Cardiovascular Diseases, Vital Organ Fibrosis, and Chronic Inflammation Associated with High-Intensity and/or High-Volume Exercise Training: Double-Edged Sword Effects of Vigorous Physical Activity in Elderly People and/or in Middle-Age Cancer-Therapy-Treated Patients
Abstract
1. Introduction
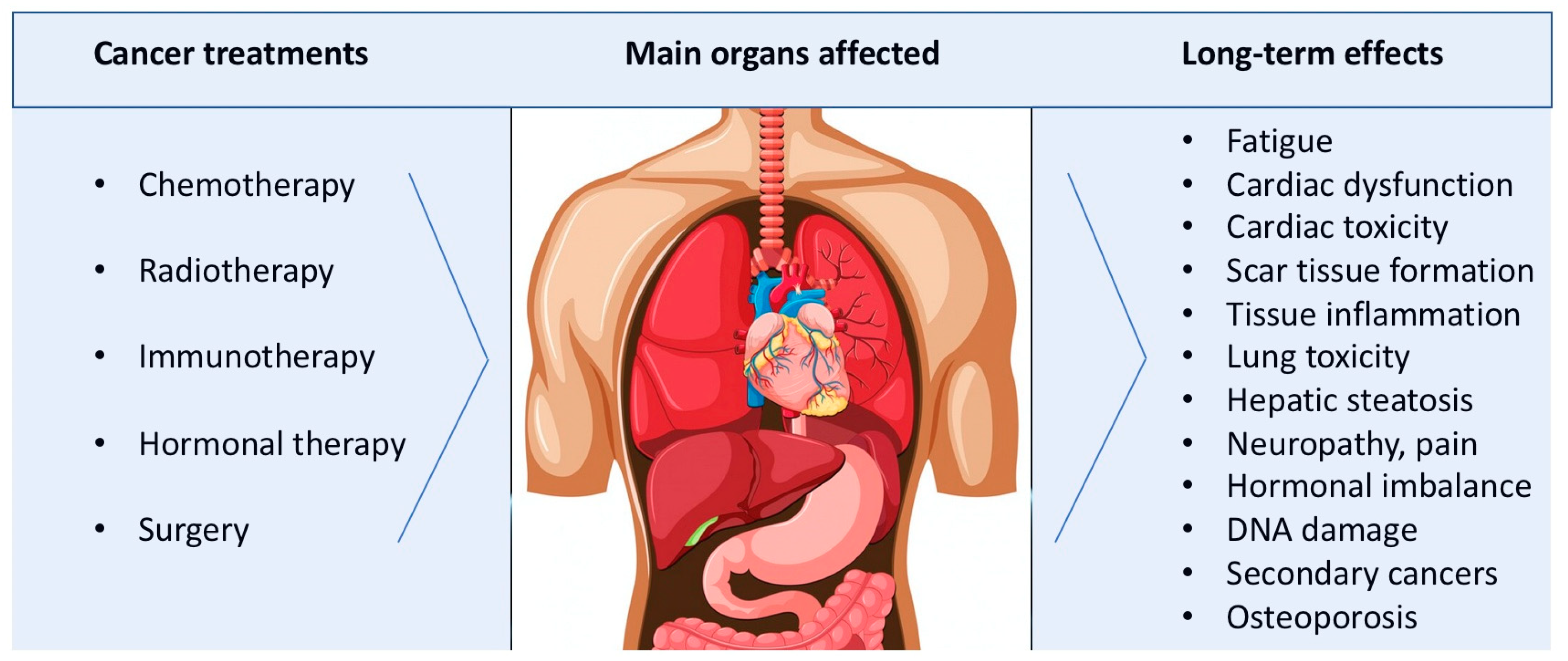
2. Long-Term Effects of Cancer Treatments
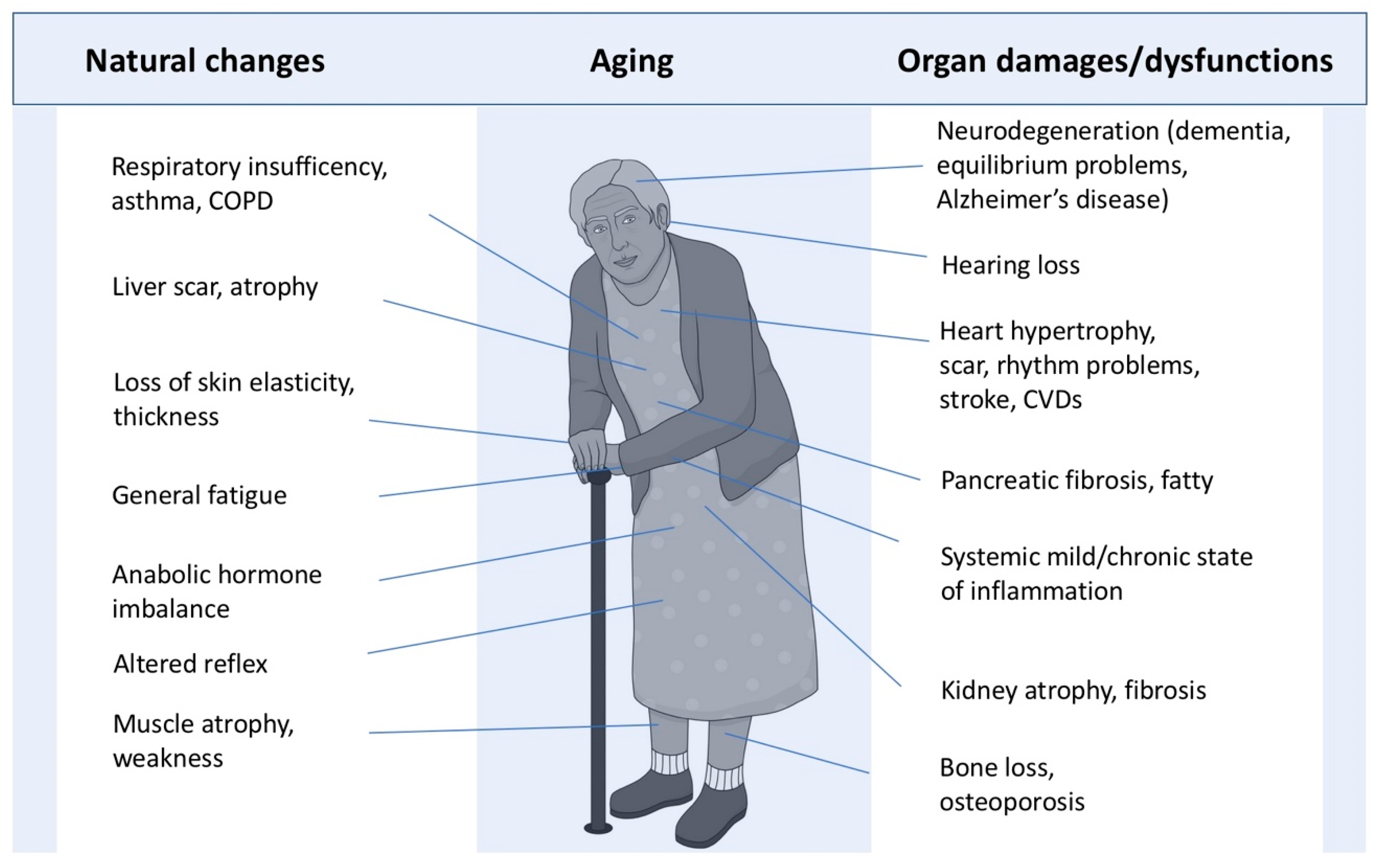
3. Natural Consequences of Aging on Systemic Functions and Vital Organs
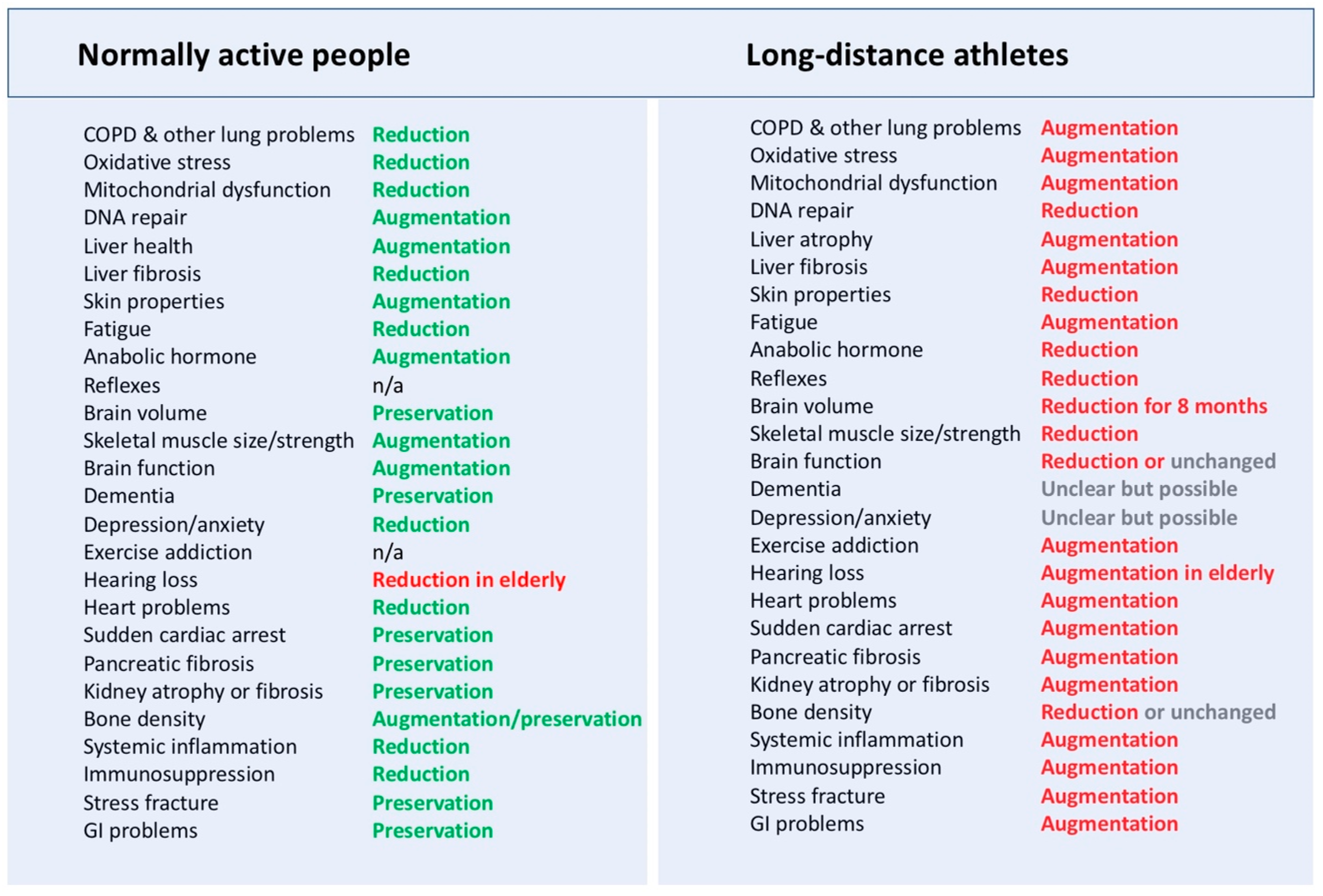
4. The Established General Benefits of Exercise Training on Health
5. Risks of Excessive Physical Activity on Organ Structures and Functions
6. Discussion
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Börzsei, D.; Hoffmann, A.; Török, S.; Veszelka, M.; Almási, N.; Varga, C.; Szabó, R. A comprehensive overview on chemotherapy-induced cardiotoxicity: Insights into the underlying inflammatory and oxidative mechanisms. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Dhamija, E.; Meena, P.; Ramalingam, V.; Sahoo, R.; Rastogi, S.; Thulkar, S. Chemotherapy-induced pulmonary complications in cancer: Significance of clinicoradiological correlation. Indian J. Radiol. Imaging 2020, 30, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Thrall, R.S.; Scalise, P.J. Bleomycin. In Pulmonary Fibrosis; Phan, S.H., Thrall, R.S., Eds.; Dekker: New York, NY, USA, 1995; pp. 231–292. [Google Scholar]
- Vahid, B.; Marik, P.E. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 2008, 133, 528–538. [Google Scholar] [CrossRef]
- Ramadori, G.; Cameron, S. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.; Jia, J.B.; Green, C.S.; Gulati, A.T.; Lall, C. Cancer therapy related complications in the liver, pancreas, and biliary system: An imaging perspective. Insights Imaging 2015, 6, 665–677. [Google Scholar] [CrossRef]
- Zorzi, D.; Laurent, A.; Pawlik, T.M.; Lauwers, G.Y.; Vauthey, J.N.; Abdalla, E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastates. Br. J. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef]
- Gebauer, J.; Higham, C.; Langer, T.; Denzer, C.; Brabant, G. Long-term endocrine and metabolic consequences of cancer treatment: A systematic review. Endocr. Rev. 2019, 40, 711–767. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, M.B.; Kuderer, N.M.; Desai, A.; Bergerot, C.; Lyman, G.H. Mitigating long-term and delayed adverse events associated with cancer treatment: Implications for survirship. Nat. Rev. Clin. Oncol. 2023, 20, 527–542. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2020, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Shahrokni, A.; Wu, A.J.; Carter, J.; Lichtman, S.M. Long term toxicity of cancer treatment in older patients. Clin. Geriatr. Med. 2015, 32, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Thong, M.S.; Doege, D.; Frick, J.; Arndt, V. Long-term organ toxicity of oncological therapies. Dtsch. Med. Wochenschr. 2025, 150, 29–36. [Google Scholar]
- United Nations. World Social Report 2023. Available online: https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2023/01/WSR_2023_Chapter_Key_Messages.pdf (accessed on 12 January 2025).
- Olivieri, F.; Biscetti, L.; Pimpini, L.; Pelliccioni, G.; Sabbatinelli, J.; Giunta, S. Heart rate variability and autonomic nervous system imbalance: Potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Res. Rev. 2024, 101, 102521. [Google Scholar] [CrossRef] [PubMed]
- Opris, C.E.; Suciu, H.; Opris, C.I.; Gurzu, S. An Update on Mitral Valve Aging. Life 2024, 14, 950. [Google Scholar] [CrossRef] [PubMed]
- Bienrnacka, A.; Frangogiannis, N. Aging and cardiac fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar]
- Vakka, A.; Warren, J.S.; Drosatos, K. Cardiovascular aging: From cellular and molecular changes to therapeutic interventions. J. Cardiovasc. Aging 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.M.; Hassan, N.H.; Samy, W.; Abdelhadi, A.A.; Saadawy, S.F.; Elsayed, S.F.; Seada, S.G.; Mohamed, S.R.A. Unraveling the hepatic stellate cells mediated mechanisms in aging’s influence on liver fibrosis. Sci. Rep. 2024, 14, 13473. [Google Scholar] [CrossRef]
- Dietrich, C.; Möller, K.; Jenssen, C.; Braden, B.; Hocke, M.; Hollerbach, S.; Ignee, A.; Faiss, S.; Iglesias-Garcia, J.; Sun, S.; et al. Pancreatic changes with lifestyle and age: What is normal and what is concerning. Endosc. Ultrasound 2023, 12, 213–227. [Google Scholar]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, E.M.; Fernandez-Guerra, P.; Nørgård, M.; Jeromdesella, S.; Kjær, P.K.; Elkjær, A.S.; Kassem, M.; Figeac, F. Senescence of skeletal stem cells and their contribution to age-related bone loss. Mech. Ageing Dev. 2024, 221, 111976. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, L.; Liu, Y. Cellular senescence in kidney fibrosis: Pathologic significance and therapeutic strategies. Front. Pharmacol. 2020, 11, 601325. [Google Scholar] [CrossRef]
- Preston, J.; Biddell, B. The physiology of ageing and how these changes affect older people. Medicine 2021, 49, 1–5. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [PubMed]
- Rohnejad, B.; Monazzami, A. Effects of high-intensity intermittent training on some inflammatory and muscle damage indices in overweight middle-aged men. Apunt. Sports Med. 2023, 58, 100404. [Google Scholar] [CrossRef]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential long-term health problems associated with ultra-endurance running: A narrative review. Sports Med. 2022, 52, 725–740. [Google Scholar] [CrossRef]
- Maron, B.J.; Shirani, J.; Poliac, L.C.; Mathenge, R.; Roberts, W.C.; Mueller, F.O. Sudden death in young competitive athletes: Clinical, demographic, and pathological profiles. JAMA 1996, 276, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Klingert, M.; Nikolaidis, P.T.; Weiss, K.; Thuany, M.; Chlíbková, D.; Knechtle, B. Exercise-associated hyponatremia in marathon runners. J. Clin. Med. 2022, 11, 6775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Malhotra, R.; Chiampas, G.; D’Hemecourt, P.; Troyanos, C.; Cianca, J.; Smith, R.N.; Wang, T.J.; Roberts, W.O.; Thompson, P.D.; et al. Cardiac arrest during long-distance running races. N. Engl. J. Med. 2012, 366, 130–140. [Google Scholar] [CrossRef]
- Ghio, F.E.; Pieri, M.; Agracheva, A.; Melisurgo, G.; Ponti, A.; Serini, C. Sudden cardiac arrest in a marathon runner. A Case Report. HSR Proc. Intensive Care Cardiovasc. Anesth. 2012, 4, 130–132. [Google Scholar]
- Webner, D.; DuPrey, K.M.; Drezner, J.A.; Cronholm, P.; Roberts, W.O. Suddent cardiac arrest and death in United States marathons. Med. Sci. Sports Exerc. 2012, 44, 1843–1845. [Google Scholar] [CrossRef]
- Del Coso, J.; Salinera, J.J.; Lara, B.; Abian-Vicen, J.; Gallo-Salazar, C.; Areces, F. A comparison of the physiological demands imposed by competing in a half-marathon vs. a marathon. J. Sports Med. Phys. Fit. 2017, 57, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J.; Stec, J.J.; Lipinska, I.; Van Cott, E.M.; Lewandrowski, K.B.; Ridker, P.M.; Tofler, G.H. Effects of marathon running on inflammatory and hemostatic markers. Am. J. Cardiol. 2001, 88, 918–920. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012, 87, 587–595. [Google Scholar] [CrossRef]
- Coylewright, M. Extreme Long-Distance Running Has Questionable Impact on Heart; American College of Cardiology News; American College of Cardiology: Washington, DC, USA, 2017. [Google Scholar]
- Jafar, O.; Friedman, J.; Bogdanowicz, I.; Muneer, A.; Thompson, P.D.; Ling, J.; Messina, A.; Yen, M.; Wakefield, D.; Varanasi, P.; et al. Assessment of coronary atherosclerosis using calcium scores in short- and long-distance runners. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tshopp, M.; Brunner, F. Diseases and overuse injuries of the lower extremities in long distance runners. Z. Rheumatol. 2017, 76, 443–450. [Google Scholar]
- Tiller, N.B.; Elliott-Sale, K.J.; Knechtle, B.; Wilson, P.B.; Roberts, J.D.; Millet, G.Y. Do sex differences in physiology confer a female advantage in ultra-endurance sport? Sports Med. 2021, 51, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Nieman, D.C.; Bermon, S.; Peeling, P. Exercise-induced illness and inflammation: Can immunonutrition and iron help. Int. Sport Nutr. Exerc. Metab. 2019, 29, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.; Bermon, S. Intensive triathtlon training induces low peripheral CD34+ stem cells. Br. J. Haematol. 2003, 120, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Knöpfli, B.H.; Luke-Zeitoun, M.; von Duvillard, S.P.; Burki, A.; Bachlechner, C.; Keller, H.; Bacharach, D.W. High incidence of exercise-induced bronchoconstriction in triathletes of the Swiss National Team. Br. J. Sports Med. 2007, 41, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V. Severe kidney injury after a 110-km trail race. Cureus 2020, 12, e7814. [Google Scholar] [CrossRef] [PubMed]
- Stuempfle, K.J.; Hoffman, M.D. GI distress is common during a 161-km ultramarathon. J. Sports Sci. 2015, 33, 1814–1821. [Google Scholar] [CrossRef]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Al Thani, A. Impact of physical exercise on gut microbiome, inflammation, and the pathobiology of metabolic disorders. Rev. Diabet. Stud. 2019, 15, 35–48. [Google Scholar] [CrossRef]
- Shin, Y.O.; Lee, J.B. Leukocyte chemotactic cytokine and leukocyte subset responses during ultra-marathon running. Cytokine 2013, 61, 364–369. [Google Scholar] [CrossRef]
- Kupchak, B.R.; Kraemer, W.J.; Hoffman, M.D.; Phinney, S.D.; Volek, J.S. The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness Environ. Med. 2014, 25, 278–288. [Google Scholar] [CrossRef]
- Kakanis, M.W.; Peake, J.; Brenu, E.W.; Simmonds, M.; Gray, B.; Hooper, S.L.; Marshall-Gradisnik, S.M. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. J. Sci. Med. Sport 2010, 13, e85–e86. [Google Scholar] [CrossRef]
- Freund, W.; Faust, S.; Birklein, F.; Gaser, C.; Wunderlich, A.P.; Müller, M.; Billich, C.; Juchems, M.S.; Schmitz, B.L.; Grön, G.; et al. Substantial and reversible brain gray matter reduction but no acute brain lesions in ultramarathon runners. BMC Med. 2012, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Krouse, R. Ultra-obligatory running among ultramarathon runners. Res. Sports Med. 2018, 26, 211. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Duffy, K.A.; Page, C.E.; Novick, A.M. Cognitive problems in perimenopause: A review of recent evidence. Curr. Psychiatry Rep. 2023, 25, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Thuany, M.; Gomes, T.N.; Villiger, E.; Weiss, K.; Scheer, V.; Nikolaidis, P.T.; Knechtle, B. Trends in participation, sex differences and age of peak performance in time-limited ultramarathon events: A secular analysis. Medicina 2022, 58, 366. [Google Scholar] [CrossRef]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systemic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- De la Corte-Rodriguez, H.; Roman-Belmonte, J.M.; Resino-Luis, C.; Madrid-Gonzalez, J.; Rodriguez-Merchan, E.C. The role of physical exercise in chronic musculoskeletal pain: Best medicine- a narrative review. Healthcare 2024, 12, 242. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Ji, X.; Chen, X.; Li, R.; Zhang, S.; Meng, Y.; Nieman, D.C.; Chen, P. The anti-inflammatory effects of exercise on autoimmune diseases: A 20-year systematic review. J. Sport Health Sci. 2024, 13, 353–367. [Google Scholar] [PubMed]
- Alvarez-Herms, J.; Odriozola, A. Microbiome and physical activity. Adv. Genet. 2024, 111, 409–450. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guertin, P.A. Cardiovascular Diseases, Vital Organ Fibrosis, and Chronic Inflammation Associated with High-Intensity and/or High-Volume Exercise Training: Double-Edged Sword Effects of Vigorous Physical Activity in Elderly People and/or in Middle-Age Cancer-Therapy-Treated Patients. J. Funct. Morphol. Kinesiol. 2025, 10, 33. https://doi.org/10.3390/jfmk10010033
Guertin PA. Cardiovascular Diseases, Vital Organ Fibrosis, and Chronic Inflammation Associated with High-Intensity and/or High-Volume Exercise Training: Double-Edged Sword Effects of Vigorous Physical Activity in Elderly People and/or in Middle-Age Cancer-Therapy-Treated Patients. Journal of Functional Morphology and Kinesiology. 2025; 10(1):33. https://doi.org/10.3390/jfmk10010033
Chicago/Turabian StyleGuertin, Pierre A. 2025. "Cardiovascular Diseases, Vital Organ Fibrosis, and Chronic Inflammation Associated with High-Intensity and/or High-Volume Exercise Training: Double-Edged Sword Effects of Vigorous Physical Activity in Elderly People and/or in Middle-Age Cancer-Therapy-Treated Patients" Journal of Functional Morphology and Kinesiology 10, no. 1: 33. https://doi.org/10.3390/jfmk10010033
APA StyleGuertin, P. A. (2025). Cardiovascular Diseases, Vital Organ Fibrosis, and Chronic Inflammation Associated with High-Intensity and/or High-Volume Exercise Training: Double-Edged Sword Effects of Vigorous Physical Activity in Elderly People and/or in Middle-Age Cancer-Therapy-Treated Patients. Journal of Functional Morphology and Kinesiology, 10(1), 33. https://doi.org/10.3390/jfmk10010033





