Mesenchymal Stem Cells Improve Muscle Function Following Single Stretch Injury: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation and Experimental Apparatus
2.2. Experimental Injury Protocol
2.3. Mesenchymal Stem Cell Culture
2.4. Histology and Immunohistochemistry
2.5. Isometric Peak Torque
2.6. Eccentric Contraction Testing
2.7. Statistical Analyses
3. Results
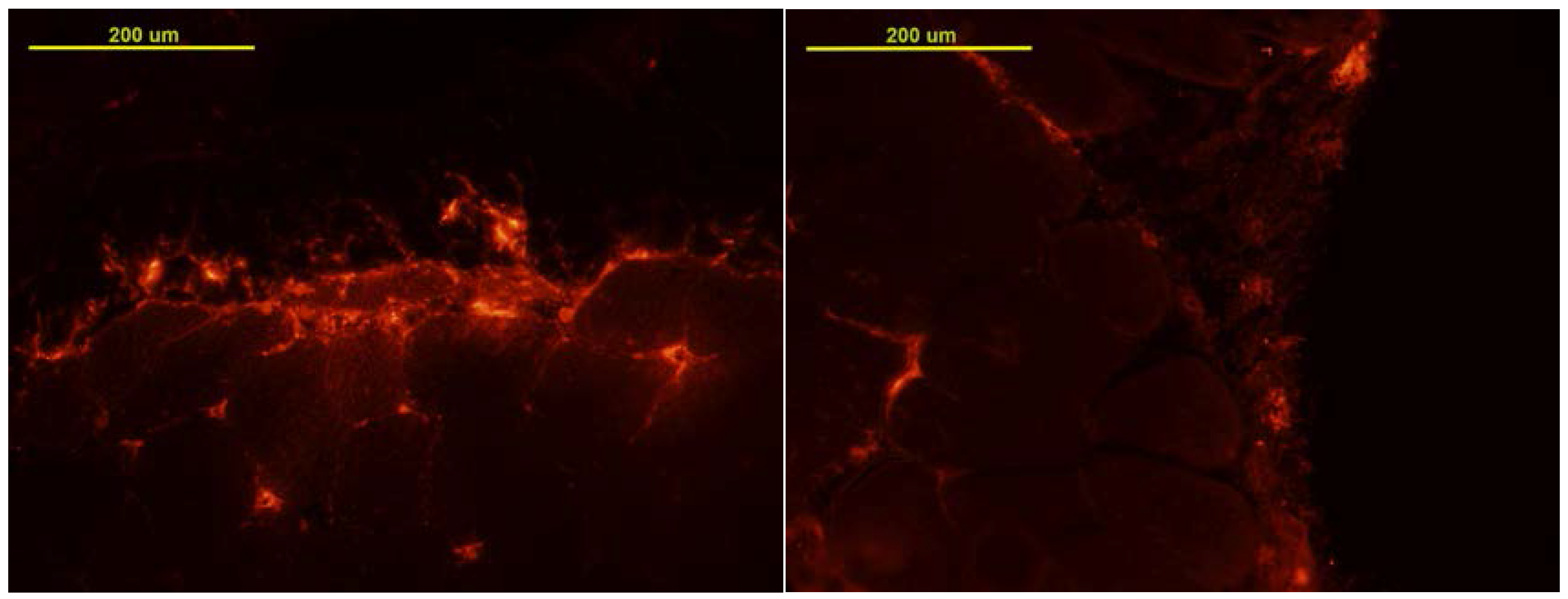
3.1. MSC Localization
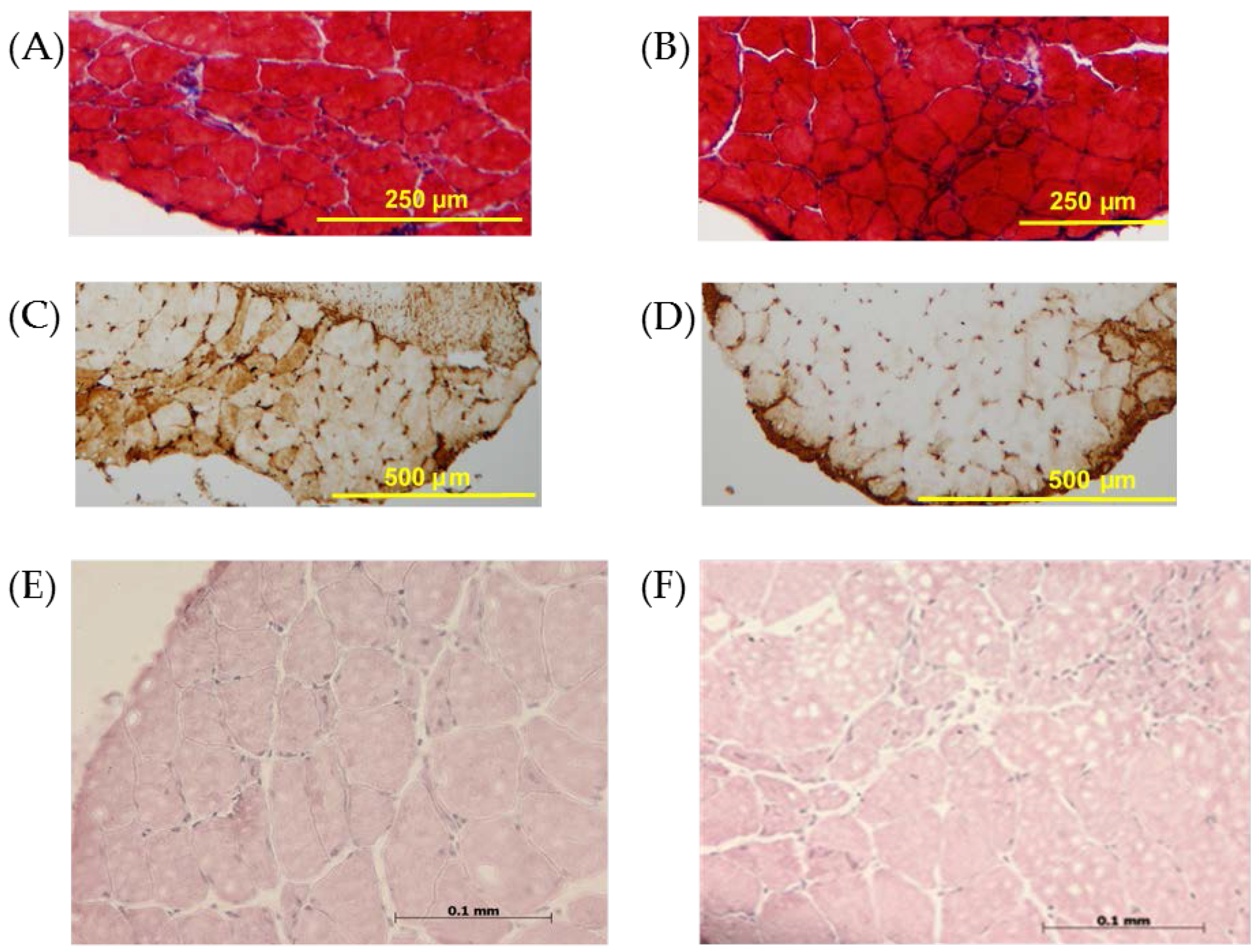
3.2. Histology and Immunohistochemistry
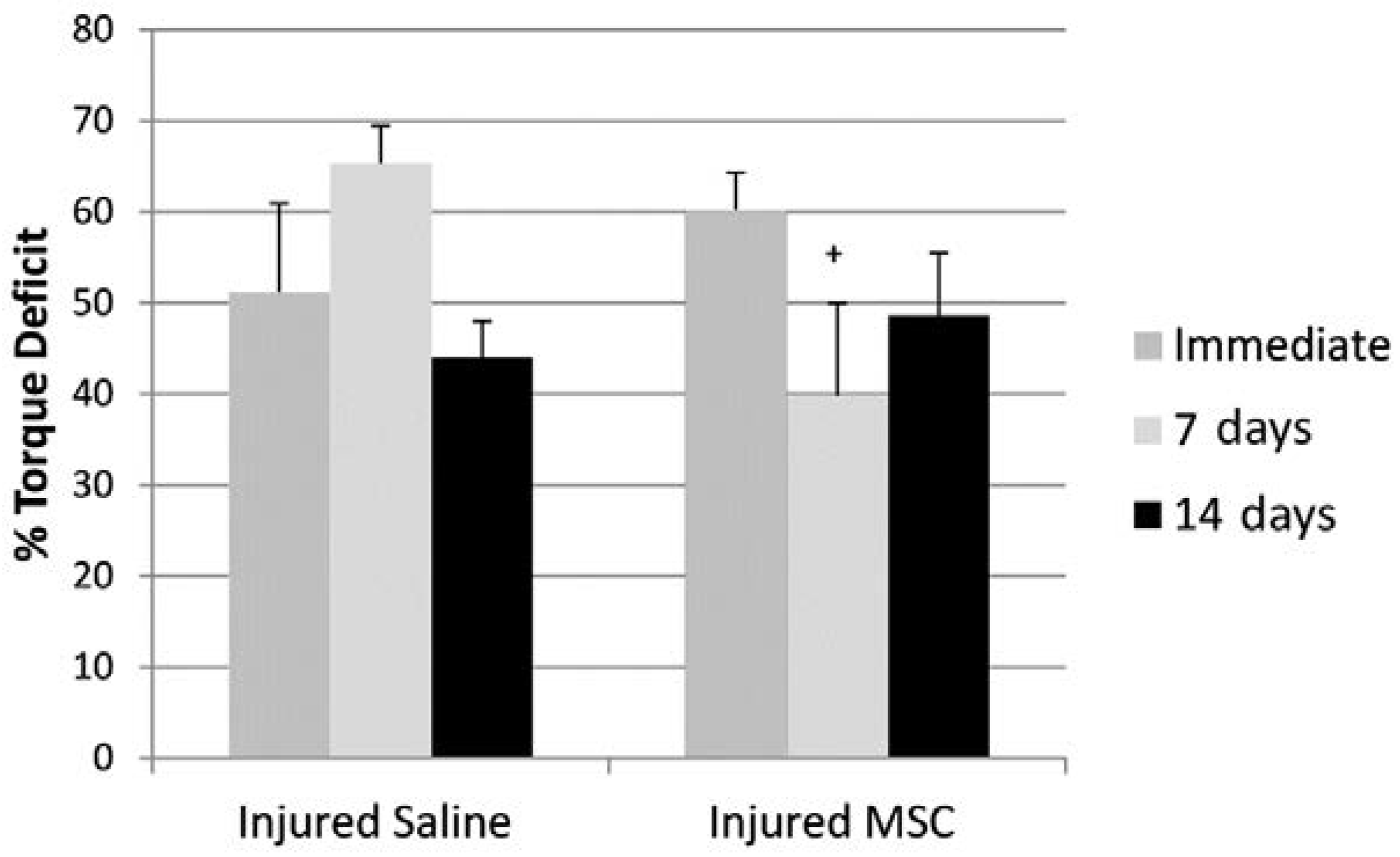
3.3. Isometric Peak Torque
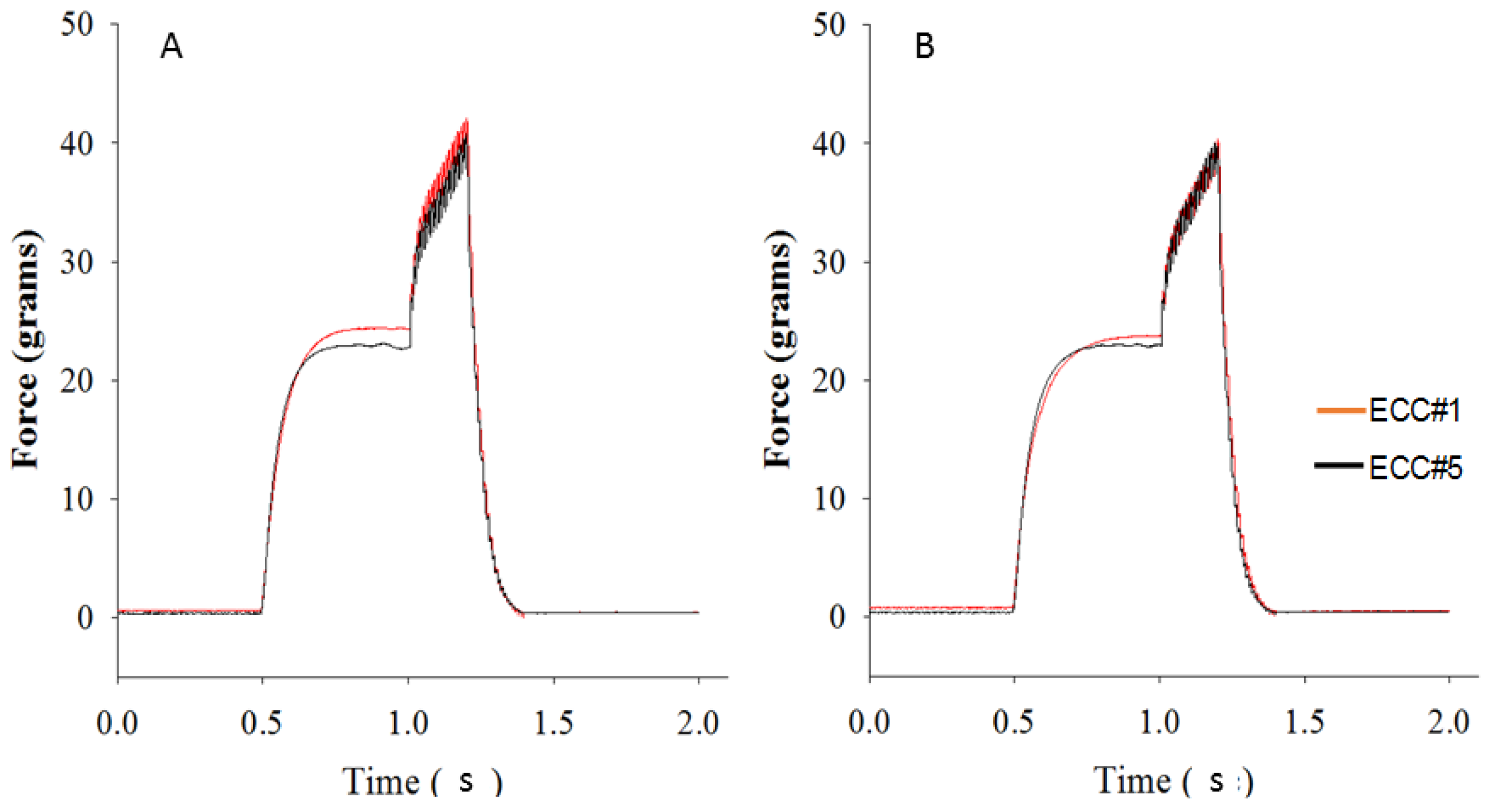
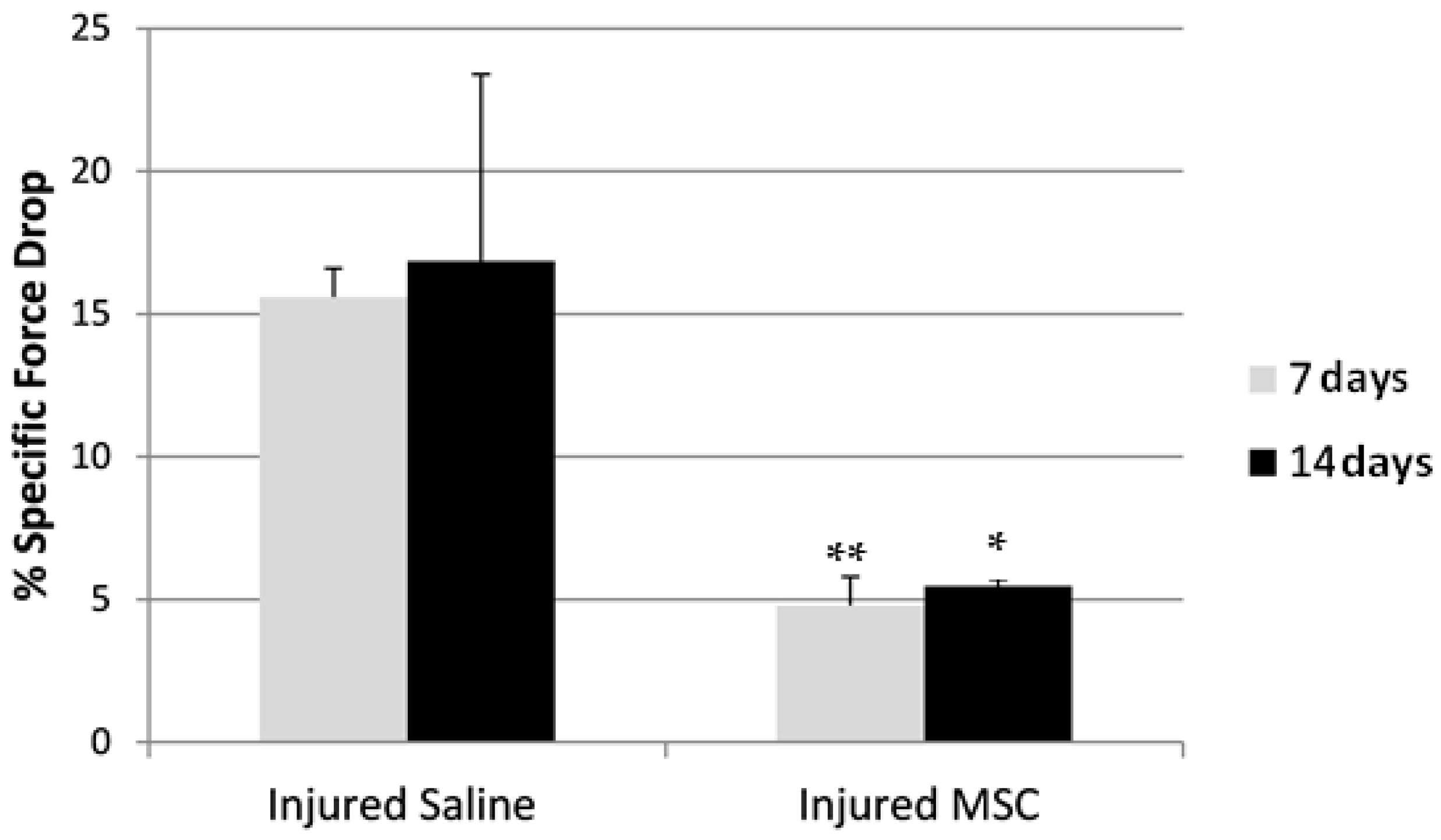
3.4. Eccentric Contraction Testing
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garrett, W.E., Jr. Muscle strain injuries. Am. J. Sports Med. 1996, 24, S2–S8. [Google Scholar] [PubMed]
- Brooks, J.H.; Fuller, C.W.; Kemp, S.P.; Reddin, D.B. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am. J. Sports Med. 2006, 34, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Joint Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Hurme, T.; Kalimo, H. Activation of myogenic precursor cells after muscle injury. Med. Sci. Sports Exerc. 1992, 24, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huard, J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am. J. Pathol. 2002, 161, 895–907. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Friden, J.; Schmitz, M.C.; Lieber, R.L. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J. Bone Joint Surg. Am. 1995, 77, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Almekinders, L.C. Anti-inflammatory treatment of muscular injuries in sport. An update of recent studies. Sports Med. 1999, 28, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, Y.; Tang, Y.; Cummins, J.; Huard, J. Ns-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am. J. Pathol. 2005, 167, 1105–1117. [Google Scholar] [CrossRef]
- Irintchev, A.; Langer, M.; Zweyer, M.; Theisen, R.; Wernig, A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J. Physiol. 1997, 500, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, R.J.; Deasy, B.M.; Huard, J. Muscle-derived stem cells. Gene Ther. 2002, 9, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Uehara, K.; Nozaki, M.; Kobayashi, T.; Terada, S.; Tobita, K.; Fu, F.H.; Huard, J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am. J. Sports Med. 2011, 39, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Matziolis, G.; Winkler, T.; Schaser, K.; Wiemann, M.; Krocker, D.; Tuischer, J.; Perka, C.; Duda, G.N. Autologous bone marrow-derived cells enhance muscle strength following skeletal muscle crush injury in rats. Tissue Eng. 2006, 12, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Matziolis, G.; Mehta, M.; Perka, C.; Duda, G.N. Dose-response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng. Part A 2009, 15, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Rathbone, C.R. Accelerated functional recovery after skeletal muscle ischemia-reperfusion injury using freshly isolated bone marrow cells. J. Surgical Res. 2014, 188, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Cir. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ishikawa, M.; Kamei, N.; Nakasa, T.; Adachi, N.; Deie, M.; Asahara, T.; Ochi, M. Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived CD133-positive cells. Stem Cells 2009, 27, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Brickson, S.L.; McCabe, R.P.; Pala, A.W.; Vanderby, R., Jr. A model for creating a single stretch injury in murine biarticular muscle. BMC Sports Sci. Med. Rehabil. 2014, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Sonnemann, K.J.; Fitzsimons, D.P.; Patel, J.R.; Liu, Y.; Schneider, M.F.; Moss, R.L.; Ervasti, J.M. Cytoplasmic γ-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev. Cell 2006, 11, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Ota, S.; Kobayashi, M.; Kobayashi, T.; Mifune, Y.; Takayama, K.; Witt, M.; Vadala, G.; Oyster, N.; Otsuka, T.; et al. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J. Bone Joint Surg. Am. 2013, 95, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Li, Y.; Zhu, J.; Ambrosio, F.; Uehara, K.; Fu, F.H.; Huard, J. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am. J. Sports Med. 2008, 36, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.A.; Shaheen, N.E.; Abu Zahra, F.A. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol. Immunotoxicol. 2016, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ota, S.; Terada, S.; Kawakami, Y.; Otsuka, T.; Fu, F.H.; Huard, J. The combined use of losartan and muscle-derived stem cells significantly improves the functional recovery of muscle in a young mouse model of contusion injuries. Am. J. Sports Med. 2016, 44, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.A.; Jarvinen, M.; Kalimo, H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2013, 3, 337–345. [Google Scholar] [PubMed]
- Winkler, T.; von Roth, P.; Radojewski, P.; Urbanski, A.; Hahn, S.; Preininger, B.; Duda, G.N.; Perka, C. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J. Tissue Eng. Regen. Med. 2012, 6, s60–s67. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.A.; Mathew, L.; Ryan, A.F.; Chen, J.; Lieber, R.L. Rapid muscle—Specific gene expression changes after a single bout of eccentric contractions in the mouse. Am. J. Physiol. Cell Physiol. 2004, 286, C355–C364. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Summan, M.; Gao, X.; Chapman, R.; Hulderman, T.; Simeonova, P.P. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J. Physiol. 2007, 582, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Gjata, B.; Lafont, H.; Sebille, A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-β 1. J. Neuroimmunol. 1996, 70, 37–44. [Google Scholar] [CrossRef]
- Heiderscheit, B.C.; Sherry, M.A.; Silder, A.; Chumanov, E.S.; Thelen, D.G. Hamstring strain injuries: Recommendations for diagnosis, rehabilitation, and injury prevention. J. Orthop. Sports Phys. Ther. 2010, 40, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Warren, G.L.; Ingalls, C.P.; Boorstein, D.B.; Armstrong, R.B. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J. Appl. Physiol. 1995, 79, 1260–1270. [Google Scholar] [PubMed]
- Warren, G.L.; Ingalls, C.P.; Shah, S.J.; Armstrong, R.B. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J. Physiol. 1999, 515, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J.P.; Lovering, R.M. A stepwise procedure to test contractility and susceptibility to injury for the rodent quadriceps muscle. J. Boil. Methods 2014, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Schmitz, M.C.; Mishra, D.K.; Friden, J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J. Appl. Physiol. 1994, 77, 1926–1934. [Google Scholar] [PubMed]
- McCully, K.K.; Faulkner, J.A. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J. Appl. Physiol. 1986, 61, 293–299. [Google Scholar] [PubMed]
- Almekinders, L.C.; Gilbert, J.A. Healing of experimental muscle strains and the effects of nonsteroidal inflammatory medicine. Am. J. Sports Med. 1986, 14, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Aarimaa, V.; Vaittinen, S.; Kalimo, H.; Jarvinen, M. Muscle injuries: Optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Shansky, J.; Wang, Z.; Mooney, D.; Vandenburgh, H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol. Ther. 2014, 22, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Willett, N.J.; Uhrig, B.A.; Guldberg, R.E.; Warren, G.L. Functional analysis of limb recovery following autograft treatment of volumetric muscle loss in the quadriceps femoris. J. Biomech. 2014, 47, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Sam, M.; Shah, S.; Friden, J.; Milner, D.J.; Capetanaki, Y.; Lieber, R.L. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am. J. Physiol. Cell Physiol. 2000, 279, C1116–C1122. [Google Scholar] [PubMed]
- Mair, S.D.; Seaber, A.V.; Glisson, R.R.; Garrett, W.E., Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am. J. Sports Med. 1996, 24, 137–143. [Google Scholar] [CrossRef] [PubMed]
- De Smet, A.A.; Best, T.M. MR imaging of the distribution and location of acute hamstring injuries in athletes. Am. J. Roentgenol. 2000, 174, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Friden, J.; Lieber, R.L. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998, 293, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Friden, J. Selective damage of fast glycolytic muscle fibers with eccentric contraction of the rabbit tibialis anterior. Acta. Physiol. Scand. 1988, 133, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Appell, H.J.; Soares, J.M.; Duarte, J.A. Exercise, muscle damage and fatigue. Sports Med. 1992, 13, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.L.; Morgan, D.L.; Gregory, J.E.; Proske, U. Damage to different motor units from active lengthening of the medial gastrocnemius muscle of the cat. J. Appl. Physiol. 2002, 92, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Denies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef] [PubMed]
- Best, T.M.; McCabe, R.P.; Corr, D.T.; Vanderby, R. Evaluation of a new method to create a standardized muscle stretch injury. Med. Sci. Sports Sci. 1998, 30, 200–205. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brickson, S.; Meyer, P.; Saether, E.; Vanderby, R. Mesenchymal Stem Cells Improve Muscle Function Following Single Stretch Injury: A Preliminary Study. J. Funct. Morphol. Kinesiol. 2016, 1, 396-406. https://doi.org/10.3390/jfmk1040396
Brickson S, Meyer P, Saether E, Vanderby R. Mesenchymal Stem Cells Improve Muscle Function Following Single Stretch Injury: A Preliminary Study. Journal of Functional Morphology and Kinesiology. 2016; 1(4):396-406. https://doi.org/10.3390/jfmk1040396
Chicago/Turabian StyleBrickson, Stacey, Patrick Meyer, Erin Saether, and Ray Vanderby. 2016. "Mesenchymal Stem Cells Improve Muscle Function Following Single Stretch Injury: A Preliminary Study" Journal of Functional Morphology and Kinesiology 1, no. 4: 396-406. https://doi.org/10.3390/jfmk1040396
APA StyleBrickson, S., Meyer, P., Saether, E., & Vanderby, R. (2016). Mesenchymal Stem Cells Improve Muscle Function Following Single Stretch Injury: A Preliminary Study. Journal of Functional Morphology and Kinesiology, 1(4), 396-406. https://doi.org/10.3390/jfmk1040396




