Abstract
This article introduces an inventive holder for endotracheal tubes designed specifically to support neonates with severe respiratory conditions during ventilation. Its primary goal is to minimize the risk of slippage of ventilator tubes in newborns, a critical concern that can lead to complications in their respiratory health. The innovation accommodates endotracheal tube equipment by offering adjustable sizing to match different dimensions. The development process employs computer-aided design (CAD) principles, while prototypes are crafted using three-dimensional (3D) printing technology. Comprising four main components—a support for the endotracheal tube header, a support for the tube unit itself, a flexible structure for tube positioning, and a stabilizing base—the innovation demonstrates structural strength and suitability within predefined parameters. It effectively supports the endotracheal tube apparatus while providing flexibility in positioning and distance adjustments. Importantly, its height can be tailored to suit the newborn’s head, offering adaptability for optimal usage. This research supports Sustainable Development Goals (SDGs) 3 and 9 relating to “Good health and well-being” and “Industry, innovation and infrastructure”.
1. Introduction
Newborns born prematurely often experience severe respiratory distress due to an underdeveloped respiratory system, which prevents them from breathing independently [1,2,3]. Therefore, it becomes necessary to provide respiratory support through the use of a breathing tube for these infants. This procedure assists newborns with respiratory issues in obtaining sufficient oxygen, increasing their chances of survival and reducing the mortality rate [4,5]. However, the endotracheal tube apparatus placed in the infant’s trachea is commonly dislodged in this patient group. This is due to the small size of the trachea in preterm infants [6].
There are several factors contributing to the dislodgement of the endotracheal tube in preterm infants [7,8]. These include frequent procedures due to repositioning or tilting of the infant, the infant’s movements and wriggling, the use of an endotracheal tube that is larger and heavier compared to the small size and weight of the preterm infant, inappropriate securing of the breathing tube, and a lack of vigilance in handling or performing procedures. The dislodgement of equipment has significant implications for care and is a major cause of risk for harm to preterm infants, leading to complications and distress from the need for repeated intubations. In severe cases, it can result in cardiac arrest and loss of life [1,9,10].
Figure 1 depicts the installation of the endotracheal tube equipment on a makeshift structure [11,12,13,14,15,16]. There is currently no specific device designed to secure this type of endotracheal tube. Therefore, problem-solving methods involve adapting locally available basic equipment, such as simple tube structures, or tying them to a headboard. However, this methodical approach results in inconvenience due to frequent adjustments and a lack of suitability. However, these makeshift solutions are inconvenient, time-consuming to assemble, and pose a risk of harm to preterm infants. The current equipment faces several issues in usage, such as the lack of support for breathing tube sets, leading to the sagging of the breathing tube set due to the weight of the equipment. The equipment is designed to work with a specific size of breathing tube, but in practice, breathing tubes come in various sizes [11,14]. Additionally, there is a challenge in adjusting the position of the breathing tube, making it difficult, particularly for very small newborns.
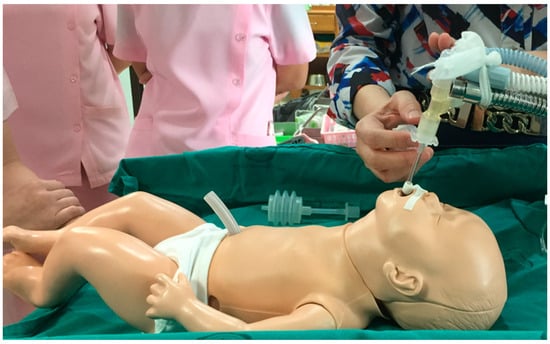
Figure 1.
The current method of securing the endotracheal tube.
Given the mentioned issues, this research aims to design and develop a device to safely hold an endotracheal tube for preterm infants that reduces the tension force on the breathing tube, is suitable for use, and can respond to the user as much as possible; increases effectiveness in preventing or reducing the rate of slippage of ventilator tubes in newborns; and reduces the risk of complications, including the death rate of newborns with respiratory problems. Furthermore, the research also involves developing a prototype medical device that is an innovative tool capable of effectively and appropriately assisting in patient treatment. This device can be locally manufactured for use in Thailand, aiming to reduce reliance on imported technology from abroad and increase access to efficient treatment within the country.
2. Materials and Methods
2.1. Conceptual Framework for the Innovation
The research approach began with identifying all usage issues from both the medical and nursing teams of the Faculty of Medicine, HRH Princess Maha Chakri Sirindhorn (HRH-PMCS) Medical Centre, Srinakharinwirot University (SWU), Thailand. Next, these issues were analyzed to inform the equipment’s design. Following this, research teams used this analysis to create prototypes of devices for users and made adjustments based on feedback from end-users until they achieved a design that aligned with the requirements.
The design and development of the working mechanism structure of a neonatal endotracheal tube support device with support and an adjustable position for the endotracheal tube is the focus of this invention. Its notable characteristics include easy and quick installation, being convenient to move, compatibility with tubes of all sizes, and the ability to adjust the position of the endotracheal tube. The endotracheal tube support device innovatively secures and supports the endotracheal tube of preterm infants, keeping them in an optimal position, preventing displacement, and ensuring ease of use. Based on the information collected from individuals who have benefited or been affected, Table 1 outlines the scope and conditions.

Table 1.
An overview of scope and specific conditions.
The structure and components are as shown in Figure 2, and the overall dimensions are as shown in Figure 3. The SolidWorks program was used to design various parts of the equipment, considering the above conditions, as described in the design section.
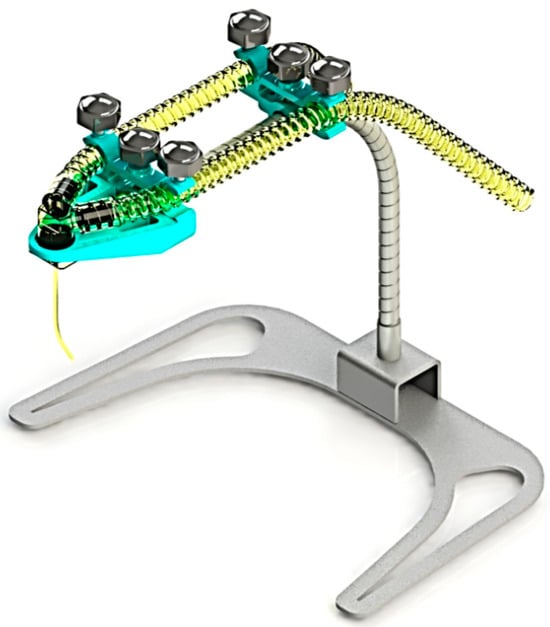
Figure 2.
The concept of 3D design for innovation.
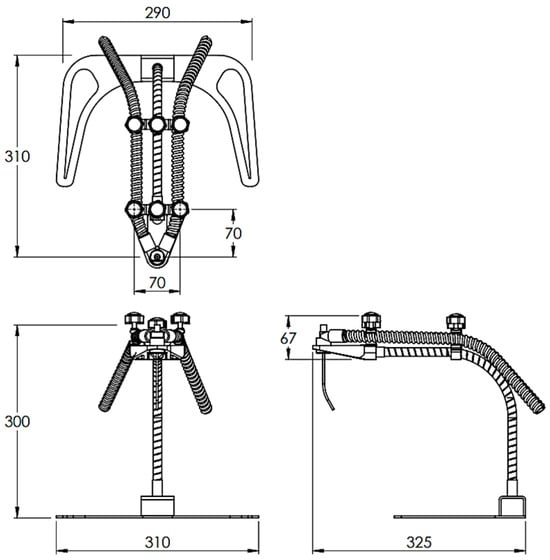
Figure 3.
The overall dimensions of the innovation (unit: mm).
Figure 4 shows the details of various components of the innovation, as well as the details of mane, materials, and manufacturing methods used for these components, as shown in Table 2.
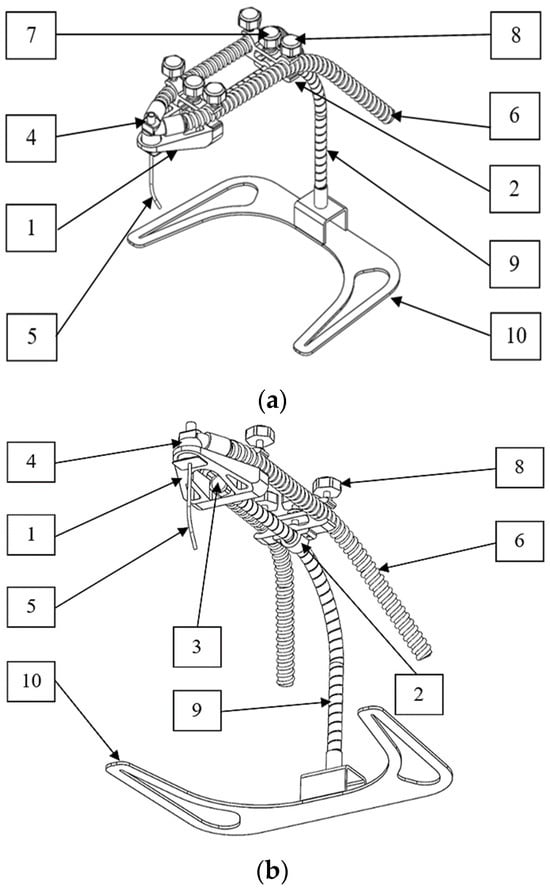
Figure 4.
The details of various components of the innovation: (a) isometric view; (b) bottom view.

Table 2.
The specific components of the innovation.
2.2. The Process of Designing and Creating a Prototype
The design emphasizes the application of computer-aided design (CAD) principles for engineering product components [25]. Illustrated in Figure 5 is the model design created using the SolidWorks program [26]. The design process also involves utilizing the program to create and simulate different structures and components of the model.
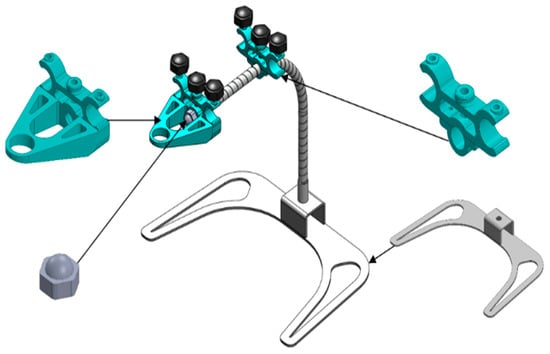
Figure 5.
The designed model with SolidWorks.
In the process of designing and simulating various structures and model components through the SolidWorks program, it is essential to consider the conditions, structure, and components outlined in Figure 2, along with the overall dimensions illustrated in Figure 3, to determine an appropriate format for innovative production. During the prototype production phase, components 1 to 3 specified in Table 2 were created using 3D printing technology [27,28,29]. Components 7 and 8 are fastened using M5 hexagon head screws, and component 9 incorporates a flexible tube with a diameter of 10 mm and a length of 300 mm. Additionally, Component 10 is constructed using a 3 mm thick stainless steel sheet under the design.
Medical devices intended for patient care must be manufactured using materials of medical grade, as exemplified in this innovative prototype. As indicated in Table 2, components 1–3 are constructed from polylactic acid (PLA) [17,18,19], components 4–6 are composed of silicone [20,21], components 7–8 are crafted from polypropylene (PP) [22,23] and stainless steel type 316, and components 9–10 utilize stainless steel type 316 [24]. These materials possess robust strength characteristics and the capacity for sterilization without compromising their physical properties, rendering them suitable for the production of medical equipment. After production, all the components are assembled to be tested and used. Prototypes were manufactured for testing and practical use following the procedures outlined in Figure 6. The design process for the innovation is demonstrated in Figure 7 through the flowchart.
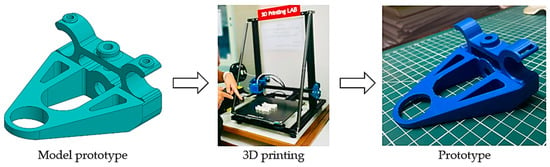
Figure 6.
Process for developing equipment with 3D printing.
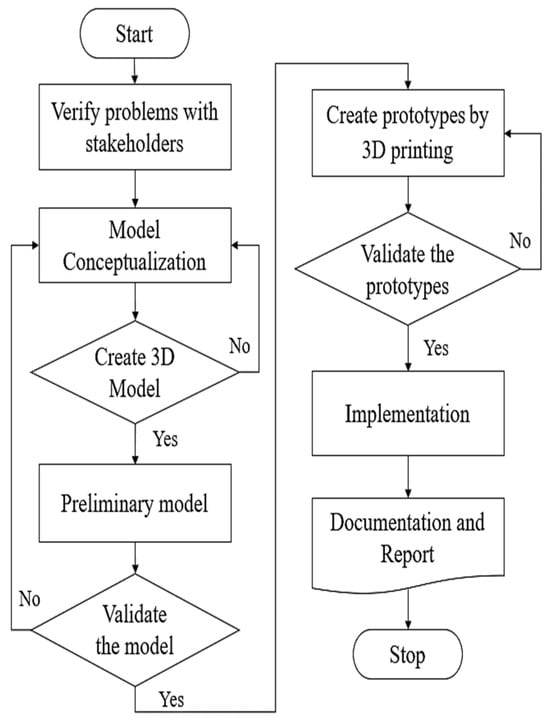
Figure 7.
The flowchart of the design process.
2.3. Innovation Assessment
This is an experimental research study that aims to test the functionality and efficiency of the developed equipment. The functionality and efficiency of the devices that have been created and the effects of the innovation on their use are studied. The population and target group consisted of essential personnel who need to use the equipment as a prototype in the Department of Pediatrics, Faculty of Medicine, HRH-PMCS Medical Centre, SWU, Thailand. In this research study, a questionnaire on satisfaction with innovation use was used as a tool for evaluation. The researchers divided the satisfaction level criteria into 5 levels, ranging from level 1 (no opinion) to level 5 (excellent). The divisions are shown in Table 3.

Table 3.
The satisfaction level criteria.
2.4. Data Reduction
For the analysis of the collected data, we calculated the percentage average of the data collected from the test group. The standard deviation (S.D.) of the assessment results for the satisfaction level with the equipment was also calculated. The equation and variable values are as shown in Equations (1) and (2) [30,31].
When S.D. is the standard deviation, xi is the value from the data, is the mean of all the data, and n is the number of data points. The analysis or interpretation of the meaning of the average satisfaction scores for this research followed the guidelines as outlined in Table 4.

Table 4.
The satisfaction level criteria.
3. Results and Discussion
3.1. The Production of the Innovative Prototype
Utilizing the design and analysis results, an innovative prototype was created to address specific problems and user requirements. The prototype was tested, and the entire structure remained robust without any damage during operation. Within the defined scope, it can perform the specified functions. Figure 8 illustrates the comprehensive structure and details of the prototype’s various components.
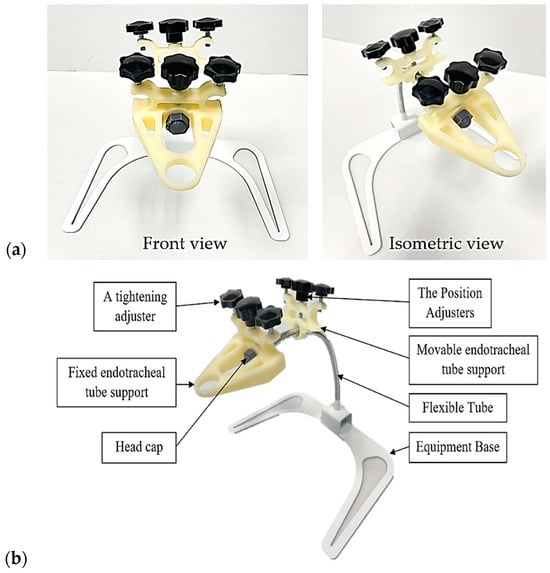
Figure 8.
The prototype of the innovation: (a) the overall structure; (b) the various components.
3.2. The Prototype’s Operation
Figure 9 illustrates the operation of the device, and Figure 10 shows how to adjust the working position of the equipment. The innovation consists of four main parts, including (1) a support for the header of the endotracheal tube unit; (2) a support for the endotracheal tube unit; (3) an adjustable flexible tube; and (4) the supporting base set. The prototype itself has a weight, excluding the endotracheal tube set, of 768 grams. The first part tightly fastens with an adjustable mechanism to lock it in place, preventing movement in the head region of the device. It serves the purpose of supporting the header of the endotracheal tube. The second part attaches to the flexible tube of the device, allowing users to adjust and move it for proper fitting. Its role is to support the endotracheal tube. The user can adjust and move the third part to the desired position. This component is for adapting to the user’s requirements. The structure of the support base allows the entire assembly to be mounted in a position that avoids contact with the patient’s head during use.

Figure 9.
The operation of the prototype.
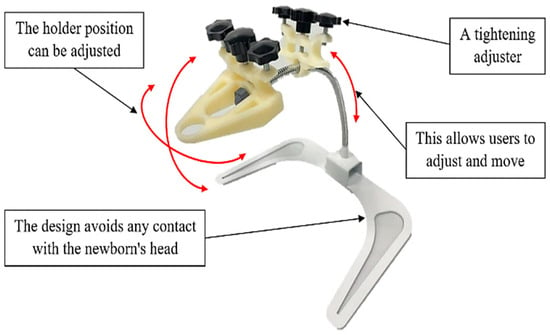
Figure 10.
The demonstration of adjusting the working positions of the prototype.
A prototype of the innovation underwent usability testing by specialist doctors, nurses, nursing students, and relevant personnel, including fifteen participants. Infant mannequins were used in a training simulation to practice installing and operating the endotracheal tube set, mimicking the sizes and anatomy of real newborns’ respiratory tracts. These mannequins help reduce potential risks in neonatal care and enable a reduction in the necessary resources, such as time and budget, used in actual patient care. Subsequently, an evaluation was conducted to gauge satisfaction with the innovation. Figure 11 depicts the testing of installation and usage with the newborn mannequin, while Figure 12 illustrates the testing with the simulated newborn mannequin. The results of the tests indicated that the device effectively secures and holds the endotracheal tube, thereby minimizing the risk of tube slippage within the small trachea of newborns. The device allows for convenient adjustment and sliding of the endotracheal tube, facilitating installation in various treatment positions. The appropriately designed tube grip ensures stability and prevents easy displacement for very small infants. Furthermore, the base of the device is engineered to be positioned without making contact with the patient’s head during use.
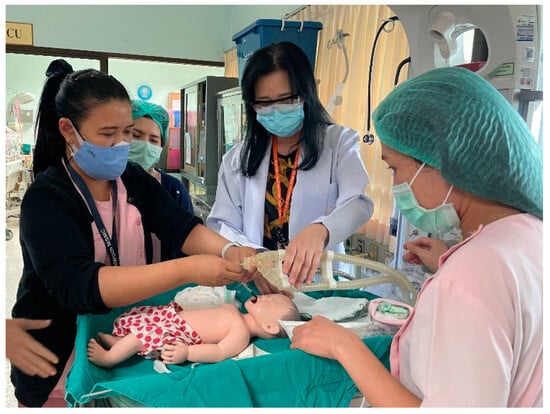
Figure 11.
The testing and installation of the prototype.
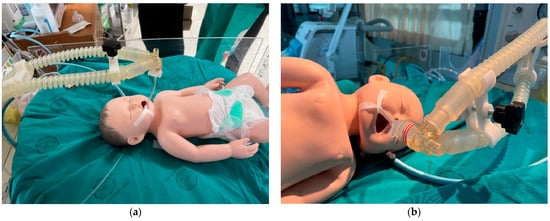
Figure 12.
Testing with the newborn mannequin (a) using the top position; (b) using a side position.
3.3. Usability Testing and Evaluation
The evaluation results for the use of the innovative prototype are presented. The usability testing involved practical applications with a newborn mannequin to simulate the installation and use of the respiratory support harness. Subsequently, a satisfaction assessment questionnaire was administered to evaluate the participants’ satisfaction with the innovation. Figure 13 presents a graph illustrating the percentage of satisfaction levels for each topic. In addition, Table 5 summarizes the evaluation results. After relevant personnel tested the equipment, they provided a prototype of the innovation for usability in the satisfaction assessment form to gather feedback and suggestions. Table 6 presents the summarized recommendations.
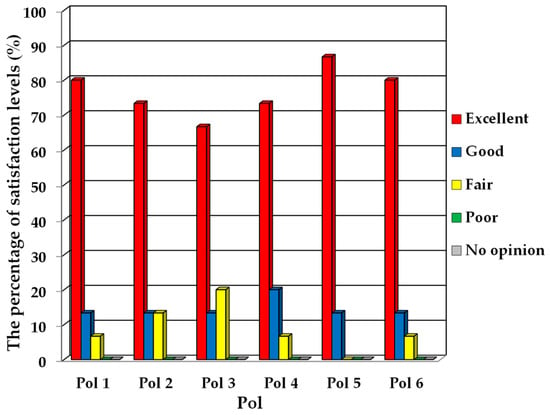
Figure 13.
The percentage of satisfaction levels for each topic.

Table 5.
An improved interpretation of the meaning.

Table 6.
Summarized recommendations.
In Figure 13, the score levels for each item in the questionnaire predominantly fall within the “excellent” category, followed by “good”, with the lowest ranking in the “fair” category. No evaluations were recorded in the “poor” or “no opinion” levels. According to Table 5, participants in the prototype testing consistently rated their overall satisfaction level as “excellent”, achieving a mean score of 4.68 with a standard deviation of 0.60. Upon examining specific items, it is noted that participants in the testing expressed a high level of satisfaction, typically at the “very satisfied” level for nearly every aspect, except for item 3, where satisfaction was rated as “satisfied”. The overall evaluation results conclude with a “very satisfied” rating, suggesting that the prototype of the innovation performs effectively in line with its design and successfully meets user expectations.
4. Conclusions
This article aims to design and develop an innovative endotracheal tube safety holder prototype for severely ill newborns with respiratory problems. The goal is to reduce the tension on the endotracheal tube set, facilitate convenient installation, and make it suitable for practical use in the field.
The prototype design consists of four main components: a support for the header of the endotracheal tube unit, a support for the endotracheal tube unit, an adjustable flexible tube, and the base set. The prototype, excluding the endotracheal tube set, weighs 768 g. The equipment demonstrates strength without damage during usage. Installation of the endotracheal tube set with the device is simple, and the device allows convenient adjustment of the working position. It helps reduce the weight tension and prevents or minimizes the slippage of the endotracheal tube. The evaluation of user satisfaction reveals an overall rating of “excellent”, with an average score of 4.68 and a standard deviation of 0.60. This indicates that participants are highly satisfied with almost every aspect, except for item 3, which still receives a “good” satisfaction rating. This suggests that the equipment performs well according to the design specifications. Therefore, this innovative prototype is suitable for production as a high-quality product that meets the defined scope and performs well in the field.
For future research endeavors, acquiring ethical clearance will be imperative to conduct trials involving real newborns. These enhancements will involve resizing the support structure for endotracheal tubes to a smaller scale, adjusting the material composition of the biocompatible component to withstand elevated temperatures, and reducing the length of the flexible tubing to optimize overall convenience.
5. Petty Patents
This innovation is currently undergoing the petty patent application process in Thailand by Srinakharinwirot University under the name “An innovative safety holder of the endotracheal tube for neonates with severe respiratory illnesses”. Invented by Phunapai, N.; Limboonruang, T.; Sriromreun, P.; Panburana, J.; Rearyal, S.; Thailand Petty Patent Application No. 2303001089.
Author Contributions
Conceptualization, N.P., J.P. and T.L.; methodology, N.P., J.P., P.S. (Parkpoom Sriromreun) and T.L.; investigation, N.P., J.P. and T.L.; resources, N.P., J.P., S.R., S.T., P.S. (Parkpoom Sriromreun), P.S. (Paranee Sriromreun) and T.L.; data curation, N.P., J.P., S.R. and T.L.; writing—original draft preparation, N.P., J.P. and T.L.; writing—review and editing, N.P., J.P., S.R., S.T., P.S. (Parkpoom Sriromreun), P.S. (Paranee Sriromreun) and T.L.; supervision, N.P., J.P., P.S. (Parkpoom Sriromreun) and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Srinakharinwirot University grant number 417/2022 and the Faculty of Engineering Alumni Association of Srinakharinwirot University.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| S.D. | (-) | The standard deviation |
| xi | (-) | The value of the data |
| (-) | The mean of all the data | |
| n | (-) | The number of data |
| Abbreviations | ||
| CAD | Computer-Aided Design | |
| PLA | Polylactic acid | |
| PP | Polypropylene | |
| SWU | Srinakharinwirot University | |
| mm | Millimeter | |
| 3D | Three-dimensional | |
| HRH-PMCS | Her Royal Highness Princess Maha Chakri Sirindhorn | |
References
- Gouyon, J.B.; Ribakovsky, C.; Ferdynus, C.; Quantin, C.; Sagot, P.; Gouyon, B. Burgundy Perinatal Network. Severe respiratory disorders in term neonates. Paediatr. Perinat. Epidemiol. 2008, 22, 22–30. [Google Scholar] [CrossRef]
- Hillman, N.H.; Lam, H.S. Respiratory disorders in the newborn. In Kendig’s Disorders of the Respiratory Tract in Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 338–366.e6. [Google Scholar]
- Warley, M.; Gairdner, D. Respiratory distress syndrome of the newborn—Principles in treatment. Arch. Dis. Child. 1962, 37, 455. [Google Scholar] [CrossRef]
- Hatch, L.D.; Grubb, P.H.; Lea, A.S.; Walsh, W.F.; Markham, M.H.; Whitney, G.M.; Slaughter, J.C.; Stark, A.R.; Ely, E.W. Endotracheal intubation in neonates: A prospective study of adverse safety events in 162 infants. J. Pediatr. 2016, 168, 62–66.e6. [Google Scholar] [CrossRef]
- Walsh-Sukys, M.C. Severe respiratory failure in neonates: Mortality and morbidity rates and neurodevelopmental outcomes. J. Pediatr. 1994, 125, 104–110. [Google Scholar] [CrossRef]
- Lanzillotti, L.D.S.; Seta, M.H.D.; Andrade, C.L.T.D.; Mendes Junior, W.V. Adverse events and other incidents in neonatal intensive care units. Cienc. Saude Coletiva 2015, 20, 937–946. [Google Scholar] [CrossRef]
- Nakrit, B. Unplanned extubation and duration of mechanical ventilation in critically ill patients on evidenced based nursing practice. Kuakarun J. Nurs. 2015, 22, 129–143. [Google Scholar]
- Peñuelas, Ó.; Frutos-Vivar, F.; Esteban, A. Unplanned extubation in the ICU: A marker of quality assurance of mechanical ventilation. Crit. Care 2011, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.S.L.; Reis, M.E.; Aguiar, V.E.; Fonseca, M.C.M. Unplanned extubation in the neonatal ICU: A systematic review, critical appraisal, and evidence-based recommendations. Respir. Care 2013, 58, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.C.R.D.; Cabral, L.A.; Schettino, R.D.C.; Ribeiro, S.N.S. Incidence and primary causes of unplanned extubation in a neonatal intensive care unit. Rev. Bras. Ter. Intensiv. 2012, 24, 230–235. [Google Scholar] [CrossRef]
- Berard, D.; Navarro, J.D.; Bascos, G.; Harb, A.; Feng, Y.; De Lorenzo, R.; Hood, R.L.; Restrepo, D. Novel expandable architected breathing tube for improving airway securement in emergency care. J. Mech. Behav. Biomed. Mater. 2021, 114, 104211. [Google Scholar] [CrossRef] [PubMed]
- Budd, R. The “Logan bow” method for securing endotracheal tubes in neonates. Crit. Care Nurse 1982, 2, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.K.; DiBlasi, R.M. Mechanical ventilation of the premature neonate. Respir. Care 2011, 56, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Chuaykarn, U. Benefit of Neosafe innovation for endotracheal tubes stabilization on incidence of unplanned extubations in the neonates at Maharaj Nakhon Si Thammarat Hospital. J. Nurs. Sci. Health 2019, 42, 112–120. [Google Scholar]
- Collaco, J.M.; McGrath-Morrow, S.A. Respiratory phenotypes for preterm infants, children, and adults: Bronchopulmonary dysplasia and more. Ann. Am. Thorac. Soc. 2018, 15, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.S.; Manley, B.J.; Davis, P.G.; Doyle, L.W. The evolution of modern respiratory care for preterm infants. Lancet 2017, 389, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Davachi, S.M.; Kaffashi, B. Polylactic acid in medicine. Polym.-Plast. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Cooper, J.D.; Todd, T.R.; Pearson, F.G. Use of the silicone tracheal T-tube for the management of complex tracheal injuries. J. Thorac. Cardiovasc. Surg. 1981, 82, 559–568. [Google Scholar] [CrossRef]
- Mahmoud, R.A.; Proquitté, H.; Hadhood, S.E.; Schmalisch, G. Effect of endotracheal tube leakage on respiratory function monitoring: Comparison of three neonatal ventilators. J. Pediatr. Intensive Care 2012, 1, 61–69. [Google Scholar] [CrossRef]
- Gopanna, A.; Rajan, K.P.; Thomas, S.P.; Chavali, M. Polyethylene and polypropylene matrix composites for biomedical applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–216. [Google Scholar]
- Murthe, S.S.; Sreekantan, S.; Mydin, R.B.S. Study on the Physical, Thermal and Mechanical Properties of SEBS/PP (Styrene-Ethylene-Butylene-Styrene/Polypropylene) Blend as a Medical Fluid Bag. Polymers 2022, 14, 3267. [Google Scholar] [CrossRef]
- Winters, G.L.; Nutt, M.J. Stainless Steels for Medical and Surgical Applications; Astm International: West Conshohocken, PA, USA, 2003; Volume 1438. [Google Scholar]
- Bi, Z.; Wang, X. Computer-Aided Design and Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Muñoz-Vásquez, S.; Mora-Pérez, Z.A.; Ospina-Henao, P.A.; Valencia-Niño, C.H.; Becker, M.; Díaz-Rodríguez, J.G. Finite Element Analysis in the Balancing Phase for an Open Source Transfemoral Prosthesis with Magneto-Rheological Damper. Inventions 2023, 8, 36. [Google Scholar] [CrossRef]
- Gloyer, P.; Schek, L.N.; Flöttmann, H.L.; Wüst, P.; Völlmecke, C. Extrusion-Based Additive Manufacturing-Driven Design and Testing of the Snapping Interlocking Metasurface Mechanism ShroomLock. Inventions 2023, 8, 137. [Google Scholar] [CrossRef]
- Sathish, T.; Vijayakumar, M.; Ayyangar, A.K. Design and fabrication of industrial components using 3D printing. Mater. Today Proc. 2018, 5, 14489–14498. [Google Scholar] [CrossRef]
- Šproch, F.; Schindlerová, V.; Šajdlerová, I. Using 3D printing technology in prototype production to control the dimensions of complexly shaped products. Manuf. Technol. 2020, 20, 385–393. [Google Scholar] [CrossRef]
- Barde, M.P.; Barde, P.J. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect. Clin. Res. 2012, 3, 113. [Google Scholar] [CrossRef]
- Livingston, E.H. The mean and standard deviation: What does it all mean? J. Surg. Res. 2004, 119, 117–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).