both modifiable and non-modifiable risk factors [2], e.g., women are more predisposed than men [13]. Furthermore, systemic diseases, such as obesity [14], hypercholesterolemia, and diabetes mellitus [15], impact tendinopathy incidence and patient response to physical therapy [16]. Genetic factors significantly influence tendon homeostasis, affecting the balance between degeneration and repair after injuries [17,18]. Other significant risk factors include restricted or excessive joint movement, muscle deficiency, and impairments in neuromuscular coordination [2].
Athletes commonly receive a diagnosis of tendinopathy, accounting for approximately 30% of all diagnosed injuries [19]. The type of sport and its intensity strongly correlate with the site of tendinopathy onset [20,21,22]; additionally, professional contexts involving high-force or repetitive activities elevate the risk [23,24,25,26].
Tendinopathy begins with repeated functional overload, damaging collagen fibrils. Normally, early lesions trigger a reparative response. However, the tendon’s limited repair capacity, combined with inadequate recovery, leads to progressive matrix damage over time. This results in a gradual loss of structural collagen and additional matrix protein deposition [27,28]. Initially asymptomatic, structural alterations accumulate. Pro-inflammatory cytokines build up, activating nociceptors and causing symptoms [2]. In damaged tendons, tenocytes and immune cells release cytokines (e.g., IL-6, TNF, IL-1β, and IFNγ) and growth factors (PDGF and TGFβ) [29,30,31]. Consequently fibroblasts adopt a pro-inflammatory phenotype [32], leading to increased collagen synthesis (mainly type III collagen) with chaotic fiber arrangement [33].
Furthermore, healthy tendons harbor clusters of stem cells and progenitor cells known as TSPCs. Prolonged inflammatory stimulation leads to TSPCs losing their ability to differentiate into tenocytes. Rather, they promote other cell subtypes (such as osteoblasts, chondrocytes, and adipocytes), further disrupting the typical tendon repair mechanisms [34].
Patients with tendinopathy commonly experience pain and morning stiffness. The onset of tendinopathy represents a vulnerable moment for individuals. Since symptoms may temporarily improve after warming up, individuals often persist with sports, work, or activities. Over time, these symptoms can escalate to constant and debilitating pain during such activities [2].
Beginning with the patient’s reported symptoms, a thorough physical examination is mandatory for diagnosis [35]. Palpation is useful for assessing tendon tenderness, especially for easily palpable tendons and to evaluate other structures such as adipose pads or tendon bursae. Performing pain-provoking tests specific to the affected tendon can be useful [36]: single leg heel raise and hop test in Achilles tendinopathy [35], single leg decline squat and Royal London Hospital test in patellar tendinopathy [37], and resisted wrist/finger extension and gripping an object in elbow tendinopathy (medial or lateral) [38]. Even if tendinopathy diagnosis relies on clinical assessment, imaging (X-ray, MRI, and ultrasonography) can aid in differential diagnosis and ruling out other causes of pain. In particular ultrasound, a widely used imaging method reveals tendon thickening, hypo-echoic regions, disrupted collagen organization, and possible neovascularization [39,40,41,42,43].
Tendinopathies can be treated using various methods, both active and passive. Among the active approaches, tendon load programs are effective as conservative treatment [44,45], including eccentric training, which induces structural adaptation in muscle-tendon units and helps protect tendons from stress and prevent re-injury [2], gradually increasing the load on the tendon to enhance its resistance [46], and isometric exercises to reduce pain [47].
Moreover, instrumental physical therapy, e.g., laser therapy, extra-corporeal shock-wave therapy (ESWT), or injection therapy, is usually employed, while surgical treatment is considered a last resort [48,49,50].
Low-energy laser therapy reduces edema and inflammation, induces analgesia, and supports healing in various musculoskeletal conditions [51].
ESWT, commonly used for calcific rotator cuff tendinopathy, plantar fasciitis, and Achilles tendinopathy [52], employs acoustic waves to enhance soft tissue healing and inhibit pain receptors [53].
Finally, injection therapy has gained ever more importance in the last years and can involve various substances, including hyaluronic acid, platelet-rich plasma (PRP), corticosteroids, and elevated-volume injections [2,11,54,55]. PRP, a centrifuged autologous blood preparation, contains an elevated platelet concentration, either paired with leukocytes or not. Platelet degranulation releases factors such as PDGF, VEGF, IGF1, TGFβ, and EGF, which stimulate tendon healing [56]. Corticosteroid injections are commonly used for tendon pathologies and have a local anti-inflammatory effect, providing pain relief and relaxing muscle spasms [57,58,59,60]. High-volume injections, frequently employed in Achilles tendinopathy, involve the injection of a significant volume of saline solution, often combined with corticosteroids and local anesthetic [61].
In this context, autologous tenocyte injection is a two-stage procedure. In the first stage, a small piece of a healthy tendon is collected under local anesthesia, with or without ultrasound guidance. The tenocytes are then isolated and cultured for 4–5 weeks. In the second phase, the autologous tenocytes obtained from in vitro culture are implanted into the damaged tendon area using ultrasound-guided injections [62,63]. Since these tenocytes are mature homologous cells that maintain their cellular phenotype unchanged, this treatment is considered safe [64].
To date, due to the paucity and heterogeneity of the results of the studies on autologous tenocyte injection in the treatment of tendinopathies, the objective of this systematic review was to investigate the current state of the art and determine the effectiveness of autologous tenocyte injection in improving pain, muscle strength, function, and MRI lesion appearance in patients with overuse and degenerative tendinopathies.
2. Materials and Methods
2.1. Protocol Design
This systematic review is performed following the guidelines set by PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-analyses) [65], and it was registered in PROSPERO with number CRD42024565211.
2.2. Search Strategy
Two authors independently performed an extensive literature search across several databases, such as PubMed Medline, Scopus, Cochrane Library, and Google Scholar. Their goal was to identify relevant articles published up to April 2024. The research meticulously examined the reference lists of the full-text studies obtained, expert documents, and congress abstracts. The search strategy incorporated the following keywords such as “tendinopathy”, “autologous tenocyte injection”, and “treatment”, along with their synonyms. Depending on the specific database, they employed Boolean operators (“AND”/”OR”) to combine these terms, according to the Cochrane [66]. No publication date filters were applied during the search process. Additionally, a third expert author meticulously reviewed the bibliographic search results and resolved any uncertainties. Table 1 illustrates the search strategy applied.

Table 1.
Search strategy.
2.3. Study Selection
Following the removal of duplicate articles, 2 authors separately assessed the abstract and the titles of all retrieved references from each database. If either author chose an article during the selection phase, it was thoroughly reviewed by both authors. Any conflicts were resolved by a 3rd author.
We adopted the following PICO (patient/population, intervention, comparison, outcome) model for the study selection: patients with chronic tendinopathy (P); autologous tenocyte injection as treatment (I); no restrictions for the control (C); quantitative and qualitative data related to pain intensity, functional recovery of the affected tendon, and significant morphological changes on imaging as outcomes (O).
Studies were included if they were case series, case reports, randomized controlled trials (RCTs), cohort studies, and case-control studies.
Exclusion criteria were (1) studies conducted on animals; (2) studies not in English; and (3) studies involving patients with recently onset tendinopathies.
2.4. Data Extraction
Two researchers separately gathered data from the selected studies using a standardized data collection form in Microsoft Excel. To address any discrepancies, a third author was consulted. We extracted the following information: (1) general study characteristics (such as authors, publication date, nationality, and study design); (2) characteristics of the treatment cohorts (including the number of participants, sex distribution, age range, type of diagnosed tendinopathy, and symptom duration); (3) a description of the intervention type; (4) data related to post-intervention outcomes (evaluated using specific rating scales and imaging techniques); and (5) main findings (including objective variations between pre- and post-intervention scales outcomes, along with mean and standard deviation for each outcome when available).
2.5. Outcomes
In this study, we focused on assessing the impact of autologous tenocyte injection therapy in patients with chronic tendinopathies across various anatomical regions. We evaluated several variables: pain, which we assessed verbally or using the visual-analogue scale (VAS); functional capacity recovery, measured with specific evaluation tools based on the location of the tendinopathy; and observable changes in MRI, which served as the reference imaging method.
2.6. Quality Assesment
In our current systematic review, we employed the Revised JBI quantitative critical assessment methods to evaluate validity and potential bias. These tools consist of a varying number of questions tailored to the specific study type—for our purposes, case reports and case series [67]. Each question offers four response alternatives: “yes” if the standard was clearly met, “no” if it was not met, “unclear” if it was not fully satisfied, and “not applicable” if the criterion does not apply to the study under examination. Within the tool, certain items address the potential bias, while alternative approaches focus on guaranteeing proper reporting and statistical analysis. A “no” response to any of the questions adversely affects the overall study quality. Importantly, these tools intentionally avoid providing specific cut-off values or scores, allowing for informed inclusion of studies with varying quality in reviews [68].
Two authors separately evaluated the risk of bias and evidence quality, resolving any concerns by consulting with a third researcher. We considered studies with a “yes” number of 6 or more.
3. Results
3.1. Study Selection
The PRISMA flow chart (see Figure 1 for further details) illustrates the article selection procedure. A total of 174 records were obtained from the initial literature search. Sixty-seven studies were eliminated due to duplication, and 107 studies were initially assessed by title and abstract. Eighty-three studies were discarded based on the title/abstract, and 19 studies did not satisfy the inclusion criteria. At least, 5 studies [62,64,69,70,71] were included. Specifically, 3 case series [64,70,71] and 2 case reports [62,69] were included in this review.
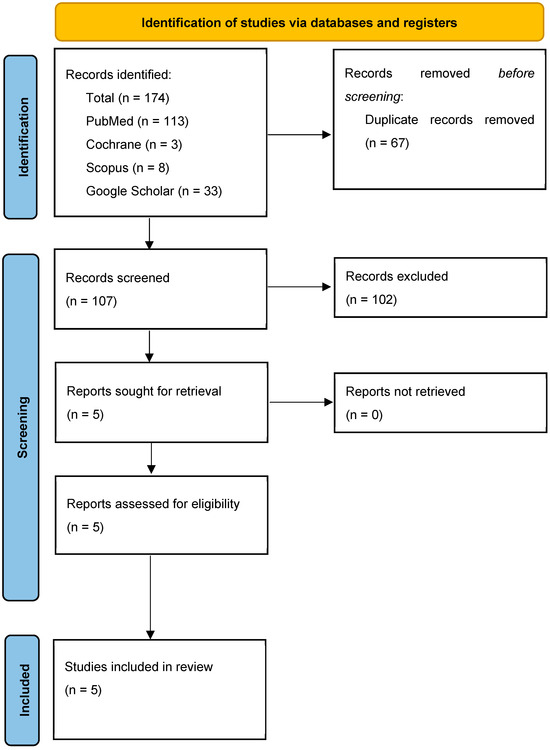
Figure 1.
PRISMA flowchart.
3.2. Characteristics of the Studies Included in the Review
All the studies featured in this review were carried out in Australia between 2013 and 2018 (2013 [69,70], 2015 [71], 2017 [64], and 2018 [62]). These studies collected data from 50 participants (22 man and 28 woman) aged between 20 and 67, all of whom were affected by various forms of tendinopathy and had ongoing symptoms for at least 6 months. The location of tendinopathy was reported in all selected articles. In particular, two studies considered lateral epicondylitis, one study considered gluteal tendinopathy and two shoulder pain. See Table 2 for further details.

Table 2.
Main characteristics of the included studies.
3.3. Intervention Protocol
Autologous tenocyte injection varied in terms of tenocyte collection site, tenocyte proliferation time, the number of tenocytes injected, and the type of needle used for injection.
Wang et al. (2013) [69] harvested tenocytes from the subject’s patella tendon under regional anesthesia, and then cellular expansion was performed in a GMP laboratory setting. About 20 days later, 2 mL of tenocyte suspension was administered to the tendon with tendinopathy, guided by ultrasound.
Wang et al. (2013) [70] took tenocytes from patellar tendon. After approximately three weeks, using ultrasound guidance and regional anesthetic, a single injection of autologous tenocytes was performed on patients directly into the tendinopathy site of the extensor carpi radialis brevis tendon.
In Wang et al.’s (2015) [71] study, around three weeks following biopsy, with the aid of an 18-gauge needle and guided by ultrasound, an injection of up to 2 mL of autologous tenocytes, in suspension with 10% autologous human serum, was performed at the tendinopathy site of the extensor carpi radialis brevis tendon.
In Bucher et al.’s [64] study, after about four weeks, a single 2 mL injection of autologous tenocyte suspension was given to the patients. The injection was administered under ultrasound guidance, using a 22-gauge needle, into the area affected by tendinopathy.
Schwab et al. [62] harvested a small section of palmaris longus tendon, which was then cultured. After 7 weeks of tenocyte proliferation, an ultrasound-guided injection of three 1 mL vials containing a total of 5 × 106 tenocytes were performed into the subscapularis tendon of the patients.
3.4. Side Effects
No patients experienced adverse effects at the biopsy site. Just three individuals declared slight pain at the tendon biopsy site in Bucher et al.’s study [64], but all these patients showed improvement with topical NSAID gel, and they had no persistent sequelae associated to the intervention site. No contamination, tendon laceration, neurotrauma, hematoma, or calcification was identified at the inoculation site.
Two articles did not evaluate side effects [62,70].
3.5. Outcome Measures Assessed
In the reviewed articles, researchers focused on three key outcomes: pain, the function of the injured tendon, and the appearance of tendinopathy on MRI. Four studies used the visual analog scale (VAS) to evaluate pain [64,69,70,71], and the tendon function was evaluated using several validated scales or tools, including the Oxford Shoulder Score [71], QuickDisabilities of the Arm, Shoulder, and Hand (QuickDASH) [69,70,71], Upper Extremity Functional Scale (UEFS) [71], Oxford Hip Score (OHS) [64], and the 36-item Short Form Health Survey (SF-36) [64]. Additionally, grip strength was quantified utilizing the Jamar dynamometer [70,71], and shoulder internal rotation muscular capacity was assessed with a Commander Power Trak II dynamometer [62]. All MRI scans were conducted using a 3 Tesla machine.
3.6. Pain
In Wang et al.’s 2013 study [69], they reported a VAS pain score of 1 out of 10 at 10 months after the injection. In Wang et al.’s 2013 study [70], the mean maximum VAS pain score was 5.94 ± 2.24 (median, 5.80). Following autologous tenocyte injection, it increased by 57% at four weeks (p < 0.001), 77% at six months (p < 0.001), and 86% at 12 months (mean, 0.76; p < 0.001). Prior to intervention in Wang et al.’s 2015 study [52], the VAS pain score was 5.94 ± 0.56. At 1-year follow-up, it improved by 86% (0.76 ± 0.14; p < 0.001). The final assessment showed a 78% improvement (1.21 ± 0.31), significantly better than the initial state but comparable to the 1-year results (p > 0.05).
Bucher et al. [64] observed progress from the starting point to three months post-injection for the VAS (change, −2.8 points; 95% CI, −4.4 to −1.2; p = 0.001). The estimated mean improvement in VAS from the starting point to 12 months was −4.1 (95% CI, −2.6 to −5.6; p < 0.001). Incremental improvement mainly occurred in the first 3 months. The improvement was sustained at 24 months, with a pain reduction from baseline to 24 months of −4.5 points (95% CI, −6.1 to −2.9; p < 0.001).
Schwab et al. [62] reported complete pain remission after 7 weeks from the injection.
3.7. Function
Wang et al. 2013 [69] reported a QuickDASH, 13/55 with sports module, 6/20 at 10 months after injection.
In Wang et al.’s 2013 study [70] the mean pre-intervention QuickDASH score was 45.88 ± 15.24. Significant (p < 0.001) progress was evident after 4 weeks, with an additional improvement to 12 months, where the mean QuickDASH score decreased to 2.88 ± 0.72 (91% improvement from pre-intervention scores). The grip strength assessment demonstrated a parallel pattern of enhancement.
In Wang et al.’s 2015 study [71], prior to the intervention, the average QuickDASH score was 45.88 ± 3.81. A substantial reduction of 91% was observed at the one-year mark, with the mean score dropping to 3.84 ± 1.05 (p < 0.001). At the final assessment, an 84% reduction was maintained, yielding a mean score of 6.61 ± 1.87 (p < 0.001). Notably, no statistically significant variation in QuickDASH scores was found between the one-year and final follow-up periods (p > 0.05).
The Upper Extremity Functional Scale (UEFS) exhibited a comparable trend. From an initial mean of 31.73 ± 3.75, a 66% enhancement was achieved at one year, resulting in a mean of 9.40 ± 0.52 (p < 0.001). This improvement was largely sustained at the final follow-up, showing a 64% enhancement with a mean of 9.20 ± 0.39 (p < 0.001 compared to baseline). Again, no significant difference was detected between the one-year and final follow-up UEFS scores.
Grip strength measurements displayed a progressive increase. From a baseline of 19.85 ± 2.81 kg, a significant improvement of 132.6% was recorded at the one-year follow-up, with the mean reaching 37.38 ± 3.30 kg (p < 0.001). This upward trend continued, culminating in a 208% improvement at the final follow-up, where the mean grip strength was 46.60 ± 3.46 kg (p < 0.001).
In Bucher et al.’s study [64], statistical analysis revealed significant gains in OHS from the pre-injection starting point to the 6-month follow-up (change, 8.3 points; 95% CI, 3.9–12.8; p = 0.009). A clinically important improvement of at least 11 points was observed in seven out of the twelve patients. The observed improvements in the OHS score were not found to be associated with patient age (Spearman rho, −0.306; p = 0.334) or symptom duration (Spearman rho, −0.182; p = 0.572).
Schwab et al. [62] also demonstrated a progressive increase in hand grip strength from the pre-autologous tenocyte injection value at three and seven weeks after the injection even though, following the return to full training, strength results did not fully recover to baseline.
3.8. MRI
Following autologous tenocyte injections, Wang et al. (2013) [69] conducted 3 Tesla MRI evaluations at both 4 and 10 months. Two experienced musculoskeletal radiologists independently assessed rotator cuff tendinopathy, partial tear thickness, and anteroposterior (AP) tear size. The study revealed that while tendinopathy, characterized by tendon thickening and persistent focal signal increase, was mitigated at the 4-month mark, this positive change did not sustain through the 10-month follow-up. In contrast, the partial-thickness rim-rent tear was not observed at either time point, indicating a successful healing process.
Wang et al., in 2013 [70], utilized magnetic resonance imaging to evaluate the severity of tendinopathy and tears at the insertion of the common extensor tendon before and after a 12-month treatment period. The mean MRI score, starting at 4.31 ± 1.14 (scale 2–6), significantly improved to 2.88 ± 0.72 (p < 0.001) after 12 months. The study demonstrated significant correlations between MRI scores and clinical outcomes, including QuickDASH (R = 0.545, p = 0.001), maximum pain (R = 0.448, p = 0.010), and grip strength (R = −0.494, p = 0.004). All patients diagnosed with grade-3 tendinosis showed a reduction in their scores following treatment. Of the sixteen patients with grade-3 tears, four experienced a decrease in tear severity, two remained unchanged, and one grade-2 tear worsened to grade 3. One patient, deemed a non-responder at 3 months, elected to undergo surgery for lateral epicondylitis and subsequently withdrew from the study.
In Wang et al. [71] in 2015, the MRI was utilized to determine the severity of tendinopathy and the size of the tear at the insertion of the common extensor tendon. Initially, the average MRI score was recorded at 4.31, with a standard deviation of 0.28. After a year, a notable reduction to 2.88 ± 0.18 was observed (p < 0.001), and this improvement persisted for five years, averaging 2.87 ± 0.19 (p < 0.001). Most participants, specifically all but two, exhibited consistent MRI scores between the one-year and final assessments. In one case, the tendinopathy component of the score showed further enhancement at 4.3 years post-intervention, moving from 2 at one year to 1, resulting in an overall improvement from 3 to 2. Conversely, another patient experienced a minor increase in the tear score, from 1 to 2, due to a minimal partial-thickness tear in the deep extensor carpi radialis brevis tendon, though this was less pronounced than pre-treatment. Interestingly, this patient continued to engage in tennis.
In the study conducted by Bucher et al. [64], the researchers aimed to determine if MRI-detected tendinopathy characteristics were associated with clinical outcomes following autologous tenocyte injections. Their analysis revealed no notable changes in tendon features, as assessed by MRI, between pre- and post-injection evaluations. The consistency of MRI measurements, as determined by intrarater agreement, was generally strong, with PABAK values ranging from 0.727 to 1.00, except for the signal intensity in the lateral gluteus medius tendon.
In Schwab et al. [62], three radiologists, working independently and blinded, confirmed a marked post-assessment change in tendon appearance on MRI. The majority, specifically two of the three, judged the tear to be entirely healed, and all observed an improvement in tendinopathy.
3.9. Other Outcomes
Bucher et al. [64] utilized the SF-36 questionnaire to assess overall health, generating both mental (MCS) and physical (PCS) component scores. The PCS subscale showed a trend of progressive enhancement, with the most significant gains occurring within the initial three months post-intervention. Prior to the intervention, the average PCS score was 28.1 (SD 8.5), ranging from 17.4 to 46.1. At 12 months, the estimated average increase in the PCS was 15.2 points (95% CI 9.8–20.5; p < 0.001). This positive change in PCS scores was sustained over a 24-month period, with an estimated average improvement of 12.8 points (95% CI 7.3–18.3; p < 0.001) from baseline.
Wang et al. (2015) [71] and Bucher et al. [64] used a 7-point scale to evaluate patient satisfaction following treatment.
In Wang et al.’s 2015 study [71], at final follow-up, a significant majority, 93% (n = 14), of patients reported high or moderate satisfaction with their autologous tenocyte injection treatment. Only one patient, whose tear worsened, expressed uncertainty, assigning a score of 5. Furthermore, a considerable number of patients (10 out of 15) resumed activities known to trigger pain in lateral epicondylitis, such as darts, tennis, weight lifting, and gardening.
Bucher et al. [64] reported that all 12 patients completed a satisfaction survey 12 months after their surgery. Of those, five expressed strong satisfaction, three reported being satisfied or quite satisfied, two were undecided, and two were dissatisfied with their results. Notably, patient satisfaction at 12 months closely aligned with the degree of improvement observed at the same time point; those experiencing the greatest improvement were highly satisfied, while those with minimal improvement were either dissatisfied or unsure.
3.10. Study Limitations
The two main limitations present in all the articles considered were the number of patients and the absence of a control group. The study design did not incorporate either a comparative alternative treatment or a placebo, precluding a definitive assessment of the treatment’s superiority or the potential influence of a placebo effect on the results [62,64,69,70,71].
Another limitation found in Bucher et al.’s study [64] is the utilization of the OHS, a clinical scale specific for hip osteoarthritis, as the main clinical outcome measure. Additionally, they explored changes detectable by MRI that correlated with clinical improvement. However, they found that clear radiological improvement was not present in the majority of cases despite the reported clinical improvement. This could be a limitation due to the lack of a validated tool to assess MRI improvement following an intervention, or it could be due to the post-injection MRI being performed too early.
3.11. Study Quality
We used the revised Joanna Briggs Institute (JBI) quantitative critical appraisal tools to evaluate validity and risk of bias. In particular, the two case reports scored 7/8 [62,69] (see Table 3).

Table 3.
Revised JBI quantitative critical appraisal tools to assess validity and risk of bias for case report.

Table 4.
Revised JBI quantitative critical appraisal tools to assess validity and risk of bias for case series.
4. Discussion
The therapeutic landscape in tendinopathies is vast and not always effective due to the wide heterogeneity of patients and the complexity of lesions and repair mechanisms underlying the pathology [57,72,73].
This review aimed to investigate the security and therapeutic effect of autologous tenocyte treatment in tendinopathies.
Autologous tenocyte implantation is a minimally invasive technique and almost always well tolerated by patients who show an early reduction in symptoms, followed by a functional recovery. This is particularly useful to allow an early rehabilitation plan [74,75].
In particular, all studies examined the safety of autologous tenocyte injection, and, throughout the follow-up period, no patient reported significant adverse effects or immunological reactions. Furthermore, a clear majority of participants showed a high degree of satisfaction after the treatment, demonstrating that not only was the treatment well tolerated but was also subjectively perceived as effective.
All areas examined, namely, pain, the recovery of function, and changes in lesion imaging, showed an improvement starting from the first month of follow-up. In all studies, the improvement continued for the first 6 months and then remained substantially unchanged up to one year, regardless of the location of the tendinopathy. However, since no control group was present in any of the studies considered, it cannot be stated with certainty that this improvement was actually due to the tenocyte injection. Nevertheless, this is an encouraging result, also considering that recent studies have shown that PRP, currently the most widely used conservative approach in clinical practice to promote tendon regeneration, has an efficacy that does not appear to be better to that of placebo in alleviating discomfort or improving tendon function [76].
However, the only study in this review that extended the follow-up time to 4.5 years [71] showed a general worsening of pain symptoms and a progressive decrease in the function of the injured tendon, although both of mild entity, without, however, returning to pre-treatment levels. Autologous tenocyte injection shows a very similar trend to corticosteroid injections but with better results and fewer side effects.
Corticosteroid treatment in insertional tendinopathies, in fact, has efficacy primarily in the short term. In the long term, progressive worsening occurs, with high rates of recurrence [77,78,79]. Furthermore, although corticosteroid injection induces a reduction in pain and inflammation and an enhanced sonographic visualization of the tendon, it has adverse effects ranging from a deficit in the strength of the injured tendon to atrophy or rupture of the same, in 82% of clinical trials [80].
Despite these problems highlighted in corticosteroid injections, some studies have shown that image-guided high-volume injections (HVIGIs) of normal saline, local anesthetic, and corticosteroid determine good results regarding pain relief and the enhancement of functional activity in patients with different types of tendinopathy. Furthermore, Maffulli et al. have highlighted positive effects also in HVIGI with normal saline, local anesthetic, and aprotinin, therefore, without the use of corticosteroids. This technique proves to be promising, with results similar to those of autologous tenocyte injection [53].
As for the evaluation of pain, specifically in the group of patients suffering from lateral epicondylitis, the studies considered have shown that, beyond the first year, it ceased to improve, remaining virtually unchanged at average VAS values of 1.2.
These data can be compared with that of the method commonly used as a last resort in chronic tendinopathies. A 2024 review [81] was analyzed that compares open, percutaneous, and arthroscopic surgery in lateral epicondylitis. From this, it can be seen that the improvement in pain is extremely variable both between the various interventions and between people undergoing the same type of intervention; however, average VAS values of 1.2 represent the lowest values among all three surgical techniques [72,82]. With autologous tenocyte injection, unlike surgical techniques, being a conservative treatment, such an outcome is strongly encouraging [83,84].
As is known, on imaging, tendinopathies classically appear with a thickening of the tendon associated with an increase in signal in T2 sequences, while concomitant tears, complete or partial, have a variable appearance: they generally present with a hyperintensity of the signal in T2 sequences, but, in the more advanced stages, the deposition of scar tissue can hide this hyperintensity of signal [85,86]. Both patients in the two case reports examined [62,69] at the MRI control of their respective lesions showed a progressive reduction in the tear in parallel with a reduction in the aforementioned signs of tendinopathy, respectively, 4 and 6 months after the tenocyte injection.
It is evident that the injection at the site of the tear not only did not determine its enlargement but, on the contrary, triggered a process of healing and repair. In Schwab et al. [62], these data are particularly significant as the patient had already undergone corticosteroid injections and, 7 weeks before the injection, an arthroscopic evaluation with PRP injection in the same site without, however, obtaining any benefit concerning pain reduction or the recovery of performance. However, it is essential to consider that, in this case, it is not possible to determine if the patient’s clinical improvement is attributable only to autologous tenocyte injection or to the combination of this method with previous therapeutic interventions.
Another aspect of this review to consider is the cost: autologous tenocyte injection, involving a laboratory phase, is certainly burdened by higher costs and longer waiting times compared to other therapeutic strategies.
This review is not free of limitations. Firstly, the included studies exhibited a heterogeneous patient population, varying in age, clinical condition, and tendinopathy site. Notably, the precise clinical condition of patients was not consistently detailed across all studies, which could significantly influence healing times. Moreover, the lack of uniformity in evaluation scales across studies, coupled with variations in intervention methods and protocols, hindered a fully objective and direct comparison of findings.
5. Conclusions
Collectively, the results of this systematic review evidenced that autologous tenocyte injection could represent a valuable option in the management of chronic tendinopathies, also considering its safety, with a minimal risk of side effects. The main effect obtained was the pain relief and, consequently, the improvement of patients’ quality of life. The clinical improvement is also evident at MRI in which it is possible to see a progressive reduction with a general disappearance of the T2 signal hyperintensity between 4 months and 1 year. However, even if the costs are higher than corticosteroid injection or PRP, autologous tenocyte injection can be considered as a second line treatment in the case of resistant tendinopathies. However, more research is necessary to confirm the efficiency of this treatment. In particular, studies conducted in other countries are necessary to obtain more globally representative data. The inclusion of a control group is essential to ensure a comparison with both untreated patients and patients treated with other injection therapies. Comparing patients with tendinopathy in the same location would be very useful so that the same evaluation scales can always be used to facilitate direct data comparability.
Author Contributions
Conceptualization, A.D. and A.d.S.; methodology, A.D., A.d.S., A.A. and C.C.; formal analysis, A.D., A.d.S. and N.M.; investigation, A.D., B.C. and M.G.; data curation, A.D., A.d.S. and A.S.; writing—original draft preparation, A.D., A.d.S. and B.C.; writing—review and editing, M.I., A.A. and C.C.; visualization, N.M., A.S., M.G. and L.L.; supervision, A.D. and A.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, T.W.T.W.; Cardenas, L.; Soslowsky, L.J.L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. Tendinopathy--from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, A.; Maffulli, N.; Saglietti, C.; Sarti, E.; Bigliardi, D.; Costantino, C.; Demeco, A. A Practical Guide to Injection Therapy in Hand Tendinopathies: A Systematic Review of Randomized Controlled Trials. J. Funct. Morphol. Kinesiol. 2024, 9, 146. [Google Scholar] [CrossRef]
- Hopkins, C.; Fu, S.-C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.-H.; Chan, K.-M. Critical review on the socio-economic impact of tendinopathy. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef]
- Demeco, A.; de Sire, A.; Salerno, A.; Marotta, N.; Palermi, S.; Frizziero, A.; Costantino, C. Dry Needling in Overhead Athletes with Myofascial Shoulder Pain: A Systematic Review. Sports 2024, 12, 156. [Google Scholar] [CrossRef]
- Demeco, A.; Salerno, A.; Gusai, M.; Vignali, B.; Gramigna, V.; Palumbo, A.; Corradi, A.; Mickeviciute, G.C.; Costantino, C. The Role of Virtual Reality in the Management of Football Injuries. Medicina 2024, 60, 1000. [Google Scholar] [CrossRef]
- Demeco, A.; Bartocci, G.; Astore, N.; Vignali, B.; Salerno, A.; Palermi, S.; Foresti, R.; Martini, C.; Costantino, C. The Efficacy of Pelvic Floor Rehabilitation in the Treatment of Urinary Incontinence in Female Athletes: A Systematic Review. Sports 2024, 12, 338. [Google Scholar] [CrossRef]
- Niemiec, P.; Jarosz, A.; Nowak, T.; Balcerzyk-Matić, A.; Iwanicki, T.; Iwanicka, J.; Gawron, K.; Kalita, M.; Górczyńska-Kosiorz, S.; Kania, W.; et al. Impact of the COL1A1 Gene Polymorphisms on Pain Perception in Tennis Elbow Patients: A Two-Year Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 13221. [Google Scholar] [CrossRef]
- Efficacy of Ultrasound-Guided Galvanic Electrolysis Technique and Physical Therapy in Patients with Achilles’ Tendinopathy: A Pilot Randomised Controlled Trial—IOS Press. Available online: https://content.iospress.com/articles/journal-of-back-and-musculoskeletal-rehabilitation/bmr230255 (accessed on 30 December 2024).
- Jarosz, A.; Nowak, T.; Szyluk, K.; Balcerzyk-Matić, A.; Iwanicki, T.; Iwanicka, J.; Kalita, M.; Gawron, K.; Kania, W.; Niemiec, P. The VEGFB Gene Variants and the Effectiveness of Platelet-Rich Plasma Treatment of Lateral Elbow Tendinopathy: A Prospective Cohort Study with a Two-Year Follow-Up. Int. J. Mol. Sci. 2024, 25, 13166. [Google Scholar] [CrossRef]
- Marotta, N.; de Sire, A.; Lippi, L.; Moggio, L.; Mondardini, P.; Sgro, M.; Bartalotta, I.; Zito, R.; Giroldini, T.; Invernizzi, M.; et al. Effectiveness of High-Power Laser Therapy via Shear Wave Speed Analysis on Pain and Functioning in Patients with Lateral Epicondylitis: A Proof-of-Concept Study. J. Clin. Med. 2024, 13, 2014. [Google Scholar] [CrossRef] [PubMed]
- Riel, H.; Lindstrøm, C.F.; Rathleff, M.S.; Jensen, M.B.; Olesen, J.L. Prevalence and incidence rate of lower-extremity tendinopathies in a Danish general practice: A registry-based study. BMC Musculoskelet. Disord. 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Zwerver, J.; Grewal, N.; de Sa, A.; Alktebi, T.; Granville, D.J.; Hart, D.A. Lipids, adiposity and tendinopathy: Is there a mechanistic link? Critical review. Br. J. Sports Med. 2015, 49, 984–988. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Occurrence of tendon pathologies in metabolic disorders. Rheumatol. Oxf. Engl. 2013, 52, 599–608. [Google Scholar] [CrossRef]
- van der Vlist, A.C.; Breda, S.J.; Oei, E.H.G.; Verhaar, J.A.N.; de Vos, R.-J. Clinical risk factors for Achilles tendinopathy: A systematic review. Br. J. Sports Med. 2019, 53, 1352–1361. [Google Scholar] [CrossRef]
- September, A.; Rahim, M.; Collins, M. Towards an Understanding of the Genetics of Tendinopathy. Adv. Exp. Med. Biol. 2016, 920, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, N.H.; Stepanyan, H.; Gallo, R.A.; Dhawan, A. Genetic Factors in Tendon Injury: A Systematic Review of the Literature. Orthop. J. Sports Med. 2017, 5, 2325967117724416. [Google Scholar] [CrossRef]
- Macedo, C.S.G.; Tadiello, F.F.; Medeiros, L.T.; Antonelo, M.C.; Alves, M.A.F.; Mendonça, L.D. Physical Therapy Service delivered in the Polyclinic During the Rio 2016 Paralympic Games. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2019, 36, 62–67. [Google Scholar] [CrossRef]
- Florit, D.; Pedret, C.; Casals, M.; Malliaras, P.; Sugimoto, D.; Rodas, G. Incidence of Tendinopathy in Team Sports in a Multidisciplinary Sports Club Over 8 Seasons. J. Sports Sci. Med. 2019, 18, 780–788. [Google Scholar]
- Francis, P.; Whatman, C.; Sheerin, K.; Hume, P.; Johnson, M.I. The Proportion of Lower Limb Running Injuries by Gender, Anatomical Location and Specific Pathology: A Systematic Review. J. Sports Sci. Med. 2019, 18, 21–31. [Google Scholar]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Ransom, J.E.; Smith, J.; Morrey, B.F. The epidemiology and health care burden of tennis elbow: A population-based study. Am. J. Sports Med. 2015, 43, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Alfredson, H.; Cucchiarini, M.; Madry, H.; Marmotti, A.; Mouton, C.; Oliveira, J.M.; Pereira, H.; Peretti, G.M.; Spang, C.; et al. Current trends in tendinopathy: Consensus of the ESSKA basic science committee. Part II: Treatment options. J. Exp. Orthop. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; de Sire, A.; Di Giacomo, G.; Paoloni, M.; Murgia, M.; Di Cesare, A.; Ammendolia, A.; Bernetti, A.; Mangone, M. Postural Evaluation and Risk of Musculoskeletal Injuries in Professional Male Rugby Players: A Proof-of-Principle Study. J. Sports Med. Phys. Fit. 2022, 62, 1675–1684. Available online: https://www.minervamedica.it/it/riviste/sports-med-physical-fitness/articolo.php?cod=R40Y2022N12A1675 (accessed on 30 December 2024). [CrossRef]
- Demeco, A.; de Sire, A.; Marotta, N.; Spanò, R.; Lippi, L.; Palumbo, A.; Iona, T.; Gramigna, V.; Palermi, S.; Leigheb, M.; et al. Match Analysis, Physical Training, Risk of Injury and Rehabilitation in Padel: Overview of the Literature. Int. J. Environ. Res. Public. Health 2022, 19, 4153. [Google Scholar] [CrossRef]
- de Sire, A.; Demeco, A.; Frizziero, A.; Marotta, N.; Spanò, R.; Carozzo, S.; Costantino, C.; Ammendolia, A. Risk of Injury and Kinematic Assessment of the Shoulder Biomechanics During Strokes in Padel Players: A Cross-Sectional Study. J. Sports Med. Phys. Fit. 2024, 64, 383–391. Available online: https://www.minervamedica.it/it/riviste/sports-med-physical-fitness/articolo.php?cod=R40Y2024N04A0383 (accessed on 30 December 2024). [CrossRef]
- Tran, P.H.T.; Malmgaard-Clausen, N.M.; Puggaard, R.S.; Svensson, R.B.; Nybing, J.D.; Hansen, P.; Schjerling, P.; Zinglersen, A.H.; Couppé, C.; Boesen, M.; et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 776–788. [Google Scholar] [CrossRef]
- Kannus, P.; Józsa, L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Jt. Surg. Am. 1991, 73, 1507–1525. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Lippi, L.; Scaturro, D.; Farì, G.; Liccardi, A.; Moggio, L.; Letizia Mauro, G.; Ammendolia, A.; Invernizzi, M. Pharmacological Treatment for Acute Traumatic Musculoskeletal Pain in Athletes. Medicina 2021, 57, 1208. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K.W. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef]
- Crowe, L.A.N.; McLean, M.; Kitson, S.M.; Melchor, E.G.; Patommel, K.; Cao, H.M.; Reilly, J.H.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; et al. S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci. Rep. 2019, 9, 1463. [Google Scholar] [CrossRef]
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Wells, G.; Appleton, L.; et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Langberg, H.; Olesen, J.L.; Gemmer, C.; Kjaer, M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J. Physiol. 2002, 542, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Seale, K.B.; El Khoury, L.Y.; Posthumus, M.; Ribbans, W.J.; Raleigh, S.M.; Collins, M.; September, A.V. Polymorphisms within the COL5A1 gene and regulators of the extracellular matrix modify the risk of Achilles tendon pathology in a British case-control study. J. Sports Sci. 2017, 35, 1475–1483. [Google Scholar] [CrossRef]
- The Utility of Clinical Measures for the Diagnosis of Achilles Tendon Injuries: A Systematic Review with Meta-Analysis—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4264655/ (accessed on 27 June 2024).
- Wan, X.-H.; Zeng, R. Handbook of Clinical Diagnostics; Springer Nature: Berlin/Heidelberg, Germany, 2019; ISBN 9789811376771. [Google Scholar]
- Malliaras, P.; Cook, J.; Purdam, C.; Rio, E. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J. Orthop. Sports Phys. Ther. 2015, 45, 887–898. [Google Scholar] [CrossRef]
- Taylor, S.A.; Hannafin, J.A. Evaluation and management of elbow tendinopathy. Sports Health 2012, 4, 384–393. [Google Scholar] [CrossRef]
- Docking, S.I.; Ooi, C.C.; Connell, D. Tendinopathy: Is Imaging Telling Us the Entire Story? J. Orthop. Sports Phys. Ther. 2015, 45, 842–852. [Google Scholar] [CrossRef]
- de Oliveira, L.N.; Durigan, J.L.Q.; Sanchez, C.R.; Mansur, H.; Rosa, A.B.B.; Marqueti, R. de C. MRI-Based Morphometric Comparison of Lower Leg Muscles and Tendons in Individuals With Medial Tibial Stress Syndrome. BioMed Res. Int. 2024, 2024, 8827692. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.; Balcerzyk-Matić, A.; Iwanicka, J.; Iwanicki, T.; Nowak, T.; Szyluk, K.; Kalita, M.; Górczyńska-Kosiorz, S.; Kania, W.; Niemiec, P. Association between Platelet-Derived Growth Factor Receptor Alpha Gene Polymorphisms and Platelet-Rich Plasma’s Efficiency in Treating Lateral Elbow Tendinopathy—A Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 4266. [Google Scholar] [CrossRef]
- The Bioinductive Collagen Implant Yields Positive Histological, Clinical and MRI Outcomes in the Management of Rotator Cuff Tears: A Systematic Review—Longo—Knee Surgery, Sports Traumatology, Arthroscopy—Wiley Online Library. Available online: https://esskajournals.onlinelibrary.wiley.com/doi/10.1002/ksa.12429 (accessed on 30 December 2024).
- Wu, K.-T.; Chen, P.-C.; Chou, W.-Y.; Chang, C.-D.; Lien, J.-J.J. Diagnostic Accuracy and Interobserver Reliability of Rotator Cuff Tear Detection with Ultrasonography are Improved with Attentional Deep Learning. Arthroscopy 2024, 24, S0749-8063(24)01088-0. [Google Scholar] [CrossRef]
- Lim, H.Y.; Wong, S.H. Effects of isometric, eccentric, or heavy slow resistance exercises on pain and function in individuals with patellar tendinopathy: A systematic review. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2018, 23, e1721. [Google Scholar] [CrossRef]
- Visnes, H.; Bahr, R. The evolution of eccentric training as treatment for patellar tendinopathy (jumper’s knee): A critical review of exercise programmes. Br. J. Sports Med. 2007, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Longo, U.G.; Loppini, M.; Denaro, V. Current treatment options for tendinopathy. Expert Opin. Pharmacother. 2010, 11, 2177–2186. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef]
- Andres, B.M.; Murrell, G.A.C. Treatment of Tendinopathy: What Works, What Does Not, and What is on the Horizon. Clin. Orthop. 2008, 466, 1539–1554. [Google Scholar] [CrossRef]
- Santilli, G.; Vetrano, M.; Mangone, M.; Agostini, F.; Bernetti, A.; Coraci, D.; Paoloni, M.; de Sire, A.; Paolucci, T.; Latini, E.; et al. Predictive Prognostic Factors in Non-Calcific Supraspinatus Tendinopathy Treated with Focused Extracorporeal Shock Wave Therapy: An Artificial Neural Network Approach. Life 2024, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Di Gesù, M.; Alito, A.; Borzelli, D.; Romeo, D.; Bonomolo, F.; Calafiore, D.; de Sire, A. Efficacy of ultrasound-guided galvanic electrolysis technique and physical therapy in patients with Achilles’ tendinopathy: A pilot randomised controlled trial. J Back Musculoskelet Rehabil. 2024, 37, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015, 2, 00068. [Google Scholar] [CrossRef]
- Rompe, J.D.; Nafe, B.; Furia, J.P.; Maffulli, N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: A randomized controlled trial. Am. J. Sports Med. 2007, 35, 374–383. [Google Scholar] [CrossRef]
- Rompe, J.D.; Maffulli, N. Repetitive shock wave therapy for lateral elbow tendinopathy (tennis elbow): A systematic and qualitative analysis. Br. Med. Bull. 2007, 83, 355–378. [Google Scholar] [CrossRef]
- Pitsilos, C.; Karachrysafi, S.; Fragou, A.; Gigis, I.; Papadopoulos, P.; Chalidis, B. The Biological Effect of Platelet-Rich Plasma on Rotator Cuff Tears: A Prospective Randomized In Vivo Study. Int. J. Mol. Sci. 2024, 25, 7957. [Google Scholar] [CrossRef]
- Agostini, F.; de Sire, A.; Paoloni, M.; Finamore, N.; Ammendolia, A.; Mangone, M.; Bernetti, A. Effects of hyaluronic acid injections on pain and functioning in patients affected by tendinopathies: A narrative review. J. Back Musculoskelet. Rehabil. 2022, 35, 949–961. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.H. PRP treatment efficacy for tendinopathy: A review of basic science studies. BioMed Res. Int. 2016, 2016, 9103792. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Maffulli, N.; Cook, J. Management of Tendinopathy. Am. J. Sports Med. 2009, 37, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.F.; Lostis, E.; Oakley, T.; Rombach, I.; Morrey, M.E.; Carr, A.J. The risks and benefits of glucocorticoid treatment for tendinopathy: A systematic review of the effects of local glucocorticoid on tendon. Semin. Arthritis Rheum. 2014, 43, 570–576. [Google Scholar] [CrossRef]
- Gaujoux-Viala, C.; Dougados, M.; Gossec, L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: A meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2009, 68, 1843–1849. [Google Scholar] [CrossRef]
- Dadgostar, H.; Fahimipour, F.; Pahlevan Sabagh, A.; Arasteh, P.; Razi, M. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: A randomized clinical trial study. J. Orthop. Surg. 2021, 16, 333. [Google Scholar] [CrossRef] [PubMed]
- Barker-Davies, R.M.; Nicol, A.; McCurdie, I.; Watson, J.; Baker, P.; Wheeler, P.; Fong, D.; Lewis, M.; Bennett, A.N. Study protocol: A double blind randomised control trial of high volume image guided injections in Achilles and patellar tendinopathy in a young active population. BMC Musculoskelet. Disord. 2017, 18, 204. [Google Scholar] [CrossRef]
- Schwab, L.M.; Blanch, P.; Young, M. Autologous tenocyte implantation into shoulder tendon pathology in an elite swimmer. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2018, 29, 19–25. [Google Scholar] [CrossRef]
- Wang, K.; Wang, A.; Cheng, T.S.; Landao-Bassonga, E.; Lee, C.; Tai, A.; Damiani, M.; Zheng, M.H. Impact of age and donor sites on bioactivities of tendon cells in autologous tenocyte implantation (OrthoATITM) for treatment of chronic tendinopathy. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2024, 9, 603–608. [Google Scholar] [CrossRef]
- Bucher, T.A.; Ebert, J.R.; Smith, A.; Breidahl, W.; Fallon, M.; Wang, T.; Zheng, M.-H.; Janes, G.C. Autologous tenocyte injection for the treatment of chronic recalcitrant gluteal tendinopathy: A prospective pilot study. Orthop. J. Sports Med. 2017, 5, 2325967116688866. [Google Scholar] [CrossRef]
- PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 25 February 2025).
- Becky Alford, M. LibGuides: Evidence Based Medicine: PICO. Available online: https://mcw.libguides.com/EBM/PICO (accessed on 25 February 2025).
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Leonardi-Bee, J.; Tufanaru, C.; Aromataris, E.; Munn, Z. Revising the JBI quantitative critical appraisal tools to improve their applicability: An overview of methods and the development process. JBI Evid. Synth. 2023, 21, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Database Syst. Rev. Implement. Rep. 2019, publish ahead of print. Available online: https://journals.lww.com/10.11124/JBISRIR-D-19-00099 (accessed on 4 July 2024). [CrossRef] [PubMed]
- Wang, A.W.; Bauer, S.; Goonatillake, M.; Breidahl, W.; Zheng, M.-H. Autologous tenocyte implantation, a novel treatment for partial-thickness rotator cuff tear and tendinopathy in an elite athlete. BMJ Case Rep. 2013, 2013, bcr2012007899. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Breidahl, W.; MacKie, K.; Zhen, L.; Qin, A.; Chen, J.; Zheng, M. Autologous tenocyte injection (ati) for treatment of severe chronic resistant lateral epicondylitis: A pilot study. Intern. Med. J. 2013, 43, 32. [Google Scholar]
- Wang, A.; Mackie, K.; Breidahl, W.; Wang, T.; Zheng, M.H. Evidence for the Durability of Autologous Tenocyte Injection for Treatment of Chronic Resistant Lateral Epicondylitis: Mean 4.5-Year Clinical Follow-up. Am. J. Sports Med. 2015, 43, 1775–1783. [Google Scholar] [CrossRef]
- Gilmor, R.; Remily, E.A.; Ingari, J.V. Management of Lateral Epicondylosis. J. Hand Surg. 2024, 49, 1124–1128. [Google Scholar] [CrossRef]
- Bahadir, B.; Sarikaya, B. Platelet-rich plasma in the management of rotator cuff tendinopathy. Jt. Dis. Relat. Surg. 2024, 35, 462–467. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Loppini, M.; Spiezia, F.; Denaro, V. New options in the management of tendinopathy. Open Access J. Sports Med. 2010, 1, 29–37. [Google Scholar] [CrossRef]
- Carr, J.B. Editorial Commentary: Platelet-Rich Plasma Shows Promise for Improving Shoulder Tendinopathy. Arthroscopy 2021, 37, 2754–2755. [Google Scholar] [CrossRef]
- Balasubramaniam, U.; Dissanayake, R.; Annabell, L. Efficacy of platelet-rich plasma injections in pain associated with chronic tendinopathy: A systematic review. Phys. Sportsmed. 2015, 43, 253–261. [Google Scholar] [CrossRef]
- Johannsen, F.; Olesen, J.L.; Øhlenschläger, T.F.; Lundgaard-Nielsen, M.; Cullum, C.K.; Jakobsen, A.S.; Rathleff, M.S.; Magnusson, P.S.; Kjær, M. Effect of Ultrasonography-Guided Corticosteroid Injection vs Placebo Added to Exercise Therapy for Achilles Tendinopathy. JAMA Netw. Open 2022, 5, e2219661. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.; Bennell, K.; Grimaldi, A.; Nicolson, P.; Kasza, J.; Hodges, P.; Wajswelner, H.; Vicenzino, B. Education plus exercise versus corticosteroid injection use versus a wait and see approach on global outcome and pain from gluteal tendinopathy: Prospective, single blinded, randomised clinical trial. BMJ 2018, 361, k1662. [Google Scholar] [CrossRef]
- Moosmayer, S.; Ekeberg, O.M.; Hallgren, H.B.; Heier, I.; Kvalheim, S.; Juel, N.G.; Blomquist, J.; Pripp, A.H.; Brox, J.I. Ultrasound guided lavage with corticosteroid injection versus sham lavage with and without corticosteroid injection for calcific tendinopathy of shoulder: Randomised double blinded multi-arm study. BMJ 2023, 383, e076447. [Google Scholar] [CrossRef]
- ACTRN12617000684325. Defining a Randomised, Controlled Study of Ortho-ATI (Trademark) vs. Corticosteroid Injection for Treatment of Rotator Cuff Tendinopathy and Tear. 2019. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12617000684325; (accessed on 29 April 2024).
- Kholinne, E.; Singjie, L.C.; Anastasia, M.; Liu, F.; Anestessia, I.J.; Kwak, J.-M.; Jeon, I.-H. Comparison of Clinical Outcomes After Different Surgical Approaches for Lateral Epicondylitis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2024, 12, 23259671241230292. [Google Scholar] [CrossRef] [PubMed]
- Burn, M.B.; Mitchell, R.J.; Liberman, S.R.; Lintner, D.M.; Harris, J.D.; McCulloch, P.C. Open, Arthroscopic, and Percutaneous Surgical Treatment of Lateral Epicondylitis: A Systematic Review. Hand 2018, 13, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Maffulli, N. Conservative management of tendinopathy: An evidence-based approach. Muscles Ligaments Tendons J. 2012, 1, 134–137. [Google Scholar] [PubMed]
- Čobec, J.; Kozinc, Ž. Conservative Treatments for Patellar Tendinopathy: A Review of Recent High-Quality Evidence. BioMed 2022, 2, 359–375. [Google Scholar] [CrossRef]
- Bruno, F.; Palumbo, P.; Arrigoni, F.; Mariani, S.; Aringhieri, G.; Carotti, M.; Natella, R.; Zappia, M.; Cipriani, P.; Giacomelli, R.; et al. Advanced diagnostic imaging and intervention in tendon diseases. Acta Bio Medica Atenei Parm. 2020, 91, 98–106. [Google Scholar] [CrossRef]
- Weinreb, J.H.; Sheth, C.; Apostolakos, J.; McCarthy, M.-B.; Barden, B.; Cote, M.P.; Mazzocca, A.D. Tendon structure, disease, and imaging. Muscles Ligaments Tendons J. 2014, 4, 66–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).