Myoelectric Manifestations of Fatigue after ACL Reconstruction: A Cross-Sectional Study after Postoperative Rehabilitation
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
- Group CG: 19 subjects with no previous history of knee injury or painful conditions of the lower limb acted as the control group;
- Group R12: 10 patients, up to 12 months after ACLr (10 ± 4 months);
- Group R24+: 23 patients, more than 24 months after ACLr (59 ± 38 months).
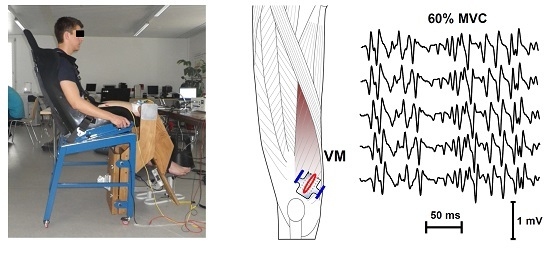
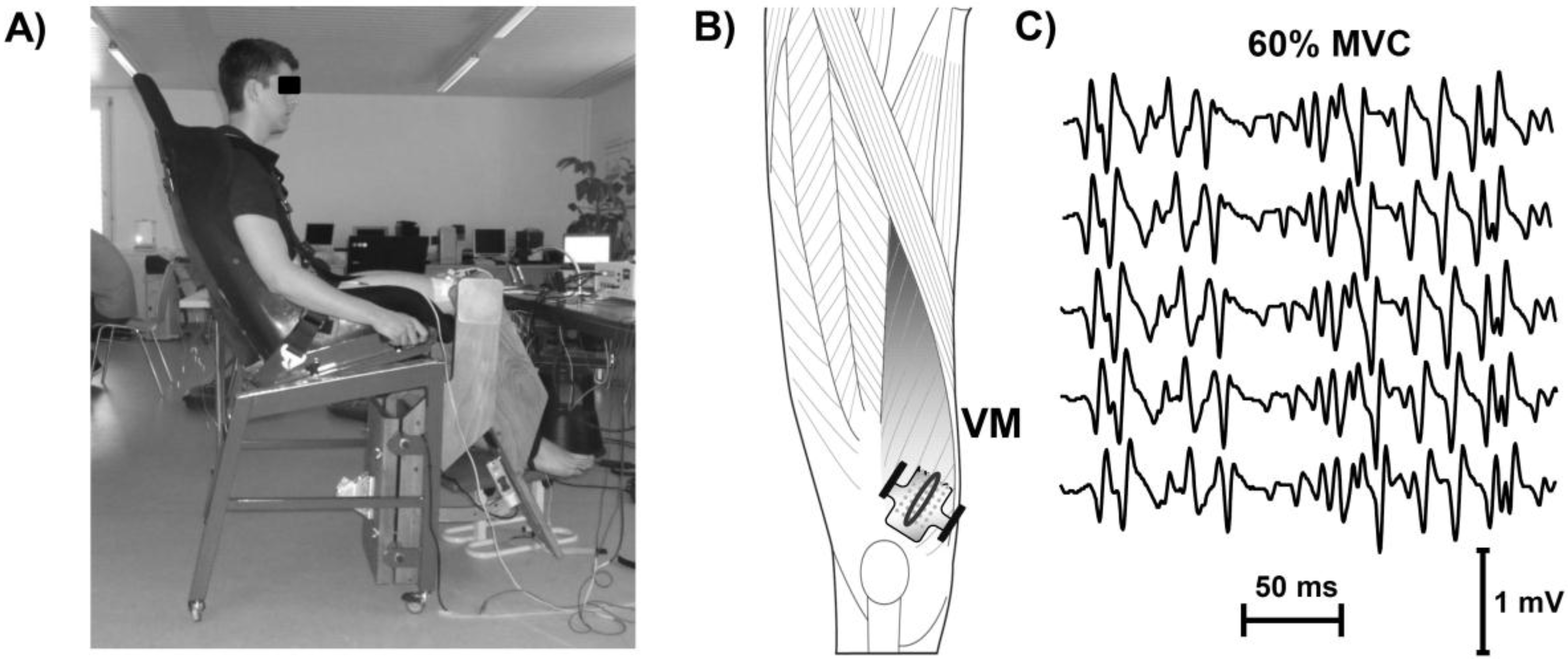
2.2. Experimental Set-Up
2.3. EMG Measurement and Signal Processing
2.4. Statistical Analysis
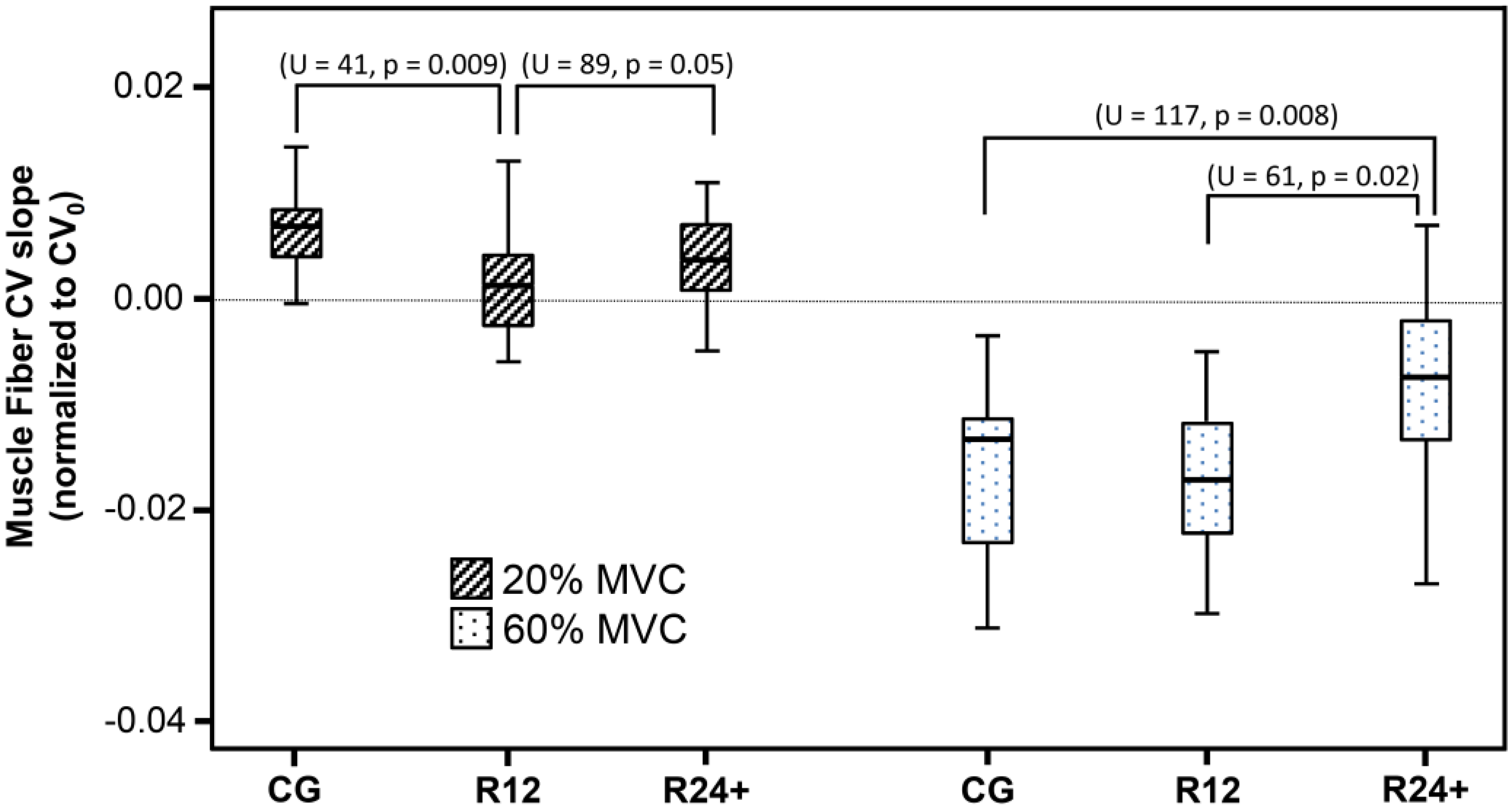
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACLr | anterior cruciate ligament reconstruction |
| CV | conduction velocity |
| MU | motor unit |
| EMG | electromyography |
| MVC | maximal voluntary contraction |
| CG | control group |
| R12 | patients that were up to 12 months after ACLr |
| R24+ | patients that were more than 24 months after ACLr |
References
- Snyder-Mackler, L.; de Luca, P.F.; Williams, P.R.; Eastlack, M.E.; Bartolozzi, A.R., 3rd. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg. Am. 1994, 76, 555–560. [Google Scholar]
- Williams, G.N.; Buchanan, T.S.; Barrance, P.J.; Axe, M.J.; Snyder-Mackler, L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am. J. Sports Med. 2005, 33, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Wojtys, E.M.; Brandon, C.; Palmieri-Smith, R.M. Muscle atrophy contributes to quadriceps weakness after ACL reconstruction. J. Sci. Med. Sport 2016, 19, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.S.; Gilsdorf, C.M.; Goerger, B.M.; Prentice, W.E.; Padua, D.A. Neuromuscular fatigue alters postural control and sagittal plane hip biomechanics in active females with anterior cruciate ligament reconstruction. Sports Health 2014, 6, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Santamaria, L.J.; McClelland, J.A.; Feller, J.A. Effect of fatigue on landing biomechanics after anterior cruciate ligament reconstruction surgery. Med. Sci. Sports Exerc. 2012, 44, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin. Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Lessi, G.C.; Serrao, F.V. Effects of fatigue on lower limb, pelvis and trunk kinematics and lower limb muscle activity during single-leg landing after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.J.; Torry, M.R.; Noonan, T.J.; Riviere, A.; Sterett, W.I. Landing adaptations after ACL reconstruction. Med. Sci. Sports Exerc. 2002, 34, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Hof, A.L.; Arnold, M.P.; Dijkstra, P.U.; Postema, K.; Otten, E. Abnormal landing strategies after ACL reconstruction. Scand. J. Med. Sci. Sports 2010, 20, e12–e19. [Google Scholar] [CrossRef] [PubMed]
- Hantes, M.E.; Tsarouhas, A.; Giakas, G.; Spiropoulos, G.; Sideris, V.; Christel, P.; Malizos, K.N. Effect of fatigue on tibial rotation after single- and double-bundle anterior cruciate ligament reconstruction: A 3-dimensional kinematic and kinetic matched-group analysis. Am. J. Sports Med. 2012, 40, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Paterno, M.V.; Schmitt, L.C.; Ford, K.R.; Rauh, M.J.; Myer, G.D.; Hewett, T.E. Effects of sex on compensatory landing strategies upon return to sport after anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2011, 41, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [PubMed]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Noninvasive Applications; IEEE Press: Piscataway, NJ, USA, 2004; p. 494S. [Google Scholar]
- Buchthal, F.; Guld, C.; Rosenfalck, P. Innervation zone and propagation velocity in human muscle. Acta Physiol. Scand. 1955, 35, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Bouissou, P.; Estrade, P.Y.; Goubel, F.; Guezennec, C.Y.; Serrurier, B. Surface EMG power spectrum and intramuscular pH in human vastus lateralis muscle during dynamic exercise. J. Appl. Physiol. 1989, 67, 1245–1249. [Google Scholar] [PubMed]
- Brody, L.R.; Pollock, M.T.; Roy, S.H.; de Luca, C.J.; Celli, B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 1991, 71, 1878–1885. [Google Scholar] [PubMed]
- Gonzalez-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, M.; Goulet, C.; Nadeau, S.; Arsenault, A.B.; Gravel, D. Comparison of the EMG power spectrum of the human soleus and gastrocnemius muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kollmitzer, J.; Ebenbichler, G.R.; Kopf, A. Reliability of surface electromyographic measurements. Clin. Neurophysiol. 1999, 110, 725–734. [Google Scholar] [CrossRef]
- Linssen, W.H.; Stegeman, D.F.; Joosten, E.M.; van’t Hof, M.A.; Binkhorst, R.A.; Notermans, S.L. Variability and interrelationships of surface EMG parameters during local muscle fatigue. Muscle Nerve 1993, 16, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sadoyama, T.; Masuda, T.; Miyata, H.; Katsuta, S. Fibre conduction velocity and fibre composition in human vastus lateralis. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Kupa, E.J.; Roy, S.H.; Kandarian, S.C.; de Luca, C.J. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J. Appl. Physiol. 1995, 79, 23–32. [Google Scholar] [PubMed]
- Maffiuletti, N.A.; Barbero, M.; Cescon, C.; Clijsen, R.; Beretta-Piccoli, M.; Schneebeli, A.; Preiss, S.; Togninalli, D. Validity of the twitch interpolation technique for the assessment of quadriceps neuromuscular asymmetries. J. Electromyogr. Kinesiol. 2016, 28, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Lepley, L.K.; Wojtys, E.M.; McLean, S.G.; Palmieri-Smith, R.M. Effects of neuromuscular fatigue on quadriceps strength and activation and knee biomechanics in individuals post-anterior cruciate ligament reconstruction and healthy adults. J. Orthop. Sports Phys. Ther. 2015, 45, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, M.; D’Antona, G.; Barbero, M.; Fisher, B.; Dieli-Conwright, C.M.; Clijsen, R.; Cescon, C. Evaluation of central and peripheral fatigue in the quadriceps using fractal dimension and conduction velocity in young females. PLoS ONE 2015, 10, e0123921. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, M.; Rainoldi, A.; Heitz, C.; Wuthrich, M.; Boccia, G.; Tomasoni, E.; Spirolazzi, C.; Egloff, M.; Barbero, M. Innervation zone locations in 43 superficial muscles: Toward a standardization of electrode positioning. Muscle Nerve 2014, 49, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R. A novel approach for estimating muscle fiber conduction velocity by spatial and temporal filtering of surface EMG signals. IEEE Trans. Biomed. Eng. 2003, 50, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- McNair, P.J.; Wood, G.A. Frequency analysis of the EMG from the quadriceps of anterior cruciate ligament deficient individuals. Electromyogr. Clin. Neurophysiol. 1993, 33, 43–48. [Google Scholar] [PubMed]
- McHugh, M.P.; Tyler, T.F.; Nicholas, S.J.; Browne, M.G.; Gleim, G.W. Electromyographic analysis of quadriceps fatigue after anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2001, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Mackler, L.; Binder-Macleod, S.A.; Williams, P.R. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med. Sci. Sports Exerc. 1993, 25, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R. Estimation of average muscle fiber conduction velocity from two-dimensional surface EMG recordings. J. Neurosci. Methods 2004, 134, 199–208. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretta-Piccoli, M.; Schneebeli, A.; Egloff, M.; Cescon, C.; Clijsen, R.; Togninalli, D.; Barbero, M. Myoelectric Manifestations of Fatigue after ACL Reconstruction: A Cross-Sectional Study after Postoperative Rehabilitation. J. Funct. Morphol. Kinesiol. 2016, 1, 193-199. https://doi.org/10.3390/jfmk1020193
Beretta-Piccoli M, Schneebeli A, Egloff M, Cescon C, Clijsen R, Togninalli D, Barbero M. Myoelectric Manifestations of Fatigue after ACL Reconstruction: A Cross-Sectional Study after Postoperative Rehabilitation. Journal of Functional Morphology and Kinesiology. 2016; 1(2):193-199. https://doi.org/10.3390/jfmk1020193
Chicago/Turabian StyleBeretta-Piccoli, Matteo, Alessandro Schneebeli, Michele Egloff, Corrado Cescon, Ron Clijsen, Danilo Togninalli, and Marco Barbero. 2016. "Myoelectric Manifestations of Fatigue after ACL Reconstruction: A Cross-Sectional Study after Postoperative Rehabilitation" Journal of Functional Morphology and Kinesiology 1, no. 2: 193-199. https://doi.org/10.3390/jfmk1020193
APA StyleBeretta-Piccoli, M., Schneebeli, A., Egloff, M., Cescon, C., Clijsen, R., Togninalli, D., & Barbero, M. (2016). Myoelectric Manifestations of Fatigue after ACL Reconstruction: A Cross-Sectional Study after Postoperative Rehabilitation. Journal of Functional Morphology and Kinesiology, 1(2), 193-199. https://doi.org/10.3390/jfmk1020193