Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Anthocyanins Extracted from Red Onion Skins Using a Central Composite Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Red Onion Skins Preparation
2.3. Conventional Solvent Extraction
2.4. Determination of the Total Anthocyanins Content (TAC)
2.5. Determination of the Antioxidant Activity (AA)
2.6. Experimental Design
2.7. Statistical Analysis
3. Results
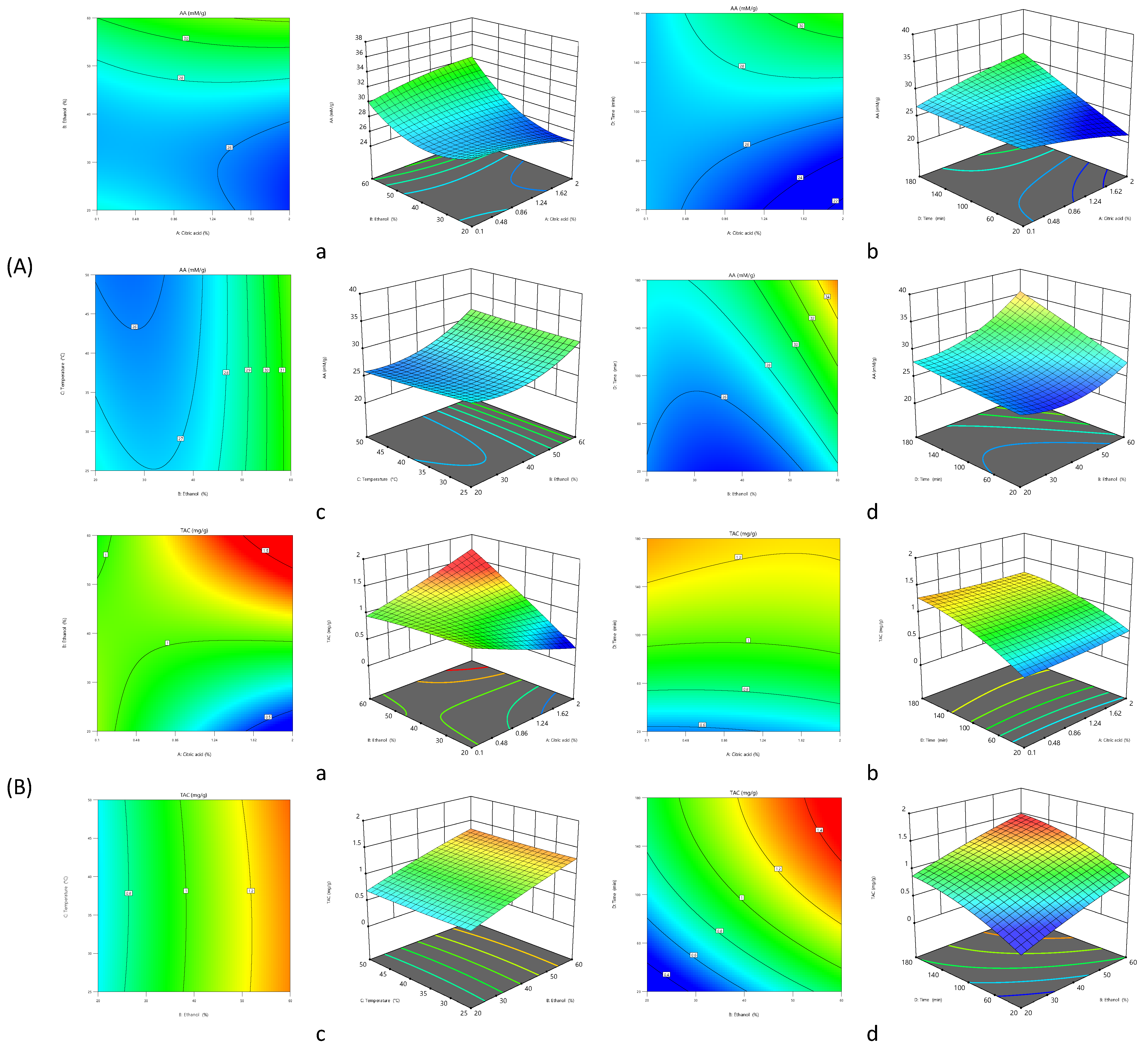
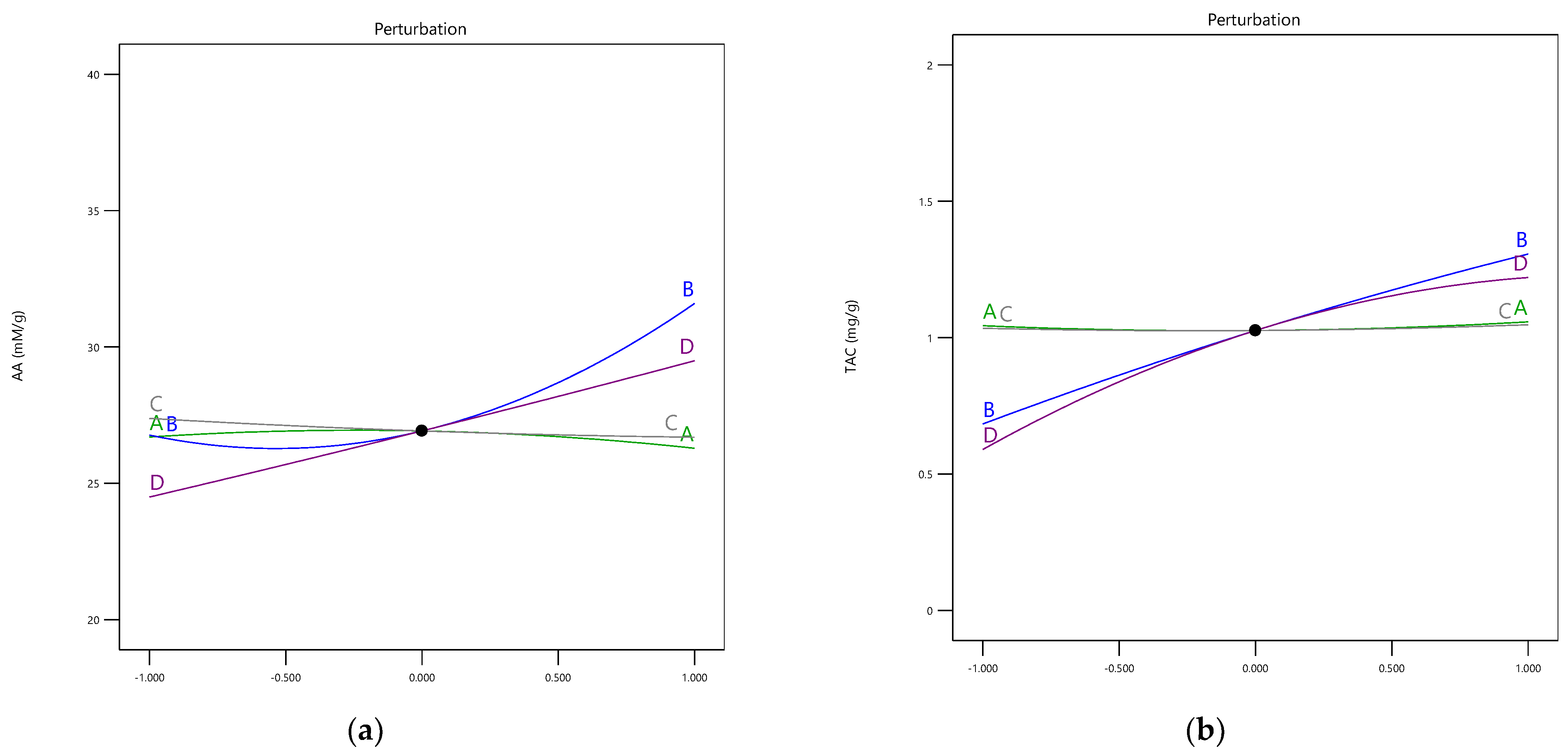
3.1. Influence of the Extraction Parameters on AA
3.2. Influence of the Extraction Parameters on TAC
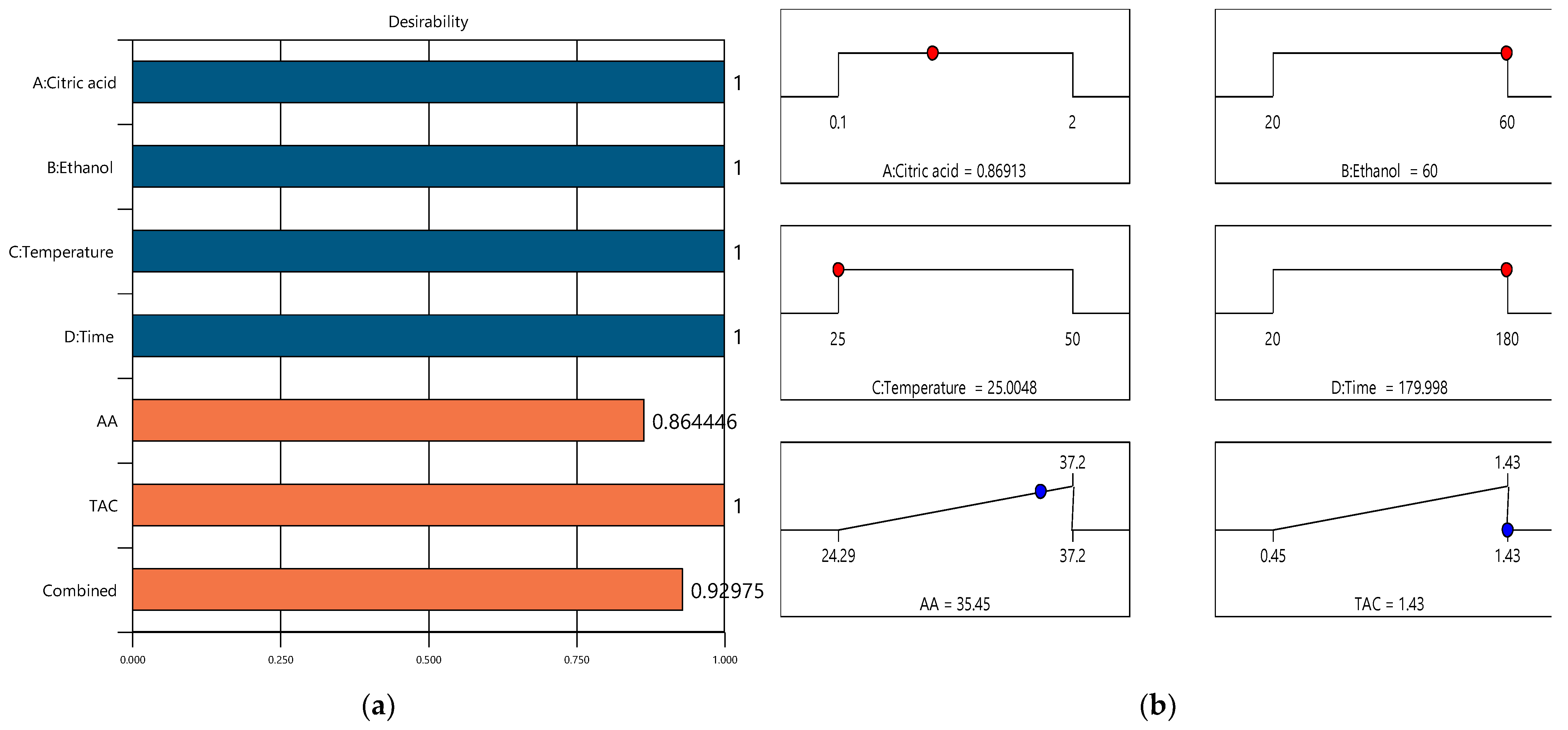
3.3. Optimization and Validation of the Extraction Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium cepa L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Zill-e-Huma; Vian, M.A.; Fabiano-Tixier, A.-S.; Elmaataoui, M.; Dangles, O.; Chemat, F. A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties. Food Chem. 2011, 127, 1472–1480. [Google Scholar] [CrossRef]
- Ali, O.-H.; Al-sayed, H.; Yasin, N.; Afifi, E. Effect of Different Extraction Methods on Stablity of Anthocyanins Extracted from Red Onion peels (Allium cepa) and Its Uses as Food Colorants. Bull. Natl. Nutr. Inst. Arab. Repub. Egypt 2016, 47, 196–219. [Google Scholar] [CrossRef][Green Version]
- Nile, A.; Gansukh, E.; Park, G.-S.; Kim, D.-H.; Hariram Nile, S. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste—In vitro and in silico approach. Food Chem. 2021, 335, 127650. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Prodromidis, P.; Grigorakis, S.; Makris, D.P.; Biliaderis, C.G.; Moschakis, T. Natural food colorants derived from onion wastes: Application in a yoghurt product. Electrophoresis 2018, 39, 1975–1983. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Freeze-dried extract from onion (Allium cepa cv. Horcal) skin wastes: Extraction intensification and flavonoids identification. Food Bioprod. Process. 2021, 130, 92–105. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Horincar, G.; Enachi, E.; Stănciuc, N.; Râpeanu, G. Extraction and characterization of bioactive compounds from eggplant peel using ultrasound—Assisted extraction. Ann. Univ. Dunarea Jos Galati. Fascicle VI-Food Technol. 2019, 43, 40–53. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Etsuo, N. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Viera, V.B.; Piovesan, N.; Rodrigues, J.B.; Mello, R.; De, O.; Prestes, R.C.; Santos, R.C.; Vaucher, A.; Hautrive, T.P.; Kubota, E.H. Extraction of phenolic compounds and evaluation of the antioxidant and antimicrobial capacity of red onion skin (Allium cepa L.)|Request PDF. Int. Food Res. J. 2017, 24, 990–999. [Google Scholar]
- Ifesan, B.O.T. Chemical Composition of Onion Peel (Allium cepa) and its Ability to Serve as a Preservative in Cooked Beef. Hum. J. 2017, 7, 25–34. [Google Scholar]
- Prokopov, T.; Slavov, A.; Petkova, N.; Yanakieva, V.; Bozadzhiev, B.; Taneva, D. Study of onion processing waste powder for potential use in food sector. Acta Aliment. 2018, 47, 181–188. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An Investigation on the Recovery of Antioxidant Phenolics from Onion Solid Wastes Employing Water/Ethanol-Based Solvent Systems. Food Bioprocess Technol. 2007, 2, 337. [Google Scholar] [CrossRef]
- Oancea, S.; Drághici, O. pH and thermal stability of anthocyanin-based optimised extracts of Romanian red onion cultivars. Czech J. Food Sci. 2013, 31, 283–291. [Google Scholar] [CrossRef]
- Hasbay, I.; Galanakis, C.M. Recovery technologies and encapsulation techniques. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 233–264. ISBN 978-0-12-813573-0. [Google Scholar] [CrossRef]
- Jeya Krithika, S.; Sathiyasree, B.; Beniz Theodore, E.; Chithiraikannu, R.; Gurushankar, K. Optimization of extraction parameters and stabilization of anthocyanin from onion peel. Crit. Rev. Food Sci. Nutr. 2022, 62, 2560–2567. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and Isolation of Phenolic Compounds. Methods Mol. Biol. 2012, 864, 427–464. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of Phenolic Compounds from Blueberry Leaves and Comparison with Other Extraction Methods. Ind. Crops Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Extraction of Anthocyanin Pigments from Purple Sunflower Hulls. J. Food Sci. 1996, 61, 600–603. [Google Scholar] [CrossRef]
- Nicoué, E.E.; Savard, S.; Belkacemi, K. Anthocyanins in Wild Blueberries of Quebec: Extraction and Identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing Conditions for Anthocyanins Extraction from Purple Sweet Potato Using Response Surface Methodology (RSM). LWT Food Sci. Technol. 2008, 41, 155–160. [Google Scholar] [CrossRef]
- Khazaei, K.M.; Jafari, S.M.; Ghorbani, M.; Kakhki, A.H.; Sarfarazi, M. Optimization of Anthocyanin Extraction from Saffron Petals with Response Surface Methodology. Food Anal. Methods 2016, 9, 1993–2001. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Bordin Viera, V.; Piovesan, N.; Mello, R.D.O.; Barin, J.S.; Fogaça, A.D.O.; Bizzi, C.A.; De Moraes Flores, É.M.; Dos Santos Costa, A.C.; Pereira, D.E.; Soares, J.K.B.; et al. Ultrasonic assisted extraction of phenolic compounds with evaluation of red onion skin (Allium cepa L.) antioxidant capacity. J. Culin. Sci. Technol. 2021, 19, 475–566. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Samir, R.M.; Osman, A.; El-Sayed, A.I.; Algaby, A.M. Physicochemical properties and antimicrobial effects of Roselle corolla, onion peels and peanut skins anthocyanins. Zagazig J. Agric. Res. 2019, 46, 769–781. [Google Scholar] [CrossRef]
- Oancea, S.; Radu, M.; Olosutean, H. Development of ultrasonic extracts with strong antioxidant properties from red onion wastes. Rom. Biotechnol. Lett. 2020, 25, 1320–1327. [Google Scholar] [CrossRef]
- Makris, D.P. Optimisation of Anthocyanin Recovery from Onion (Allium cepa) Solid Wastes Using Response Surface Methodology. J. Food Technol. 2010, 8, 183–186. [Google Scholar] [CrossRef]
| Code | Independent Variables | Units | Minimum | Maximum | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| A | Citric acid | % | 0.0500 | 2.64 | −1 = 0.10 | +1 = 2.00 |
| B | Ethanol | % | 6.36 | 73.63 | −1 = 20.00 | +1 = 60.00 |
| C | Temperature | °C | 16.47 | 58.52 | −1 = 25.00 | +1 = 50.00 |
| D | Time | min | 10.00 | 234.54 | −1 = 20.00 | +1 = 180.00 |
| Run | Factor 1 A: Citric Acid % | Factor 2 B: Ethanol % | Factor 3 C: Temperature °C | Factor 4 D: Time min | Response 1 AA mM TE/g DW | Response 2 TAC mg C3G/g DW |
|---|---|---|---|---|---|---|
| 1 | 1 | 40 | 37 | 100 | 26.92 | 1.02 |
| 2 | 1 | 40 | 16.47 | 100 | 27.71 | 1.05 |
| 3 | 2 | 60 | 50 | 20 | 27.24 | 1.34 |
| 4 | 1 | 73.63 | 37 | 100 | 37.2 | 1.43 |
| 5 | 1 | 40 | 37 | 100 | 26.84 | 1.03 |
| 6 | 0.1 | 20 | 50 | 20 | 28.7 | 0.6 |
| 7 | 2 | 60 | 25 | 20 | 25.01 | 1.32 |
| 8 | 0.1 | 60 | 25 | 180 | 32.53 | 1.18 |
| 9 | 1 | 40 | 37 | 100 | 26.74 | 1.02 |
| 10 | 1 | 40 | 58.52 | 100 | 26.73 | 1.09 |
| 11 | 2.64 | 40 | 37 | 100 | 25.32 | 1.11 |
| 12 | 0.1 | 20 | 25 | 20 | 30.39 | 0.62 |
| 13 | 2 | 20 | 25 | 180 | 29.51 | 0.52 |
| 14 | 1 | 6.36 | 37 | 100 | 29.41 | 0.45 |
| 15 | 0.1 | 60 | 50 | 180 | 31.07 | 1.21 |
| 16 | 1 | 40 | 37 | 10 | 24.29 | 0.52 |
| 17 | 1 | 40 | 37 | 100 | 26.73 | 1.04 |
| 18 | 0.05 | 40 | 37 | 100 | 26.63 | 1.05 |
| 19 | 1 | 40 | 37 | 234.54 | 31.14 | 1.22 |
| 20 | 1 | 40 | 37 | 100 | 27.64 | 1.01 |
| 21 | 2 | 20 | 50 | 180 | 27.24 | 0.51 |
| AA | TAC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | p-Value | Source | SS | df | MS | F-Value | p-Value |
| Model | 172.39 | 14 | 12.31 | 112.73 | <0.0001 a | Model | 1.84 | 14 | 0.1316 | 868.48 | <0.0001 a |
| A-Citric acid | 0.1126 | 1 | 0.1126 | 1.03 | 0.3491 | A-Citric acid | 0.0001 | 1 | 0.0001 | 0.9003 | 0.3793 |
| B-Ethanol | 32.49 | 1 | 32.49 | 297.46 | <0.0001 | B-Ethanol | 0.5410 | 1 | 0.5410 | 3569.82 | <0.0001 |
| C-Temperature | 1.63 | 1 | 1.63 | 14.91 | 0.0083 | C-Temperature | 0.0006 | 1 | 0.0006 | 3.65 | 0.1045 |
| D-Time | 19.45 | 1 | 19.45 | 178.09 | <0.0001 | D-Time | 0.3096 | 1 | 0.3096 | 2043.28 | <0.0001 |
| AB | 4.22 | 1 | 4.22 | 38.59 | 0.0008 | AB | 0.3111 | 1 | 0.3111 | 2052.61 | <0.0001 |
| AC | 1.19 | 1 | 1.19 | 10.92 | 0.0163 | AC | 3.422 × 10−7 | 1 | 3.422 × 10−7 | 0.0023 | 0.9636 |
| AD | 19.08 | 1 | 19.08 | 174.65 | <0.0001 | AD | 0.0049 | 1 | 0.0049 | 32.31 | 0.0013 |
| BC | 2.80 | 1 | 2.80 | 25.60 | 0.0023 | BC | 0.0008 | 1 | 0.0008 | 5.28 | 0.0613 |
| BD | 4.54 | 1 | 4.54 | 41.59 | 0.0007 | BD | 0.0000 | 1 | 0.0000 | 0.1077 | 0.7540 |
| CD | 2.28 | 1 | 2.28 | 20.87 | 0.0038 | CD | 0.0001 | 1 | 0.0001 | 0.3299 | 0.5866 |
| A2 | 1.27 | 1 | 1.27 | 11.59 | 0.0144 | A2 | 0.0042 | 1 | 0.0042 | 27.54 | 0.0019 |
| B2 | 76.35 | 1 | 76.35 | 698.99 | <0.0001 | B2 | 0.0144 | 1 | 0.0144 | 95.30 | <0.0001 |
| C2 | 0.1926 | 1 | 0.1926 | 1.76 | 0.2325 | C2 | 0.0033 | 1 | 0.0033 | 21.48 | 0.0036 |
| D2 | 0.0442 | 1 | 0.0442 | 0.4045 | 0.5483 | D2 | 0.1175 | 1 | 0.1175 | 775.31 | <0.0001 |
| Residual | 0.6554 | 6 | 0.1092 | Residual | 0.0009 | 6 | 0.0002 | ||||
| Lack of Fit | 0.0767 | 2 | 0.0383 | 0.2649 | 0.7797 b | Lack of Fit | 0.0004 | 2 | 0.0002 | 1.50 | 0.3271 b |
| Pure Error | 0.5787 | 4 | 0.1447 | Pure Error | 0.0005 | 4 | 0.0001 | ||||
| Cor Total | 173.05 | 20 | Cor Total | 1.84 | 20 | ||||||
| Dependent Variable | Predicted Value | 95% Confidence Intervals | Experimental Value |
|---|---|---|---|
| AA (mM TE/g DW) | 35.45 | 24.29–37.20 | 37.20 |
| TAC (mg C3G/g DW) | 1.43 | 0.45–1.43 | 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, F.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Bahrim, G.E.; Râpeanu, G. Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Anthocyanins Extracted from Red Onion Skins Using a Central Composite Design. Inventions 2022, 7, 89. https://doi.org/10.3390/inventions7040089
Stoica F, Constantin OE, Stănciuc N, Aprodu I, Bahrim GE, Râpeanu G. Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Anthocyanins Extracted from Red Onion Skins Using a Central Composite Design. Inventions. 2022; 7(4):89. https://doi.org/10.3390/inventions7040089
Chicago/Turabian StyleStoica, Florina, Oana Emilia Constantin, Nicoleta Stănciuc, Iuliana Aprodu, Gabriela Elena Bahrim, and Gabriela Râpeanu. 2022. "Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Anthocyanins Extracted from Red Onion Skins Using a Central Composite Design" Inventions 7, no. 4: 89. https://doi.org/10.3390/inventions7040089
APA StyleStoica, F., Constantin, O. E., Stănciuc, N., Aprodu, I., Bahrim, G. E., & Râpeanu, G. (2022). Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Anthocyanins Extracted from Red Onion Skins Using a Central Composite Design. Inventions, 7(4), 89. https://doi.org/10.3390/inventions7040089








