Abstract
The shift from centralized to distributed generation and the need to address energy shortage and achieve the sustainability goals are among the important factors that drive increasing interests of governments, planners, and other relevant stakeholders in microgrid systems. Apart from the distributed renewable energy resources, fuel cells (FCs) are a clean, pollution-free, highly efficient, flexible, and promising energy resource for microgrid applications that need more attention in research and development terms. Furthermore, they can offer continuous operation and do not require recharging. This paper examines the exciting potential of FCs and their utilization in microgrid systems. It presents a comprehensive review of FCs, with emphasis on the developmental status of the different technologies, comparison of operational characteristics, and the prevailing techno-economic barriers to their progress and the future outlook. Furthermore, particular attention is paid to the applications of the FC technologies in microgrid systems such as grid-integrated, grid-parallel, stand-alone, backup or emergency power, and direct current systems, including the FC control mechanisms and hybrid designs, and the technical challenges faced when employing FCs in microgrids based on recent developments. Microgrids can help to strengthen the existing power grid and are also suitable for mitigating the problem of energy poverty in remote locations. The paper is expected to provide useful insights into advancing research and developments in clean energy generation through microgrid systems based on FCs.
1. Introduction
The global electricity systems are currently witnessing a paradigm shift from the traditional centralized to distributed generation technologies [1,2]. This development, coupled with the necessity to address the concerns of an energy shortage, ensures energy security and realizes the environmental sustainability is part of the critical factors responsible for growing interests in microgrid systems across the world [3,4,5]. In addition, it is necessary to develop more diversified electrical energy production resources beyond the current solar, wind, hydro, biomass, diesel, and battery technologies for microgrid systems. Interestingly, fuel cell (FC) systems are considered as promising energy resources on the basis of being clean, pollution-free, and efficient, including their potential to store higher calorific value, in the hydrogen form, compared to the chemical energy that may be stored by using most other materials [6,7,8,9], and the capability to supply energy for a relatively longer time [10]. It was of interest in this paper to discuss the potential of the FC technologies for microgrid system applications. One way to engage the technologies is by integrating them with the renewable energy resources, in which they operate as a storage device for harnessing relatively high renewable energy; another option is to use them as the source of energy in microgrid systems.
Microgrid systems are being employed both for on-grid and islanded purposes in several developed countries and are fueled by several resources like solar, wind, biomass, hydro, diesel, and natural gas [11]. While the on-grid configuration seeks to support the existing grid, the islanded mode is used for serving remote or grid-independent applications since the microgrid is disconnected from the grid [12,13,14,15,16]. However, microgrid systems are limited to the grid-independent application in several communities in developing countries due to the challenge of poor and inefficient power grid infrastructure, as is the case in Nigeria [3,17]. Off-grid microgrids, in this instance, are not only employed for powering remote houses or business premises but also used for meeting a proportion of the energy demand of those houses or facilities that also have a connection with the national grid since the supply from the network is erratic in several parts of the country [18]. This paper was, therefore, motivated by the developments in microgrids and the possibility of powering them by FCs. Such efforts can help achieve energy security by growing a diversified energy system.
The issue of reliability of some of the existing microgrid systems in developing countries, especially the solar-photovoltaic (PV) microgrids, using Nigeria as a typical example [18], is another compelling factor that motivates exploring the potential of other energy resources such as FCs. A lack of understanding of the intermittent characteristics of solar irradiation and poor technical design is part of the key factors responsible for systems’ failure. FCs can provide continuous operation, that is, they can be operated all the time, as long as the fuel is fed to the system [19], making them a highly reliable energy option that can serve as a backup for variable characteristics in renewable energies and the limitation on battery systems by the charge/discharge characteristics. The abundance of hydrogen fuel is one critical factor that favors the energy generation capability of fuel cell technologies [7].
Several studies exist in the literature that examined the application of microgrid systems for electrification purposes based on solar, wind, hydro, and biomass. However, this paper pays attention to the application of FC technologies in microgrid systems. Therefore, reviewing current contributions on the energy-generation capacity of stationary FC systems was among the major goals of this paper. Relevant background on FCs was discussed, which provided an introduction of inherent features of the technology, developmental status, and the types that are recognized in the market and being researched by scientists in the energy research community [20]. A review was published on fuel cell systems with a power electronic interface, which focused on different FC technologies and their operating principles [21]. The study also examined the advantages, shortcomings, and possible application of the technologies for grid-connected systems in household, mobile, industrial, and commercial systems. Another research study also examined the potential of solid oxide FC systems for micro-power sources in portable devices [22].
A comparative study was conducted that discussed the different FC technologies [23]. The authors first presented the general working principle of the technologies and then compared them on the basis of fuel types, operational specifications, and technical characteristics. The concept of hydrogen and fuels was discussed with the intent of providing insights into hydrogen production methods, distribution, delivery, storage, and applications [24]. The paper also discussed the principle of hydrogen FCs and the stand-alone and co-generation applications. The study published in [25] presented the review of FC technologies that are engaged for built environment applications. It examined the potential of FCs for co-generation and tri-generation applications, including their maintenance and benefits such as emissions savings and reliable energy and heat generation.
The review on direct liquid FC technologies was published with major emphasis on the different types and their principles, applications, and problems affecting their commercial developments [26]. The use of hydrogen and FC systems for decarbonizing the energy system was discussed [27]. The authors presented a detailed review of the potential use of hydrogen in electrical power and heat production, including their applications in the industry, transport, and energy storage systems. A review of solid oxide FCs for electricity generation was also presented [28]. The applications discussed were extended to marine, transportation, and small-scale residential systems. An overview of FC technologies was discussed, focusing on the principle and thermodynamic analysis in FCs, including new perspectives and the irreversibility of the technologies [29]. A study discussed the applications and future outlook of hydrogen FCs [30], while the study in [31] considered the emerging FC systems and their applications. The application of FC technologies as an alternative to traditional energy production resources was presented [32].
The fundamental principles and use of FC systems was discussed [33]. The authors focused on providing a review of FCs, history, types, advantages and challenges, developments, and different applications. The state of the art, technologies, and market positions of hydrogen and FC systems was discussed [34], while an overview of the stationary FCs was presented in [35]. The theory and types of fuels engaged both for mobile and stationary applications were discussed [36]. A detailed overview of the direct carbon FC system was presented [37], while a handbook on FC technologies was also published [38], focusing on the introduction, applications, thermodynamics, and electrochemical kinetics, fueling problems, and FC cycles.
A review was presented on a hybrid energy configuration of solar-hydrogen and FC systems with the main emphasis on electricity production applications [39]. Another study also considered the review of FC technologies employed for mobile and stationary systems. This work examined the low- and high-temperature technologies, as well as their applications in transport and co-generation systems. The review of power-to-gas pilot plants was discussed with emphasis on the production of hydrogen from renewable electricity. The paper focused on the processes involved in converting excess electricity into hydrogen fuel, storing and reconverting the fuel into electricity when needed, using FC systems. A study also examined stationary FC technologies with insights into their commercialization. This work provided oversight of the current status of commercialization and also examined the key economic and market segmentation challenges. The review of the development trends of high-temperature solid oxide FCs was discussed [40]. The authors considered the technical and commercial status of this technology and those of direct carbon FCs.
A study was also conducted that concentrated on the history of FC systems [41], while the research study in [42] examined the life-cycle evaluation of the FC system’s components. The paper focused on FCs engaged for stationary and mobile applications, which were then compared to conventional energy systems on an environmental basis. A review of FC stack design was published with a particular interest in high-performance solid oxide FCs [43]; it also examined the development status and trends in high-performance solid oxide FC cell/stack models. Furthermore, the progress in battery storage, the FC system, and the hydrogen storage for clean energy systems were discussed in [44]. The authors also discussed the key problems associated with energy systems.
A recent paper was published that focused on the achievement of battery charge management for a hybrid energy system, which is based on solar PV, wind, and FCs with a battery storage system [45]. The authors recognized the fact that the charging process in batteries needs to be well coordinated using an adaptive control, a form of a nonlinear control system. An artificial intelligence (AI) technique was used in the paper, which also improved the performance of the battery-charging controller device. A comparison was done for various energy management strategies for minimizing the H2 utilization in a hybrid design based on FCs, ultracapacitors, and battery components [46]. The authors proposed two novel approaches referred to as the salp swarm algorithm (SSA) and mine-blast optimization (MBO), which were reported to perform better than the traditional strategies, like the fuzzy logic, proportional integral (PI) control, and the state machine, in terms of fuel consumption and system efficiency.
A sizing strategy was proposed for a hybrid power plant that was based on the FC design for weight minimization [47]. The idea put forward by the authors was that the reduction in the size of the power plant could also lead to a minimization of the power and energy requirements, and the enhancement of system’s techno-economic performance. A study was presented on the use of microbial FCs in bio-sensors [48]. The authors discussed the state-of-the-art review of the technology application, some of which included the monitoring of microbial activity, testing of biochemical O2 demand, detection of toxicants, and detection of microbial biofilms that are responsible for the bio-corrosion effect. A priority-based energy management strategy was discussed for a hybrid configuration of PV, FCs, and battery in the grid-independent direct current (DC) microgrid system [49]. The strategy was introduced by the author to coordinate the energy interchange between the mentioned components in the microgrid.
These existing studies stand as relevant background for this current paper. The papers considered several aspects of FCs with extensive scopes that can help in understanding the technologies as they are employed for stationary and mobile applications. However, this current study presents a comprehensive review of FC systems, with emphasis on the developmental statuses of the different types of technologies, technical and operational characteristics including their comparison, the prevailing techno-economic barriers to the technologies’ progress, and the future outlook, including their prospects and applications in microgrid systems. The relevance of microgrid systems is that they can provide ancillary service, thus supporting and strengthening the existing grid and are also useful for addressing the issue of energy poverty and shortage in off-grid locations.
The transition from the centralized to distributed generation system is occasioned by the need for energy security and sustainability. The possibility that the existing power grid is in one way or the other affected by an eventuality such as earthquake, hurricane, cyber-attack, pandemic, etc., justifies the necessity of microgrids. In such a situation, microgrids, whether operated in islanded mode or as an autonomous system, may be used to supply electricity to some parts of the area, region, or country affected [50,51,52]. Examples of the autonomous generation are roof-mounted, building-attached, or ground-mounted microgrid systems. These advantages motivated this paper.
The potential of FCs may be combined with those of renewable energy resources to achieve the desired sustainable energy system. When hydrogen is produced from renewable energies, it is said to be a renewable resource, which is then used to drive the operation of fuel cells [53]. Microgrids based on FCs can provide a reliable energy supply. Therefore, this paper derives its significance from the potential of FC technologies in microgrid systems, which is expected to provide useful insights into future directions toward advancing research in clean energy generation through microgrids based on FCs.
The remaining aspect of the paper is outlined as follows: Section 2 concentrates on the background and different kinds of FCs; Section 3 discusses the technical comparisons of the FC systems, possible configurations in microgrid applications, advantages, barriers, FC control mechanisms, and hybrid designs, including the impact of FCs in a microgrid system; Section 4 presents the developmental trends of the technologies and future research directions and outlook, while Section 5 concludes the paper.
2. Background on FCs
2.1. Historical Developments
The idea of FCs was discovered in 1839 by a scientist called William R. Grove [38,54]. This technical breakthrough was achieved while reversing water electrolysis to produce direct current (DC) output from hydrogen (H2) and oxygen (O2). The electrochemical process involved in FCs is essentially a reversed electrolysis reaction [23]. A fuel cell was initially called a gaseous voltaic battery (GVB) before it was eventually named “fuel cell”. The GVB technology employed platinum electrodes, sulfuric acid electrolyte, and the hydrogen and oxygen reactants. The platinum material in this arrangement was used to catalyze the reaction between H2 and O2. Several discoveries followed and the historical trends of FCs are summarized in Table 1, which emphasizes the technology, progress made, scientists or organizations, and the year of contribution. There were other achievements in the area of automotive applications, but this paper is focused on FCs for electrical power applications.

Table 1.
History of stationary FC developments.
2.2. Description of Fuel Cell Systems
FCs may be described as a kind of “electrochemical” device, which can offer a continuous conversion of chemical energy to electrical energy, while the thermal energy developed and the water formed in the process are the by-products [38,62,63,64,65]; the condition for continuous energy generation being the constant supply of the fuel and oxidant. FC technologies are made up of four major components, viz., cathode, anode, electrolyte, and the external circuit, as shown in Figure 1 [23].
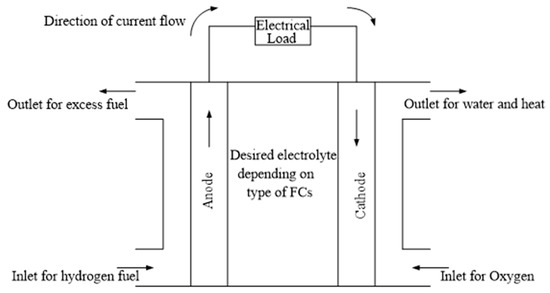
Figure 1.
Basic diagram of the FC.
The hydrogen fuel is oxidized into protons and electrons at the anode, while oxygen is reduced to oxides and the reaction to form water occurs at the cathode [23,56]. Besides, either protons or oxide ions travel via the electrolyte, while the electrons are transported by way of the external circuit to generate direct current (DC) as output. At the interface with the cathode, a reaction is created with oxygen which led to the formation of water and development of heat, owing to the exothermic process [35]. The excess H2 is fed back to the fuel tank through the outlet as shown in Figure 1, which may then be re-used by the FC.
The reactions are represented by Equations (1)–(3) [66,67]. The reaction at the anode, i.e., negative or hydrogen electrode, is described by Equation (1), while the reaction at the cathode or positive or oxygen electrode of the FC is represented by Equation (2). The overall reaction is described by Equation (3).
The quantity of direct current delivered by FCs is limited by a very small “contact” area among the electrodes, electrolyte, and the reactants. Another challenge is that of the distance between the cathode and anode of the FC. Due to this, a thin layer of electrolyte is integrated with flat “porous electrodes” to maximize the contact area and the efficiency of cells [56]. A single cell produces a low output of about 1 V; a series arrangement of some cells leads to the development of the FC “stack”, which can produce higher voltage output. The fuel cell power plant arrangement comprises the stack, balance of plant (BOP), inverter, and, if required, a fuel reformer may also be integrated [35]. The BOP refers to the parts of the FC system other than the generating component, just like balance of system (BOS) in the photovoltaic plant. The DC output of the FC stack may also be used to power DC appliances or load without the need for an inverter or employed to develop a DC microgrid [68].
The FC systems are similar to battery systems by their electrochemical characteristics and the process of generating electricity. They are also similar to engines by their capability to generate electricity continuously while consuming fuel. On the other hand, they differ from battery technologies by the fact that they do not require recharging, while they differ from engines by their quiet operation [32,64]. FCs operate efficiently and are emissions-free compared to battery technologies and engines; though some form of thermodynamic processes is associated with FCs, they differ from thermal engines by not being limited by the Carnot efficiency [38,62,69]. These features are of interest in modern energy planning, generation, and development, toward achieving decarbonized and sustainable energy future.
The FC technologies are usually categorized by the kind of electrolyte used in their working arrangements [25,70]. The classification is being done by the kind and the level of purity of the fuel and oxidant used, and the working temperature. There are different kinds of chemical reactions between the fuel (i.e., hydrogen) and oxidant (i.e., oxygen) according to the different kinds of FC systems. There exist six categories of FC technologies in the market such as proton exchange FC, alkaline FC, direct methanol FC, phosphoric acid FC, molten carbonate FC, and solid oxide FC [25,55,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. They are further classified as low, medium, and high temperature, and liquid FCs, which will be described in detail in subsequent subsections.
2.3. Different FC Technologies
2.3.1. Alkaline Fuel Cell Technology
The alkaline FCs employ the “alkaline” electrolyte, namely, potassium hydroxide (KOH), diluted in water and operate with pure hydrogen fuel, while pure oxygen serves as the oxidant [35,80]. In their operation, hydrogen fuel is supplied to the anode but oxygen is transported to the cathode. The exchange of ions is permitted between the cathode and the anode in the liquid KOH, which leads to the generation of direct current [80]. Materials such as potassium titanate, ceria, asbestos, and zirconium phosphate gel have been used as “microporous” separators in alkaline FCs [67]. However, asbestos is not used nowadays because of its toxicity and health hazard. Though early fuel cells of this type had their operating temperatures between 100 °C and 250 °C, operating temperatures for current devices are about 70 °C, which is why the alkaline FCs are categorized as a low-temperature technology [89]. Nickel (Ni) is commonly used as the catalyst in alkaline FCs instead of the traditional platinum material because of its low-temperature property. Ni and Ag (silver) are used as anode and cathode. Equations (4) and (5) describe the anode and cathode reactions in alkaline FCs [80].
One of the advantages of alkaline FCs is that they offer relatively high efficiencies of up to 0.6 in some of the applications. According to Alhassan et al. [90], a single alkaline FC can achieve a voltage output of 0.5 to 0.9 V with an efficiency as high as 0.65 but this depends on the system design. Furthermore, alkaline FCs can deliver electrical output in the range of 5–150 kW [80], and newer systems may even be operated at temperatures lower than 70 °C [35]. This implies that they can operate within a wider range of temperatures [80]. One of the disadvantages of the technology is that the electrolyte (KOH) is corrosive and, because of its liquid state, the sealing of the anode and cathode gases becomes a very difficult task compared to the use of a solid electrolyte [35]. Another challenge is that the KOH adsorbs the carbon dioxide, thus reducing the conduction power of the electrolyte; this is referred to as carbon dioxide “poisoning” of the electrolyte [80,91]. The design of alkaline FCs is classified into three categories, viz., mobile electrolyte, static electrolyte, and the dissolved fuel type, and are used in space and submarine applications [80].
2.3.2. Direct Methanol Fuel Cell Technology
The direct methanol FCs are a kind of low-temperature system that employs a fuel directly that is not hydrogen, unlike most other technologies [35]. These are usually classified as subcategory polymer electrolyte membrane FCs because of their similar design and internal configuration with operating temperatures between 60 and 130 °C [35,80]. However, the waste products resulting from the reaction of the direct methanol FCs include carbon dioxide, which differentiates them from the polymer electrolyte membrane (PEM) cell technology. A “polymer” membrane is used as an electrolyte in direct methanol FCs. Though they are a variant of the PEM, the catalyst on their negative electrode (i.e., anode) extracts hydrogen from the liquid methanol [35]. This development essentially eliminates the necessity for a “fuel reformer”. Thus, the pure methanol can be used as a fuel. The pure methanol in combination with the steam is fed into the direct methanol FC at the anode and the resulting reaction is such that the methanol is transformed into CO2 and hydrogen ions. The electrons then flow through the external circuit to generate the current, i.e., before flowing back to the cathode, in which case the protons are transported to the cathode through the electrolyte. Water is formed at the cathode by the reaction between the protons and electrons with oxygen. The anode and cathode reactions in direct methanol FCs are described by Equations (6) and (7) [80]:
With a typical operating temperature value of about 120 °C, direct methanol FCs could develop an efficiency of around 0.4 [35]. This low operating efficiency is due to “methanol crossover”, which is the cause of unproductive methanol consumption in the process [67]. The transformation of methanol to H2 and CO2 occurs at a low temperature and, as a result, a noble metal catalyst is required in direct methanol FC operational arrangements [80]; the anode and cathode materials are platinum or platinum-ruthenium (PtRu) and platinum (Pt), respectively, while perfluoro-sulfonic acid (Nafion membrane) is the electrolyte in direct methanol FCs. One advantage of the technology is that methanol is cost effective, easy to produce, and could be employed directly in the cell operation. This allows a simple cell structure and design with relatively low weights, which is why they are employed as a low-weight alternative to battery technologies for military and other applications. Another advantage is that it is easy to store methanol devoid of the risk of explosions as in the case of hydrogen fuel; the technology is apt for portable power for laptops and mobile appliances, including small plants that are less than 5 kW [67,80]. The major shortcoming of direct methanol FCs is that they have an efficiency of less than 0.4, which is considered the lowest of all FC technologies [80].
2.3.3. Molten Carbonate Fuel Cell Technology
The molten carbonate FCs, unlike the alkaline and the direct methanol FCs, is a kind of high-temperature technology, which uses a molten carbonate salt as an electrolyte [35]. The technology has a working temperature of about 650 °C and employs liquid carbonate salts such as lithium carbonate, potassium carbonate, and sodium carbonate in its technical operation [35,67,91,92]. The FCs have an electrical efficiency of about 0.6; however, they can achieve an efficiency of around 0.8 if the waste thermal energy is harnessed for co-generation purposes. When heated to 650 °C, the salts in molten carbonate FCs melt to form carbonate ions (CO32−) [80]. These ions migrate from the cathode to the anode and then combine with hydrogen to form water, CO2, and electrons [35]. The electrons developed are collected at the anode and made to travel to the cathode through an external circuit, thus generating direct current and heat [23,35,80]. To replenish or restore the electrolyte in molten carbonate FCs, oxygen and CO2 combine with electrons to produce carbonate ions [35]. The electrode materials in molten carbonate FCs are Ni-5Cr and NiO (Li), which are used for anode and cathode, and the reactions at these two electrodes are represented by Equations (8) and (9), respectively, and the overall reaction is described by Equation (10) [80]:
The molten carbonate FCs, through their high-temperature operation (between 600 and 800 °C), can improve “reaction kinetics”, which implies that they can achieve a high rate of electrode reactions without the need for platinum catalysts [67]. An external reformer is not also required in their structure to convert fuels like natural gas and biogas to hydrogen, meaning that they possess a capability to convert the methane gas and light hydrocarbons or petroleum products in the fuel to hydrogen in the cell arrangements through a process known as internal reforming [91]. These developments lead to cost reduction compared to low-temperature technologies such as alkaline, phosphoric acid, and proton exchange membrane FCs. Another advantage of the molten carbonate FCs is that they are less susceptible to CO poisoning compared to the low-temperature technologies [35], and it is possible to efficiently harness the reaction heat for producing more electrical energy [67]. The molten carbonate FCs are employed for co-generation in decentralized energy systems and could achieve electrical power output in the range of 0.1 to 2 megawatt (MW) [80]. The issue of durability is one major shortcoming of the molten carbonate FCs. This problem is attributed to the high temperature associated with the workings of the cells, which leads to a reduction of performance. The “Gibbs free energy”, for realizing the oxidation of hydrogen by oxygen, reduces as the operating temperature is increased, and the electrolyte initiates component degradation and corrosion in the process that eventually leads to a decrease in cell lifespan [35,67,80,91]. It is reported that 1.23 electron volt (eV), 1.06 eV, and 0.85 eV are achieved at 25 °C, 600 °C, and 1000 °C, respectively, illustrating the thermodynamic indices and performance of molten carbonate FCs with temperature [67].
2.3.4. Solid Oxide Fuel Cell Technology
The solid oxide FCs are part of the high-temperature technologies, which use nonporous “solid ceramic”, namely, zirconium oxide, stabilized with yttrium oxide (yttria-stabilized zirconia) as an electrolyte, under the operating temperature between 800 and 1000 °C [35,91]. In this technology, oxygen air is fed to the positive electrode, and the mobility of the oxygen ions from the positive to the negative electrode is being initiated by the nonporous solid ceramic; electrons then flow through an external circuit to generate current [35]. Water is formed through the reaction between oxygen ions and hydrogen at the negative electrode. The anode, cathode, and overall reactions in solid oxide FCs are represented by Equations (11)–(13), respectively [67,80]:
The solid oxide FC technology has classes of designs, namely, planar and tubular technologies, and can realize fuel-to-electrical efficiencies of >0.6 but could achieve an efficiency of 0.85 if the waste thermal energy is utilized for co-generation application [35,91]. Just as is the case of molten carbonate FCs, the high-temperature process in solid oxide FCs allows direct internal reforming that provides the opportunity of using some of the fuels that contain hydrogen such as natural gas, biogas, coal gas, propane, etc., and there is no need for a noble catalyst in their operational arrangements [80]. This way, the cost is reduced in the cell structure design. Solid oxide FCs are employed for co-generation in the aspect of distributed generation applications with power capacity in the range of 100 to 250 kW [80], and the electrode materials are nickel-yttria-stabilized zirconia (Ni–YSZ) and lanthanum strontium manganite (LSM), which are used for anode and cathode, respectively. However, the challenge of high-temperature corrosion is also prominent in this technology, and one way of mitigating such a problem is by using expensive protective layers and materials in the cell arrangements [35].
2.3.5. Phosphoric Acid Fuel Cells
The phosphoric acid FCs may be classified as a technology that falls between the low- and the high-temperature systems (medium-temperature), and they operate with liquid phosphoric acid (H3PO4) electrolyte and operating temperatures between 150 and 220 °C [35,67,80,93]. The electrolyte is essentially an acid in a Teflon-bonded SiC structure [91]. The positively charged hydrogen ions are transported to the cathode via the electrolyte. The electrons produced at the anode flow to the cathode through the external circuit, thus giving rise to direct current. Water is also formed through the reaction of electrons and hydrogen ions with oxygen at the cathode. The anode and cathode reactions in phosphoric acid FCs are described by Equations (14) and (15) [80]:
The electrode materials in phosphoric acid FCs are platinum or platinum-ruthenium and platinum for anode and cathode, respectively, and the fuel-to-electrical efficiencies between 0.35 and 0.40 could be achieved in this technology [35,80]. However, it is possible to realize the efficiency of about 0.85 when the technology is engaged for combined heat and power applications. The phosphoric acid FCs have a simple structure design and are less prone to carbon monoxide poisoning and electrolyte volatility and are engaged for small- to medium-sized plants between 50 kW and 11 MW [80], but one shortcoming is that their efficiency is found to be lower than those of other FC technologies and they are weighty [35,80,91]. Another disadvantage is that of an increase in system cost due to the requirements of integrating corrosion-resistant components to mitigate the effect of acid for the electrolyte.
2.3.6. Polymeric Electrolyte Membrane Fuel Cell Technology
The polymeric electrolyte membrane FCs are a low-temperature technology that uses a thin, permeable, polymetric membrane as a solid electrolyte and are essentially a part of the family of low-temperature systems [67,91]. The technology has a working temperature of around 80 °C with less warm-up time, and because its membrane is thin and very light and the need for catalysts, platinum materials, is employed on one or two sides of the permeable membrane [35]. The hydrogen ions are supplied at the anode of this technology, which is then broken into protons and electrons. The protons migrate to the cathode across the electrolyte, while the electrons are made to flow through the external circuit to generate direct current. Water is formed when oxygen air at the cathode reacts with the hydrogen ions [35]. The materials used as anode and cathode electrodes in polymeric electrolyte membrane FCs are platinum or platinum-ruthenium and platinum, and Equations (16) and (17) describe the reactions at the anode and cathode [80]:
The polymeric electrolyte membrane FCs can achieve a range of efficiencies between 0.4 and 0.6 and have a capability for varying the system output to balance load demand patterns. The technology can realize electrical power in the range of 5 to 250 kW with a tightly packed and lightweight structure and is suitable for space and military applications [35,80]. This technology has a high-power density; a value of >1000 W/kg was reported in the literature [36]. Because a solid electrolyte is used in this technology, it is easier to seal gases at the anode and cathode terminals, thus making the FC system cheaper to manufacture compared to some other technologies. Besides, the technology is less susceptible to corrosion, but in some instances, a low working temperature of 80 °C may not be sufficient to achieve useful combined heat and power (CHP) purposes, and a noble metal catalyst will be needed for separating the hydrogen protons and electrons [35,91]. The use of a platinum catalyst in this technology also increases costs.
2.4. Techno-Economic Comparison of Fuel Cell Technologies
The comparison of the FC technologies is shown in Table 2, while the merits and challenges of the different technologies are summarized in Table 3. The technical values presented in Table 2 are based on different applications that have been reported in the literature.

Table 2.
Comparison of evaluative metrics of the FC technologies.

Table 3.
Merits and challenges of fuel cell technologies.
3. Fuel Cells in Microgrid Systems
The application of microgrid technologies in electrical systems may be classified as grid-integrated and grid-independent systems. Whether the FC technologies are employed for grid-connected or off-grid purposes, it is necessary to establish the fact that an FC power plant is more the FC stack. This is due to the constant supply of reactants, i.e., fuel and oxidant, that is critical for achieving continuous production of electrical power [36]. Therefore, the nexus between FC stack and microgrid is of interest in this subsection. The fuel and the oxidant, for instance, need to meet a certain predetermined level of impurity before being employed to operate the FCs. As a result, the FC power plant is structured containing some components such as fuel processing, oxidant conditioning, electrolyte management, and heat energy management units, including the one employed for reaction product removal, etc. These components were illustrated and detailed with a schematic in [36], but the FC microgrid in this current paper is based on Figure 2, which is used for the purpose of illustration in line with fundamental electrical principles. Figure 2 illustrates the additional subsystems required for processing the energy from the FC stack to the users. The FC microgrids are capable of producing electrical power from as low as <1 W to hundreds of kW [111].
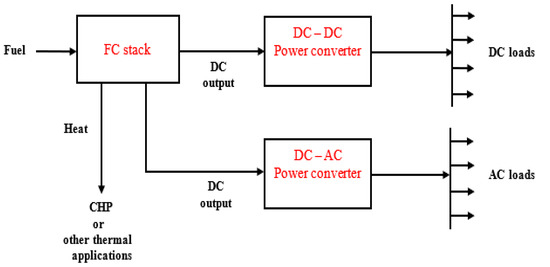
Figure 2.
Schematic of the FC microgrid system.
The FC stack produces direct current but most of the appliances within the residential, commercial, and industrial premises, for example, are alternating current (AC) powered. Therefore, a power conditioning unit, usually regarded as an inverter or otherwise called the DC-AC converter, is employed to convert the DC output of the FC stack to AC power. FCs also make use of DC–DC power converters in addition to DC–AC power converters for power conditioning purposes [112]. The inverter, being a developed power electronic device, has efficiency as high as around 0.96 for MW-rated power generation systems [36]. Also, the FC power unit generates waste heat. This thermal energy is usually coordinated by an arrangement of heat exchangers to the FCs to enhance the system’s efficiency and performance. Besides, some FC technologies make use of the waste heat as input for CHP application or bottoming cycles for additional generation of electric power [25,35]. One of the advantages of the CHP application is that it increases the efficiency of the FCs to as high as 0.85 or even more [36,80]. Another merit of the CHP is that the waste thermal energy may be harnessed for household use such as water and space heating, food processing, drying, and preservation, including raising of steam for industrial applications.
In FC systems, there are two types of fuels, which are the primary and the secondary fuels. The primary fuels include natural gas, methanol, low-sulfur extract, solid waste, naphtha, heavy oils, coal, biomass, etc., while the secondary fuels are hydrogen and carbon monoxide [36]. The conversion of primary to secondary fuels is necessary because the secondary fuels, hydrogen and/or carbon monoxide, are considered to be more electrochemically functional in the FCs’ arrangements than the primary fuels. Therefore, the role of the FC processor is to convert the primary to secondary fuels, and it usually accounts for about 33% of the plant’s weight and capital cost, especially the one based on hydrocarbon [36].
The output power, efficiency, water balance, heat utilization, quick start-up, long dormancy, size, weight, and fuel supply are specific requirements for operating and managing FC technologies [111]. However, realizing improved efficiency and minimized emissions are major market drivers for power and automobile applications. The expectation is that the FCs used in automobile systems are to have an operating lifespan between 3000 and 5000 h, i.e., less than one year, while those used for stationary applications have an operating lifespan between 40,000 and 80,000 h, i.e., about five to 10 years [111]. This is one of the factors that position FCs used for stationary purposes in a greater market and business opportunity.
The applications of a stationary system with emphasis on FC microgrid system include the following:
- Grid-connected [111,113]
- Grid-parallel [111]
- Stand-alone power [111,113]
- Emergency or backup power [97,111,114,115,116,117]
- DC microgrid [68]
3.1. Application of Fuel Cells in Microgrid Systems
3.1.1. Grid-Connected
The energy flow in this application is allowed in three different paths such as from the electrical grid or network to the users’ load, from the FC microgrid to the users’ load, and from the FC microgrid to the electrical grid [118,119]. By using a “load-following” strategy, the microgrid system may be designed as a constant energy source to meet the users’ maximum electricity consumption. In this case, the excess electricity production from the microgrid system may be exported to the electrical grid. Two sources of energy supply are available to the users in this scenario, i.e., electrical grid and FC microgrid. The economic benefit of exporting the surplus energy from the FC microgrid system to the electrical grid is expected to yield a reduction in the electricity bill from the utility [120].
A net metering system is also expected to be part of the electrical system for evaluation purposes. This design requires standard codes such as the Institute of Electrical and Electronics Engineers (IEEE) 1547 standards for integrating distributed generation systems [121]. A grid-tie hybrid power system based on FCs and microturbine or a parallel arrangement of three separate FC units is presented in [122], which was modeled to manage the thermal and electrical loads in a residential premise where the thermal load is being served by the gas supply and the waste heat from the FCs.
However, the schematic of a grid-connected application of FCs is shown in Figure 3, where the heavy-current load is being served by the heat from the FCs and the electrical supply, which is different from the idea presented by the authors in [122]. The heavy-current load such as electric cookers, water heaters or boiling rings, space heaters, and oven may be operated by the electrical supply. Energy meter M is employed to quantify the energy purchased from and sold to the power grid. The microgrid system in Figure 3 was designed with the aim of satisfying the total load requirement in a typical household by the fuel cell system and the grid. The total load includes the heavy-current load and the other loads such as the inductive load, TVs, lighting, DVD player, etc. It is a conventional practice to have an interface between the electrical arrangement and the grid, which depends on the capacity and configuration. The interface could be a power converter for a domestic application or transformer for heavy applications.
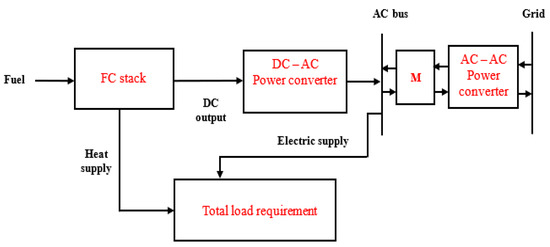
Figure 3.
Grid-integrated application of FC power for residential loads.
3.1.2. Grid-Parallel
In this application, energy may be purchased from the electrical grid to meet the users’ load when required; however, the FC microgrid is not allowed to export any excess energy to the electrical grid [111]. This configuration also implies that two energy sources are available to the users, which are the existing electrical grid and the FC microgrid systems. Therefore, the energy flow is allowed in two different directions. The microgrid could be designed to balance the users’ demand, while energy could be imported from the existing grid in cases where there is an increase in users’ demand. An increase in demand is expected as the users can climb the energy ladder [123]. Since there is no energy flow from the FC microgrid to the existing power grid, the interconnection codes are not required. Therefore, Figure 4 represents the grid-parallel application. Also, a battery system (auxiliary power source) is not included in the arrangement as it has been replaced by the grid, which is also able to provide the start-up requirements [111].
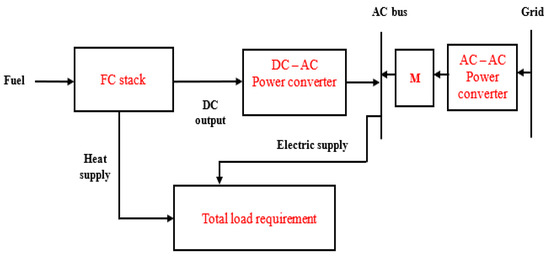
Figure 4.
Grid-parallel application of FC power for residential loads.
3.1.3. Stand-Alone Power
In this application, there is no interaction between the users’ load and the grid, unlike the grid-tied and grid-parallel modes, nor is there any electrical connection between the FC microgrid system and the existing power grid [124]. Therefore, energy flow is only available from the on-site power system to the load. The on-site power system could be a single-source, stand-alone power system that is entirely based on FC technology or a hybrid system that is based on the combination of FCs and other technologies like solar PV, biomass, battery, diesel generator, microturbines, etc. A conceptual remote hybrid electrification system was described in [125]. However, this paper designed Figure 5, Figure 6 and Figure 7 for illustrating different configurations of FC-based, stand-alone power systems.
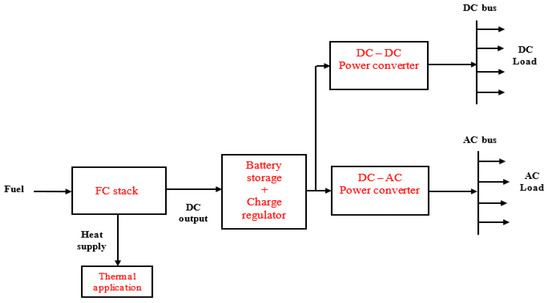
Figure 5.
Stand-alone application based on FC power source.
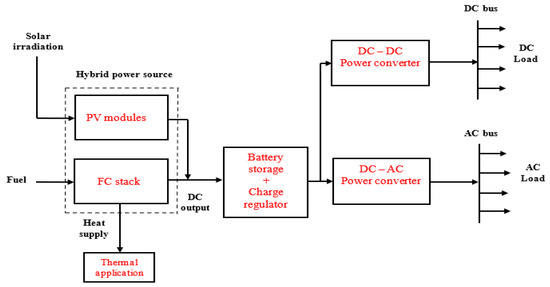
Figure 6.
Stand-alone application based on the FC and PV power sources.
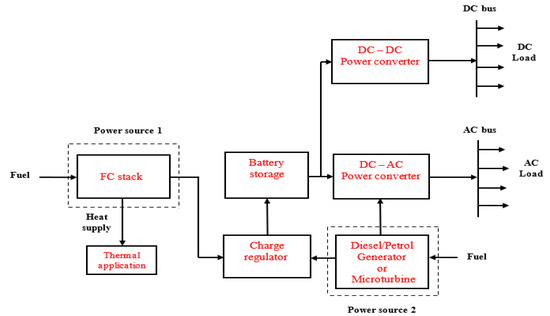
Figure 7.
Stand-alone application based on FCs and fossil-fueled power sources.
Figure 5 shows a single-source system based on FCs, while Figure 6 and Figure 7 illustrate hybrid systems of FCs + PV and FCs + diesel generator. A microturbine generator may also be used instead of diesel generator in Figure 7, which implies that power sources 1 and 2 could be served from a single primary fuel—natural gas. Furthermore, a “load-following” approach is usually employed in stand-alone power systems, which is why battery storage is integrated with the energy generation arrangement to respond to rapid variations in users’ load demand at some point in the system operation [111,126].
The stand-alone power has to have the technical capability to satisfy the start-up requirements of inductive loads such as water pumps, motors, and fans, for instance. This implies that the FC microgrid (FCs + battery storage) must be modeled to meet maximum continuous load demand for reliability purposes. In the system presented in Figure 5, the FC stack can provide the charging current for the battery bank at times when there is low energy demand by the users. However, the users will experience loss of energy supply if the FC stack is faulty or not available to supply power. This problem may be addressed by using the complementary characteristics of the hybrid systems, shown in Figure 6 and Figure 7, as the users will benefit from the hybrid energy generation resources. In this case, some form of energy management strategy is integrated with the power electronic converters to control the energy interchange between the power sources and the battery storage system.
3.1.4. Emergency or Backup Power
In the emergency application, the microgrid system must not only possess the technical capability to meet a fast start-up but also be connected with a battery bank or another peaking plant [111]. The battery bank can offer a low backup power for a short duration (usually seconds to a few minutes); however, the FC stack can provide higher backup power in several kW with a longer duration of >30 min [111,118].
Both the electrolyzer and the hydrogen tank can be integrated with the FC microgrid used in backup power mode. The significance of this is that the system will be capable of producing its fuel (i.e., H2) at those periods when electricity is imported from the grid [111]. The electrolyzer consumes electricity to produce hydrogen by the process of electrolysis of water, while the purpose of the hydrogen tank is the storage of hydrogen generated by the electrolyzer for operating a hydrogen-fueled generator [127]. Such a system that makes use of an electrolyzer is known as the regenerative FC. An example of backup power is the uninterruptible power supply (UPS), which can supply power for 30 to 60 min, and it finds applications in telecom, Information and Communications Technology (ICT), manufacturing, security, utility substations, and traction (i.e., railway) systems [111].
3.1.5. DC Microgrid Application
The DC generated by the FC stack may be used to power DC appliances, without the need for an inverter, or employed for developing a direct current (DC) microgrid. Figure 8 illustrates the DC microgrid with a connection with the existing power grid [68]. The DC microgrid is composed of a DC voltage source, i.e., an FC stack, which is connected to the source DC-DC converter for achieving a higher or lower voltage output depending on the design requirements. A load DC-DC converter is required, as shown in Figure 8, for interfacing the load with the DC bus, which is used to bring the bus voltage to the value required by the load or appliance. The common voltage levels that have been employed for telecom systems are 24 V and 48 V [68,128,129]. It is necessary to provide an interface between the existing power grid and the DC microgrid; this is achieved by using the bidirectional AC-DC converter [130]. Through this it is possible to sell back the excess electricity from the DC microgrid to the existing power grid, as well as purchase energy from the power grid when the output of the microgrid system is lower than the load demand [131]. The gateway unit requires an AC power input as it is meant to provide a bidirectional link between the microgrid and the existing grid or other generation sources in the arrangement.
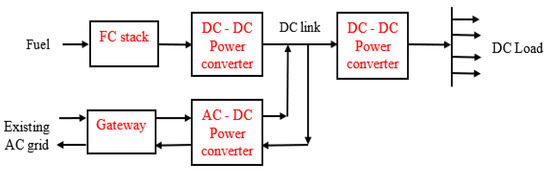
Figure 8.
Schematic of a DC microgrid based on FC.
3.2. Comparison of Fuel Cell Microgrid Applications
FC systems may be employed for on-grid and off-grid purposes in situations where the microgrid system is co-located with the load. Suppose that energy supply is being provided mainly by the FC stack, as shown in Figure 3. Then the microgrid can potentially compete with or serve as an alternative to the grid supply or provide electricity for grid-independent applications in isolated communities. Furthermore, operating the FC microgrid in parallel with the existing grid could provide the opportunity for meeting peak load or baseload requirements since continuous energy generation is a property of the energy source.
The system may be operated in combination with variable energy generation systems, like the solar photovoltaic plant, wind generator, or biomass power system, to produce electricity at periods when these systems are not available to power the load, as illustrated in Figure 5. The system may also be employed as an emergency energy generator in a situation whereby the existing grid is down or the other source of electricity is unavailable. Table 4 presents the comparison of the FC microgrid applications, while the applications of FCs based on rated power output are presented in Table 5.

Table 4.
Comparison of the FC microgrid applications [68,111,118,119,121,124].

Table 5.
Applications of FC systems based on rated power output.
3.3. Other Fuel Cell Technologies
3.3.1. Regenerative Fuel Cell Technology
The regenerative FCs possess a dual operating mode. That is, they may be operated as an electrolyzer and alternately as FCs, thus providing the opportunity to operate either in the electrolysis or the FC mode, respectively [111,132]. The electrolyzer consumes electrical energy to generate H2 water electrolysis; the hydrogen that is produced is then fed to the hydrogen storage tank for operating a hydrogen-fueled generator [127]. The electrolyzer and the FC systems may be integrated to form a single cell stack; the regenerative FC may also employ two separate cell stacks where one is being used as the FC while the other is used as an electrolyzer [111]. While the single-cell stack is referred to as a unitized system, the two separate cell stacks are regarded as a discrete system [132]. Figure 9 illustrates a microgrid based on solar PV modules and regenerative FCs with master and slave application of the FCs. Such a configuration is based on a dedicated controller for fuel supply and timing of operation for the two FCs.
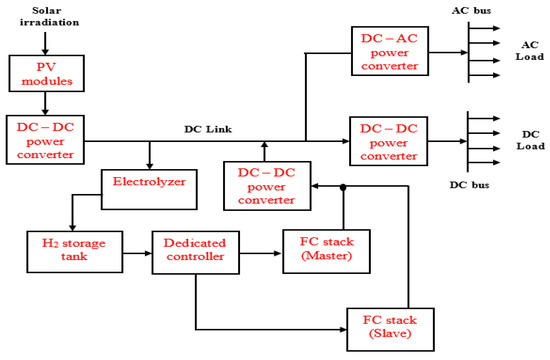
Figure 9.
Microgrid system based on solar PV and regenerative FC.
The cell stack produces H2 and oxygen from electrical energy in the electrolysis mode. However, it produces electrical energy from the stored H2 (and O2 that may be obtained from the air) in the fuel cell mode [111]. By this characteristic (similar to a secondary battery), regenerative FC is an energy storage system that utilizes H2 as the storage medium. It possesses the highest specific energy that may be reached of all rechargeable energy storage technologies. For instance, specific energy of 400 to 1000 Wh kg−1 (watt hour per kilogram) may be achieved for a practical system [111,133].
The technology may be used in applications where it is required to store a large quantity of energy such as the following [133]:
- Employed as an energy storage system for grid-independent electrification systems.
- Used in combination with variable power sources such as solar and wind.
- Used as emergency power.
- Employed in marine systems, such as “unmanned” underwater vehicle.
- Used in spacecraft as hybrid energy storage or propulsion systems.
- Used as solar rechargeable aircraft for the high-altitude, long-endurance purpose.
The diagram illustrating the use of a regenerative FC system as storage in a solar photovoltaic microgrid was published in [133]. The overall specific energy of regenerative H2/O2 FCs that ranges from 400 to 1000 Wh/kg is a multiple of the value achieved for any battery system [111]. For instance, specific energy values of 100–300 Wh/kg [134,135], 30–50 Wh/kg [69,118], 100–200 Wh/kg [69], and 10–50 Wh/kg [95] for lithium-ion, lead-acid, sodium-nickel chloride also known as ZEBRA, and vanadium redox battery (VRB), respectively. These, including the fact that regenerative FCs are independent of hydrogen infrastructure like the other FC technologies, are important factors that attract their use in the mentioned applications.
However, certain trade-offs are usually considered for planning and designing regenerative FCs. These include the choice between oxygen and air feed, single-stack and/or discrete systems, feed, and the choice of working pressure [111,133]. Each of the mentioned trade-offs may be weighed on different grounds, but the economic factor, efficiency, and the duty cycles are usually the driving factors for selecting an oxidant for a particular purpose, for instance.
3.3.2. Biofuel Cell Technology
Biofuel cells (BFCs) are a type of low-temperature technology in which “biological catalytic” reactions are used to produce DC from the electrolysis of reactants (fuel and oxidant), unlike in conventional low-temperature FCs where metal catalysts are used [136]. BFCs can use fuels like H2, methanol, and organic materials to generate electricity. They are categorized into those systems that use bacteria and algae (living cells) and those that use enzymes and mitochondria-catalysts extracted from cells (biological catalysts). The operation of enzymatic FCs involves the utilization of enzymes to realize fuel and oxidant oxidation at the anode and cathode, respectively, at neutral pH and ambient temperature considered to be favorable compared to conventional systems. Besides, unlike the traditional FCs, there is no need for separator and compartments in enzymatic FCs, which is why they can be designed as miniature cells and membrane-less systems in µm scale [136,137,138]. The output of such a technology may be useful for operating electronic devices such as sensors, etc.
3.3.3. Hybrid Systems and Control Mechanisms
It is proven that FCs find applications in the grid and off-grid systems. Using the FC microgrid as a single-source power system in connection with the existing grid implies that electricity may be exported from the grid when the microgrid output is not enough to satisfy the load requirements. Such an electrical arrangement benefits from the complementary characteristics of the two sources of energy–the FCs and the grid. However, certain limitations exist when technology, such as the solid oxide FCs, for instance, is employed as the only power source in grid-independent applications. These are reflected in the degradation of the system’s life span, especially when the operation is dynamic, and the high capital cost, which affects the proliferation of FC systems [139]. The degradation of FCs’ lifetime will unavoidably affect their durability. These are among the notable factors that gave a hybrid system an edge over a single-source system in stationary applications. Then, it is of interest to put in place some control strategies to coordinate the operation of microgrid systems that are based on multiple energy systems. [140]
A recent study considered a CHP system that is based on solid oxide and proton exchange membrane FCs integrated with a hybrid storage configuration for off-grid purposes [139]. The authors proposed the hybrid CHP solutions that seek to overcome the mentioned limitations, in which case the solid oxide FCs are used to satisfy the system’s baseload, while the production and storage of pure H2 from the solid oxide FC anode off-gas was achieved by integrating a purification unit with the system arrangements. The stored H2 may then be employed for driving proton exchange membrane FCs when the electricity demand peaks. The main idea of the research was the application of a hybrid energy storage system, the battery bank and the H2 in this case, to reduce peak electricity demands. This increased the solid oxide FC’s average “load” and also reduced the installed size, leading to a nearly constant load operation of the solid oxide FCs and improved durability.
The load-following capability of a hybrid energy system (HES) has been discussed recently for microgrid operation, which is based on FCs and microturbine [141]. The authors identified a major limitation of the microgrids that are entirely based on FCs, which is the lack of load-following capability. This shortcoming has been attributed to the slow “electrochemical” and “thermodynamic” reactions associated with the internal operational characteristics of the FCs. Proton exchange membrane FC system, for instance, is one technology that demonstrates appreciable potential when employed in microgrids, but it requires the integration of energy storage such as the battery or the supercapacitor to realize a rapid electrical load following [142]. The control mechanism is such that the storage system responds to the transient load, while the FCs responds to the steady-state load [141].
However, relatively low life span and high capital costs are part of the shortcomings of battery and ultracapacitor technologies engaged for load following, just like the limitations earlier mentioned for FCs used for single-source microgrid systems. In light of this, a microturbine (microT) may also be integrated with the FCs to satisfy both the steady-state and the transient requirements of the load both in on-grid and off-grid applications [141]. The microT, though similar to a conventional gas turbine, possesses a quick response because of its favorable compact design, which allows a faster rate of flow of gas. These properties make the microturbine a suitable option for achieving load-following capability in FC microgrid systems and the authors employed a conventional loop gain control strategy for the microgrid operation.
The application of FCs and heterogeneous energy storage systems was recently discussed for the energy management of data centers [143]. The authors identified the “limited” load-following capability of FCs. This implies that the FC technology is slow in responding to a rapid increase in load demand due to a limitation placed on fuel delivery. The technology also has a delay in responding to and adjusting electrical power to a decrease in load, thus wasting energy. The combination of multiple batteries with FCs has been was introduced in the work to address the issue of limited load-following capability by providing electricity supply when the output of the FCs is less than the load requirement and employing the excess energy of the FCs to charge the batteries when the demand is low. An online algorithm was proposed by the authors, which was based on the principle of classical control policy (ccP) for dynamic capacity consideration in the specified datacenters.
The dynamic power and cooling generation have been achieved for household purposes by integrating a solid oxide FC with an organic Rankine cycle (oRc) and absorption chiller systems [144]. The authors proposed a dynamic, physical model that integrates the solid oxide FCs, oRc, and the absorption chiller to simulate the residential dynamic loads. It was reported that the hybrid design incorporating dynamic models of oRc and absorption chiller provided a capability to follow the residential dynamic load and the use of the solid oxide FC dynamic waste heat to generate additional electrical power or cooling for the households. The idea of such a study was to explore the high thermal property of the solid oxide FCs in conjunction with bottoming cycles (as illustrated in Figure 3) to produce extra electrical energy, thermal energy, or cooling for enhancing the system’s efficiency compared to when an individual system, solid oxide FCs, or oRc and absorption chiller, is operated in a stand-alone configuration.
The performance analysis of a co-generation system based on proton exchange membrane FCs and the lithium-ion battery system was achieved, using a typical residential premise in North China as a case study [145]. The authors recognized the good dynamic performance and the inherent charge/discharge properties of lithium-ion battery technology as a means to enhance the dynamic response when integrated with the CHP system, especially in a situation where the electrical generation output does not match the load. The proton exchange membrane FCs were employed as the prime mover in the study, and the entire system design had a coordinated thermal and electrical load-following strategy. It was reported that the hybrid design incorporating the lithium-ion battery produced an efficiency of 81.24% compared to the configuration without the battery that produced an efficiency of 70.22%.
The hybrid energy management (HEM) system model was presented, which was based on proton exchange membrane FCs, solar PV, and wind generator. [146]. The authors proposed a novel adaptive control strategy for coordinating the energy flow or interchange in stand-alone microgrid systems. The HEM system coordinates the hybrid energy sources (HES) and the storage device is based on the artificial neural network control (ANNc) and fuzzy logic control (fLC) mechanisms. Besides, the ANNc is used to attain the maximum power point (MPPT) of the solar PV modules, while the fLC is employed for energy sharing between the HES and the charge/discharge characteristics for optimizing the overall performance. The fLC is also adopted in the study to enhance the energy-generating performance of the FCs and its lifespan by controlling the FC stack temperature.
A direct hybrid design of polymer exchange membrane surface FC was achieved with small aqueous ultracapacitors [147]. It was already established that the proton exchange membrane FCs possess a high-power density, but this property may be degraded by the size and the weight of the associated supports or ancillaries required to manage and control the flow rates of air, H2, and the humidity. A hybrid design based on batteries or ultracapacitors was introduced by several researchers to overcome the challenges of limitation of fuel cell dynamics by fuel supply mechanism consisting of the compressor, flow control device, and/or the humidifiers.
However, the authors posited that the FC system’s performance enhancement offered by a hybrid design with ultracapacitors raises a technical question about the best configurations and control approaches. In that way, it was desirable not to implement hybrid designs at the expense of the FC’s durability and reliable operation. Though trade-offs may be entertained in certain situations, the system complexities and capital cost also need to be rationally moderate. In most existing studies, for power management, the FCs/ultracapacitor hybrid systems usually include one or two DC-to-DC power electronic converters. It is also possible to have a hybrid design that directly connects the FCs with the ultracapacitors without the need for a power management mechanism and its associated power converters. Such design is referred to as passive hybrid design, and how this may be employed to enhance system performance and reliability with steep load demand variations was the idea proposed by the authors in [147].
The load-following capability of solid oxide FCs was achieved by using a time delay control mechanism [148]. The authors also identified the limitation of load following, which affects the solid oxide FC system’s commercialization. In light of this, they introduced a time delay control with an observer in the gas supply system to improve the FC’s dynamics–in terms of load following without curtailing or blocking the fuel supply. The initial approach of a time delay with an observer was enhanced by integrating a filter to forestall the unwanted effects, such as fuel disruptions, i.e., external disturbances, on the operation of the FCs. A 5-kW solid oxide FC was developed in the work as a test case and it was reported that the proposed control approach provided good dynamic performance when applied to the fuel supply part of the system design.
The optimal fault-tolerant control approach has been introduced in the operation of solid oxide FCs [149]. The authors recognized that certain critical issues such as load tracking, thermal management, air excess ratio constraint, high efficiency, low capital cost, and fault detection are key factors required for developing solid oxide FCs. It was also found that there are little or no research studies focused on the control strategies that are based on optimization and fault diagnosis in solid oxide FCs. It was reported that the proposed control strategy can track the electrical, operating temperature, and the air excess ratio, realizing optimum efficiency, and cost under normal and abnormal (air compressor fault, for instance) conditions.
The energy management of a HES has been achieved based on the tidal turbine and hydrogen microgrid system [150]. The idea of the work is to use an EM strategy to convert the energy produced by the tidal energy converter to H2 efficiently and ensuring the operation of the system components, and a reduction of losses in the system. Two approaches that are based on the maximum torque/ampere and the loss minimization were introduced to control the tidal generator to quantify the surplus H2 that may be generated.
The hierarchical energy management system was proposed for the hybrid design of solar PV, H2, and battery island, direct current microgrid systems [151]. The authors first presented some of the advantages of DC microgrids such as the fact that they are not being affected by issues of power quality, synchronization, and the reactive power flow, which are common challenges of the AC microgrid design. These were part of the factors that motivated a strategy that was introduced to enhance the performance of the electrical system in terms of cost and robustness. The energy management system consists of the local control (LC) and the system control (SC) layers. In the LC layer, the components in the DC microgrid system are managed and controlled based on their working characteristics and features, while the strategy for minimizing the associated fuel utilization is realized in the SC layer, including the energy interchange between the FC and battery.
A two-level energy management (EM) strategy was also realized for a DC microgrid system based on solar PV, FCs, and battery systems [152]. The authors mentioned the shortcoming of the conventional, distributed-control technique, which is its limitation in satisfying the energy management requirements for operating the multiple-source DC microgrid system. A two-level EM approach was then proposed, which was similar to the idea presented in [151]. The strategy is segregated into two, viz., the device control (dC) and system control (SC), levels. At the (dC) level, the maximum power point-droop, dual-mode control, and the droop control were introduced to improve the reliability of the microgrid system, while the power flow between the FCs and the battery was managed at the (SC) level. The authors posited that the proposed two-level EM approach can achieve lower H2 fuel consumption compared to the traditional PI control and the state machine control mechanisms.
3.3.4. Impact of Employing FCs in Microgrids
The microgrid, a low voltage (LV) network, can integrate distributed energy resources and different kinds of loads, which may be operated in grid-integrated or grid-independent modes [153]. Distributed energy resources include solar, wind, small hydro, biomass, fuel cells, battery, etc. The output of the microgrid system is usually affected by the behavior of the energy source(s) it contains. In a practical microgrid, the FC technology is affected by the fuel supply, for instance, and it also requires the addition of a storage system. The utilization of the FCs in conjunction with the battery and/or other sources of energy requires some form of control mechanism. This and the impact of utilizing FCs in microgrids have been detailed in the preceding section based on recent scholarly works.
4. Fuel Cells: Status and Future Outlook
4.1. Developmental Status
The direct methanol FCs are in the early phase of technological developments and, as such, they are underdeveloped, unlike the other systems [36], Phosphoric acid FCs are, and are regarded as the first-generation fuel cells that are technically matured and are considered to be ready for commercialization. Polymeric electrolyte membrane FCs are likely to be in the commercial phase in the next five to 10 years. Molten carbonate FCs are referred to as second-generation FC systems that have reached the early demonstration phase with the possibility of attaining the commercial status after the phosphoric acid FCs within the next five to less than 10 years. Solid oxide FCs are regarded as the third-generation FC systems, as their attainment of the commercial status is expected to be after the phosphoric acid and molten carbonate FC technologies, and they are in the developmental phase and are also likely to be commercialized within the next 5 to 10 years.
4.2. Future Research Direction and Outlook
This study reviewed the FC technologies. It has also paid attention to the exciting potentials and applications of these technologies in microgrid-based power systems both in grid-tied and off-grid configurations. However, the future research study will consider the design and comprehensive analysis of FC-based microgrids for remote communities. This will then be compared with the authors’ previous work on PV microgrids for remote communities, to compare the technical and economic performance of FC microgrids and solar microgrids.
The finite nature of fossil fuels and the environmental issues associated with their power generation systems are major factors that led to the efforts by the global community to adopt energy resources that are eco-friendly and sustainable. One of the products of these efforts is the utilization of renewable energy resources, solar, wind, biomass, etc., which are already deployed in on-grid and off-grid microgrid systems around the globe. Batteries are popular storage technologies used to mitigate the effect of intermittencies in renewable energies [118].
However, FCs have a greater capability for power applications compared to battery technologies, as they can also provide the ancillary services [154,155,156,157] such as voltage and frequency regulation, power quality support, etc. The capacity of FCs to deliver continuous power makes them a suitable source of power generation and emergency/backup supply. For instance, in energy management systems, FCs can provide backup for a longer period than batteries [111]. They can provide constant power supply, unlike renewable energy sources that have variable characteristics. They are environmentally friendly, unlike a constant power source such as the internal combustion engines that produce noise and emissions [38]. FC systems have higher specific energy compared to batteries.
Based on the mentioned factors, there is the possibility that electrical power systems will experience a wider application of FCs within the next five to 10 years, as they have the technical characteristics and advantages to compete with other energy resources. This is also dependent on the market dynamics and developments in R&D that can optimize performance and cost. This is because high capital cost is one factor that limits the proliferation of the FC power system in the market. With this in mind, FC systems have to be cost effective in terms of capital and installation costs to compete with the conventional electrical power production systems [80]. In addition, intensive research is required to enhance the lifespan of FCs and also minimize the complexities in fuel processing and system operation.
5. Conclusions
This paper presented a detailed review of FC technologies such as proton exchange membrane, alkaline, direct methanol, phosphoric acid, molten carbonate, and solid oxide FCs, which are engaged for stationary applications. The molten carbonate and the solid oxide FCs are categorized as high-temperature systems, while alkaline, direct methanol, proton exchange membrane, and phosphoric acid FCs are classified as low-temperature technologies.
The paper also discussed other technologies like the regenerative FCs and the biofuel cells. The regenerative FCs find applications in energy storage of grid-independent electrifications, in combination with variable power sources, emergency power or backup systems, marine, spacecraft, and aircraft systems, while biofuel cells such as enzymatic FCs are used in sensors and electronic systems. The paper also concentrated on the comparison of operational characteristics of the FCs based on technical and economic metrics such as operating temperature, efficiency, specific energy, specific power, energy density, power density, energy cost, and power cost. Generally, the specific energy of regenerative FCs of 400–1000 Wh/kg is very much higher than the values of those of rechargeable batteries based on the literature.
The study finds that proton exchange membrane FCs have a relatively high specific power with a value of >1000 W/kg compared to solid oxide FCs that have a value of 1.05–1.67 W/kg and other systems. The proton exchange membrane, solid oxide, phosphoric acid, alkaline, and molten carbonate FCs have efficiencies of 35–45%, 50–70%, 37–45%, 35–60%, >50%, and 10–40%, respectively, but direct methanol FCs have the lowest efficiency of all the technologies with a value of around 35%, based on the literature. Besides, solid oxide and molten carbonate FCs being high-temperature technologies have a working temperature range of 800–1000 °C and 600–800 °C, respectively, compared to proton exchange membrane, phosphoric acid, alkaline, and direct methanol FCs that have a working temperature of 50–100 °C, 150–220 °C, 60–120 °C, and 70–100 °C.
The specific energy of proton exchange membrane, solid oxide, molten carbonate, and direct methanol FCs are in the range of 100–450, 410–1520, 369–607, and 140.3–960 Wh/kg. The power cost of PEMFCs, SOFCs, PAFCs, AFCs, MCFCs and DMFCs are up to $10,200/kW, up to $1500/kW, about $3000/kW, >$200/kW, $2000–4000/kW, and $15,000–125,000/kW, respectively, which is higher than the cost of most of the other power-generation technologies. The power density of proton exchange membrane, solid oxide, molten carbonate, and direct methanol FCs is in the range of 4.20 and above, 4.20–19.25, 1.05–1.67, and 1.00–300 kW/m3, respectively. The energy density of proton exchange membrane, solid oxide, molten carbonate, and direct methanol FCs is in the range of 112.2–770 kWh/m3, 172–462.09 kWh/m3, 25–40 kWh/m3, and 29.9–274 kWh/m3. Furthermore, the comparative assessments reveal that proton exchange membrane, solid oxide, phosphoric acid, alkaline, molten carbonate, and direct methanol FCs are in the range of up to 3000, 1000, >50,000, 8000, 7000–8000 and 1000 h, respectively, as obtained from the literature.
Besides, particular attention was paid to different technical applications of the FC technologies such as grid-integrated, grid-parallel, emergency or standby power, stand-alone power, and DC microgrid configurations, which are of interest both in the on-grid and off-grid microgrid systems. The study also considered the FC control mechanisms and hybrid designs, and the technical challenges posed when employing FCs in microgrids based on recent developments.
Furthermore, the advantages of the various technologies were presented including the technical barriers associated with the mode of operation of each system. The study also discussed the developmental trends of different technologies. The direct methanol FCs are in the early phase of technological developments and, as such, they are not as developed as other systems. Phosphoric acid FCs are technically matured systems that are ready for commercialization and they are regarded as the first-generation FCs, while the proton exchange methanol FCs are likely to be in the commercial phase in the next five to 10 years. Molten carbonate FCs are referred to as second-generation systems that have reached the early demonstration phase, with their developments and possibility of attaining the commercial status after the phosphoric acid FCs within the next 5 to less than 10 years. Solid oxide FCs are regarded as the third-generation systems as their attainment of the commercial status is expected to be after the phosphoric acid and the molten carbonate FCs, and they are in the developmental phase and are also likely to be commercialized within the next five to 10 years.
Based on the mentioned factors, there is the possibility that electrical power systems will experience a wider application of FCs within the next five to 10 years as they have the technical characteristics and advantages to compete with other energy resources. This is also dependent on the market dynamics and developments in R&D that can optimize performance and cost. This is because high capital cost is one factor that limits the proliferation of the FC power system in the market. With this in mind, FC systems have to be cost effective in terms of capital and installation costs to compete with the conventional electrical power production system. Also, intensive research is required to enhance the lifespan of FCs and also minimize the complexities in fuel processing and system operation and ensuring the safety of users given the volatility nature of hydrogen.
The paper demonstrated interests in the application of FCs in microgrid systems based on some attractive features such as being clean, pollution-free, highly efficient, and flexible and promising energy resource for microgrid applications that need more attention in research and development terms. Furthermore, they can offer continuous operation and do not require recharging. Since microgrid systems can help to strengthen the existing power grid and are suitable for solving the problem of energy poverty or shortage in remote locations, the research paper is expected to provide useful insights into advancing research and developments in clean energy generation through microgrid systems based on FC technologies.
Funding
This work was carried out with the aid of a grant from the International Development Research Centre, Ottawa, Canada, www.idrc.ca, and with financial support from the Government of Canada, provided through Global Affairs Canada (GAC), www.international.gc.ca under the framework of the Mathematical Sciences for Climate Change Resilience (MS4CR) program administered by AIMS-NEI, www.nexteinstein.org.
Acknowledgments
This work was carried out with the aid of a grant from the International Development Research Centre, Ottawa, Canada, www.idrc.ca, and with financial support from the Government of Canada, provided through Global Affairs Canada (GAC), www.international.gc.ca under the framework of the Mathematical Sciences for Climate Change Resilience (MS4CR) program administered by AIMS-NEI, www.nexteinstein.org.
Conflicts of Interest
The authors declare that there is no conflict of interest on this research study.
References
- Vezzoli, C.; Ceschin, F.; Osanjo, L.; M’Rithaa, M.K.; Moalosi, R.; Nakazibwe, V.; Diehl, J.C. Designing Sustainable Energy for All. Sustainable Product-Service System Design Applied to Distributed Renewable Energy; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Agüero, J.R.; Takayesu, E.; Novosel, D.; Masiello, R. Grid modernization: Challenges and opportunities. Electr. J. 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Salihu, T.Y.; Akorede, M.F.; Abdulkarim, A.; Abdullateef, A.I. Off-grid photovoltaic microgrid development for rural electrification in Nigeria. Electr. J. 2020, 33, 106765. [Google Scholar] [CrossRef]
- Khodayar, M.E. Rural electrification and expansion planning of off-grid microgrids. Electr. J. 2017, 30, 68–74. [Google Scholar] [CrossRef]
- Moka, S.; Pande, M.; Rani, M.; Gakhar, R.; Sharma, M.; Rani, J.; Bhaskarwar, A.N. Alternative fuels: An overview of current trends and scope for future. Renew. Sustain. Energy Rev. 2014, 32, 697–712. [Google Scholar] [CrossRef]
- Michaelides, E.E.S. Alternative Energy Sources; Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Markowitz, M. Hydrogen: An energy powerhouse with unlimited potential. Open Access Government. 21 February 2019. Available online: https://www.openaccessgovernment.org/hydrogen-energypowerhouse/59506/#comment-167675 (accessed on 6 May 2020).
- Squadrito, G.; Andaloro, L.; Ferraro, M.; Antonucci, V. Hydrogen fuel cell technology. In Advances in Hydrogen Production, Storage and Distribution; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Fuel Cell Technologies, Applications, and State of the Art. A Reference Guide. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Theraja, B.L.; Theraja, A.K. A Textbook of Electrical Technology; S.Chand: New Delhi, India, 2003; Volume 2. [Google Scholar]
- Hirsch, A.; Parag, Y.; Guerrero, J. Microgrids: A review of technologies, key drivers, and outstanding issues. Renew. Sustain. Energy Rev. 2018, 90, 402–411. [Google Scholar] [CrossRef]
- Jain, M.; Gupta, S.; Masand, D.; Agnihotri, G.; Jain, S. Real-time implementation of islanded microgrid for remote areas. J. Control Sci. Eng. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Lee, J.H.; Baek, S.T.; Jung, H.J.; Kang, H.H.; Chung, J.M.; Suh, I.Y. Development of a 250kW power conditioning system for molten carbonate fuel cell power generation system. In Proceedings of the International Conference on Electrical Machines and Systems, ICEMS 2007, Seoul, Korea, 8–11 October 2007. [Google Scholar]
- Segura, F.; Durán, E.; Andújar, J.M. Design, building and testing of a stand alone fuel cell hybrid system. J. Power Sources 2009, 193, 276–284. [Google Scholar] [CrossRef]
- Castañeda, M.; Cano, A.; Jurado, F.; Sánchez, H.; Fernández, L.M. Sizing optimization, dynamic modeling and energy management strategies of a stand-alone PV/hydrogen/battery-based hybrid system. Int. J. Hydrogen Energy 2013, 38, 3830–3845. [Google Scholar] [CrossRef]
- Castañeda, M.; Fernández, L.M.; Sánchez, H.; Cano, A.; Jurado, F. Sizing methods for stand-alone hybrid systems based on renewable energies and hydrogen. In Proceedings of the 2012 16th IEEE Mediterranean Electrotechnical Conference—MELECON, Yasmine Hammamet, Tunisia, 25–28 March 2012. [Google Scholar]
- Nnaji, E.C.; Adgidzi, D.; Dioha, M.O.; Ewim, D.R.E.; Huan, Z. Modelling and management of smart microgrid for rural electrification in sub-saharan Africa: The case of Nigeria. Electr. J. 2019, 32, 106672. [Google Scholar] [CrossRef]
- Akinyele, D.; Belikov, J.; Levron, Y. Challenges of microgrids in remote communities: A STEEP model application. Energies 2018, 11, 432. [Google Scholar] [CrossRef]
- Markowitz, M. Fuel cells: Delivering reliable power when needed for emergency response efforts. Open Access Government. 6 December 2019. Available online: https://www.openaccessgovernment.org/fuel-cells-reliable-power-emergency-response/79014/ (accessed on 6 May 2020).
- Coralli, A.; Sarruf, B.J.M.; De Miranda, P.E.V.; Osmieri, L.; Specchia, S.; Minh, N.Q. Fuel cells. In Science and Engineering of Hydrogen-Based Energy Technologies: Hydrogen Production and Practical Applications in Energy Generation; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Chiabrera, F.; Garbayo, I.; Alayo, N.; Tarancón, A. Micro solid oxide fuel cells: A new generation of micro-power sources for portable applications. In Proceedings of the Smart Sensors, Actuators, and MEMS VIII, Barcelona, Spain, 8–11 May 2017. [Google Scholar]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J. Hydrogen and Fuel Cells; Green Energy Technology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Elmer, T.; Worall, M.; Wu, S.; Riffat, S.B. Fuel cell technology for domestic built environment applications: State of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 913–931. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.P.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, J.K. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Kendall, K. Hydrogen fuel cells. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sørensen, B.M. Hydrogen and fuel cells: Emerging technologies and applications. Choice Rev. Online 2006, 43, 43. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Töpler, J.; Lehmann, J. Hydrogen and Fuel Cell: Technologies and Market Perspectives; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Adamson, K.A. Stationary Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Li, X. Fuel cells. In Energy Conversion, 2nd ed.; CRR Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A.; Munnings, C. A comprehensive review of direct carbon fuel cell technology. Prog. Energy Combust. Sci. 2012, 38, 360–399. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A review on solar-hydrogen/fuel cell hybrid energy systems for stationary applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Munnings, C.; Kulkarni, A. Review of progress in high temperature solid oxide fuel cells. ChemInform 2014, 50, 23–37. [Google Scholar] [CrossRef]
- Ortiz-Rivera, E.I.; Reyes-Hernandez, A.L.; Febo, R.A. Understanding the history of fuel cells. In Proceedings of the 2007 IEEE Conference on the History of Electric Power, HEP 2007, Newark, NJ, USA, 3–5 August 2007. [Google Scholar]
- Pehnt, M. Life-cycle analysis of fuel cell system components. In Handbook of Fuel Cells; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A review on cell/stack designs for high performance solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Bendary, A.F.; Ismail, M.M. Battery charge management for hybrid PV/wind/fuel cell with storage battery. Energy Procedia 2019, 162, 107–116. [Google Scholar] [CrossRef]
- Rezk, H.; Nassef, A.M.; Abdelkareem, M.A.; Alami, A.H.; Fathy, A. Comparison among various energy management strategies for reducing hydrogen consumption in a hybrid fuel cell/supercapacitor/battery system. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- López-Pérez, M.; Claudio-Sánchez, A.; Cano-Castillo, U.; Loyola-Morales, F. Hybrid electric power plant sizing strategy based on ab-initio fuel cell design for weight minimization. Int. J. Hydrogen Energy 2020, 45, 21738–21753. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Liu, M.; Yang, W.; Gu, T. Microbial fuel cells for biosensor applications. Biotechnol. Lett. 2015, 37, 2357–2364. [Google Scholar] [CrossRef]
- Shamshuddin, M.A.; Babu, T.S.; Dragicevic, T.; Miyatake, M.; Rajasekar, N. Priority-based Energy Management Technique for Integration of Solar PV, Battery, and fuel cell systems in an autonomous dc microgrid. Electr. Power Compon. Syst. 2017, 45, 1881–1891. [Google Scholar] [CrossRef]
- Papaioannou, D.I.; Papadimitriou, C.N.; Dimeas, A.L.; Zountouridou, E.I.; Kiokes, G.C.; Hatziargyriou, N.D. Optimization & sensitivity analysis of microgrids using HOMER software—A case study. In Proceedings of the MedPower 2014, Athens, Greece, 2–5 November 2014. [Google Scholar]
- Singh, A.; Baredar, P.; Gupta, B. Techno-economic feasibility analysis of hydrogen fuel cell and solar photovoltaic hybrid renewable energy system for academic research building. Energy Convers. Manag. 2017, 145, 398–414. [Google Scholar] [CrossRef]
- Hawkes, A.; Staffell, I.; Brett, D.; Brandon, N. Fuel cells for micro-combined heat and power generation. Energy Environ. Sci. 2009, 2, 729–744. [Google Scholar] [CrossRef]
- Kassem, N. Externalities affecting the viability of wind power for hydrogen production. In Proceedings of the 2004 New and Renewable Energy Technologies for Sustainable Development, Evora, Portugal, 28 June–1 July 2007. [Google Scholar]
- Ota, K.I. Fuel cells: Past, present and future. IEEJ Trans. Fundam. Mater. 2008, 128, 329–332. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Stratistics Market. Stationary Fuel Cells—Global Market Outlook (2017–2026); PRNewswire: Telangana, India, 2020. [Google Scholar]
- Breakthrough Technologies Institute. 2010 Fuel Cells Technologies Market Report; U.S. Department of Energy—Energy Efficiency and Renewable Energy: Washington, DC, USA, 2011. [Google Scholar]
- Satyapal, S.S.D. Fuel Cell Technologies Overview; States Energy Advisory Board: Washington, DC, USA, 2012. [Google Scholar]
- Energy & Environmental Services. Number of Fuel Cells Shipped Globally from 2010 to 2019, by Application. Available online: https://www.statista.com/statistics/732220/shipments-of-fuel-cells-worldwide-by-application/ (accessed on 5 August 2020).
- Bente, V. (Holland Innovation Network China). Overview of Hydrogen and Fuel Cell Developments in China. 2019. Available online: https://www.nederlandwereldwijd.nl/binaries/nederlandwereldwijd/documenten/publicaties/2019/03/01/waterstof-in-china/Holland+Innovation+Network+in+China++Hydrogen+developments.+January+2019.pdf (accessed on 5 August 2020).
- Hoogers, G. Stationary power generation. In Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Wang, C.; Nehrir, M.H. Distributed generation applications of fuel cells. In Proceedings of the 2006 Power Systems Conference 2006: Advanced Metering, Protection, Control, Communication and Distributed Resources, PSC, Clemson, SC, USA, 14–17 March 2006. [Google Scholar]
- Huang, X.; Zhang, Z.; Jiang, J. Fuel cell technology for distributed generation: An overview. In Proceedings of the 2006 IEEE International Symposium on Industrial Electronics, Montreal, QC, Canada, 9–13 July 2006. [Google Scholar]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Fuel Cells: Problems and Solutions; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Burmester, D.; Rayudu, R.; Seah, W.; Akinyele, D. A review of nanogrid topologies and technologies. Renew. Sustain. Energy Rev. 2017, 67, 760–775. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- O’hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Liu, D.; Case, S. Durability study of proton exchange membrane fuel cells under dynamic testing conditions with cyclic current profile. J. Power Sources 2006, 162, 521–531. [Google Scholar] [CrossRef]
- Kasahara, K.; Morioka, M.; Yoshida, H.; Shingai, H. PAFC operating performance verified by Japanese gas utilities. J. Power Sources 2000, 86, 298–301. [Google Scholar] [CrossRef]
- Dillon, R.; Srinivasan, S.; Aricò, A.S.; Antonucci, V. International activities in DMFC R&D: Status of technologies and potential applications. J. Power Sources 2004, 127, 112–126. [Google Scholar] [CrossRef]
- Lokurlu, A.; Grube, T.; Höhlein, B.; Stolten, D. Fuel cells for mobile and stationary applications - Cost analysis for combined heat and power stations on the basis of fuel cells. Int. J. Hydrogen Energy 2003, 28, 703–711. [Google Scholar] [CrossRef]
- Wee, J.H. Applications of proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2007, 11, 1720–1738. [Google Scholar] [CrossRef]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization strategies for Solid Oxide Fuel Cell (SOFC) application: A literature survey. Renew. Sustain. Energy Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Appleby, A.J. Fuel cell technology: Status and future prospects. Energy 1996, 21, 521–653. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Daud, W.R.W.; Rosli, R.E.; Majlan, E.H.; Hamid, S.A.A.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Giorgi, L. Fuel cells: Technologies and applications. Open Fuel Cells J. 2013, 6. [Google Scholar] [CrossRef]
- Bischoff, M.; Huppmann, G. Operating experience with a 250 kWel molten carbonate fuel cell (MCFC) power plant. J. Power Sources 2002, 105, 216–221. [Google Scholar] [CrossRef]
- Kunusch, C.; Puleston, P.; Mayosky, M. PEM fuel cell systems. In Advances in Industrial Control; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Masaaki, T.; Nobuyuki, Z.; Toshiya, M.; Shogo, S.; Yoshio, Y. Development of Molten Carbonate Fuel Cell. Ishikawajima-Harima Giho/IHI Eng. Rev. 2003, 36, 5–13. [Google Scholar]
- Fuerte, A.; Valenzuela, R.X.; Daza, L. Preparation and characterisation of SOFC anodic materials based on Ce-Cu. J. Power Sources 2007, 169, 47–52. [Google Scholar] [CrossRef]
- Lin, B.Y.S.; Kirk, D.W.; Thorpe, S.J. Performance of alkaline fuel cells: A possible future energy system. J. Power Sources 2006, 161, 474–483. [Google Scholar] [CrossRef]
- Sundmacher, K. Fuel cell engineering: Toward the design of efficient electrochemical power plants. Ind. Eng. Chem. Res. 2010, 49, 10159–10182. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R. Direct methanol fuel cell. In Methanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://www.sciencedirect.com/topics/engineering/direct-methanol-fuel-cell (accessed on 5 August 2020).
- Tang, D.P.; Pan, J.; Lu, S.F.; Zhuang, L.; Lu, J.T. Alkaline Polymer electrolyte fuel cells: Principle, challenges, and recent progress. Sci. China Ser. B Chem. 2010, 53, 357–364. [Google Scholar] [CrossRef]
- FuelCellToday. Alkaline Fuel Cells (AFC). Available online: http://fuelcelltoday.com/technologies/afc (accessed on 10 June 2020).
- Alhassan, M.; Umar Garba, M. Design of an alkaline fuel cell. Leonardo Electron. J. Pract. Technol. 2006, 5, 99–106. [Google Scholar]
- Office of Renewable Energy. Types of Fuel Cells|Department of Energy; U.S. Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2017. [Google Scholar]
- Watanabe, T. Molten carbonate fuel cells. In Handbook of Climate Change Mitigation and Adaptation, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Williams, M.C. Fuel cells. In Fuel Cells: Technologies for Fuel Processing; National Energy Technology Laboratory: Morgantown, WV, USA, 2011. [Google Scholar]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, S.; Park, J.I.; Park, C.W.; Lee, J.; Song, I.; Naesung, L.; Kim, K.; Park, J. Lifetime prediction of a polymer electrolyte membrane fuel cell via an accelerated startup-shutdown cycle test. Int. J. Hydrogen Energy 2012, 37, 9775–9781. [Google Scholar] [CrossRef]
- Eska, B.; Corneille, M. Planning Guideline for Fuel Cell Back-up power supplies—Uninterruptible Power Supply (UPS) and Emergency Power Systems (EPS) with Fuel Cells. 2018. Available online: http://www.cleanpowernet.de/wp-content/uploads/2019/03/Planning-Guideline-UPS-and-EPS-with-Fuel-Cells.pdf (accessed on 3 July 2020).
- Fuelcell.co.uk. Fuel Cell Guide—Alkaline Fuel Cells. Available online: http://www.fuelcell.co.uk/alkaline-fuel-cells/ (accessed on 30 June 2020).
- Jamb, M.; Suryawanshi, Y.; D’Abreo, M.; Goswami, P. Polymer Electrolyte Membrane Fuel Cells for Sustainable Energy Production. Res. J. Eng. Technol. 2017, 8, 89–96. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Shih, N.C.; Weng, B.J.; Lee, J.Y.; Hsiao, Y.C. Development of a small fuel cell underwater vehicle. Int. J. Hydrogen Energy 2013, 38, 11138–11143. [Google Scholar] [CrossRef]
- PEMFC. Available online: http://www.fuelcelltoday.com/technologies/pemfc (accessed on 3 July 2020).
- Gittleman, M.; Jorgensen, C.D.M.; Waldecker, S.; Hirano, J.; Mehall, S. Automotive Fuel Cell R&D Needs. 2010. Available online: https://www.energy.gov/sites/prod/files/2014/03/f10/fuelcell_presolicitation_wkshop_mar10_gittleman.pdf (accessed on 3 July 2020).
- Melorose, J.; Perroy, R.; Careas, S. Fuel Cell Technology Reaching Towards Commercialization; Springer: London, UK, 2015. [Google Scholar]
- Sammes, N.; Smirnova, A.; Vasylyev, O. Fuel Cell Technologies: State and Perspectives; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Chen, Y.; Nie, X.; Wang, B.; Xia, C.; Dong, W.; Wang, X.; Wang, B.Z. Tuning La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite cathode as functional electrolytes for advanced low-temperature SOFCs. Catal. Today 2019. [Google Scholar] [CrossRef]
- US DOE (Energy Efficiency & Renewable). Comparison of Fuel Cell Technologies. Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/fuelcells/pdfs/fc_comparison_chart.pdf (accessed on 3 July 2020).
- Business, F.I. Phosphoric Acid Fuel Cell Market to Hit USD 1.12 Bn by 2026; Rising Focus on Bringing Down Carbon Emissions Worldwide to Boost Market Prospects: Fortune Business Insights. 2020. Available online: https://www.globenewswire.com/news-release/2020/02/17/1985659/0/en/Phosphoric-Acid-Fuel-Cell-Market-to-Hit-USD-1-12-Bn-by-2026-Rising-Focus-on-Bringing-Down-Carbon-Emissions-Worldwide-to-Boost-Market-Prospects-Fortune-Business-Insights.html (accessed on 3 July 2020).
- McLean, G.F.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Hilmi, M.; Yuh, A.; Farooque, C. Fuel cells—Molten carbonate fuel cells. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 454–461. [Google Scholar]
- Barbir, F. Fuel cell applications. In PEM Fuel Cells; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Yu, X.; Starke, M.R.; Tolbert, L.M.; Ozpineci, B. Fuel cell power conditioning for electric power applications: A summary. IET Electr. Power Appl. 2007, 1, 643–656. [Google Scholar] [CrossRef]
- İnci, M.; Türksoy, Ö. Review of fuel cells to grid interface: Configurations, technical challenges and trends. J. Clean. Prod. 2019, 213, 1353–1370. [Google Scholar] [CrossRef]
- Ferraro, M.; Sergi, F.; Cretì, P.; Brunaccini, G.; Andaloro, L.; Antonucci, V. Direct hydrogen 5.0 kW PEM fuel cell system supplying an uninterruptible power supply. ECS Trans. 2019, 5, 791–801. [Google Scholar] [CrossRef]
- Müller, M.; Kimiaie, N.; Glüsen, A.; Stähler, M.; Stolten, D. Development of a 2 kW direct methanol fuel cell system for backup power. In Proceedings of the EFC 2013—5th European Fuel Cell Piero Lunghi Conference, Rome, Italy, 11–13 December 2013. [Google Scholar]
- Jiang, W.; Brunet, J.; Fahimi, B. Application of active current sharing control in fuel cell-battery off-line UPS system. In Proceedings of the PESC Record—2008 IEEE Annual Power Electronics Specialists Conference, Rhodes, Greece, 15–19 June 2008. [Google Scholar]
- Müller, M.; Kimiaie, N.; Glüsen, A. Direct methanol fuel cell systems for backup power—Influence of the standby procedure on the lifetime. Int. J. Hydrog. Energy 2014, 39, 21739–21745. [Google Scholar] [CrossRef]
- Akinyele, D.O.; Rayudu, R.K. Review of energy storage technologies for sustainable power networks. Sustain. Energy Technol. Assess. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Carrasco, J.M.; Franquelo, L.G.; Bialasiewicz, J.T.; Galvan, E.; Guisado, R.C.; Prats, A.M.; Leon, J.I.; Moreno-Alfonso, N. Power-electronic systems for the grid integration of renewable energy sources: A survey. IEEE Trans. Ind. Electron. 2006, 53, 1002–1016. [Google Scholar] [CrossRef]
- Emmanuel, M.; Akinyele, D.; Rayudu, R. Techno-economic analysis of a 10 kWp utility interactive photovoltaic system at Maungaraki school, Wellington, New Zealand. Energy 2017, 120, 573–583. [Google Scholar] [CrossRef]
- Basso, T.S.; DeBlasio, R. IEEE 1547 series of standards: Interconnection issues. IEEE Trans. Power Electron. 2004, 19, 1159–1162. [Google Scholar] [CrossRef]
- Azmy, A.M.; Erlich, I. Intelligent operation management of fuel cells and micro-turbines using genetic algorithms and neural networks. In 2004 New and Renewable Energy Technologies for Sustainable Development; Wspc: Evora, Portugal, 2007. [Google Scholar]
- Louie, H.; Dauenhauer, P.; Wilson, M.; Zomers, A.; Mutale, J. Eternal light: Ingredients for sustainable off-grid energy development. IEEE Power Energy Mag. 2014, 12, 70–78. [Google Scholar] [CrossRef]
- IEEE. IEEE Guide for Array and Battery Sizing in Stand-Alone Photovoltaic (PV) Systems; IEEE Std 1562-2007; IEEE: New York, NY, USA, 2008; p. 22. [Google Scholar]
- Generation, D.; Storage, E. IEEE Guide for Optimizing the Performance and Life of Lead-Acid Batteries in Remote Hybrid Power Systems; IEEE: New York, NY, USA, 2008. [Google Scholar]
- Gao, D.W. Applications of ess in renewable energy microgrids. In Energy Storage for Sustainable Microgrid; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lambert, T.; Gilman, P.; Lilienthal, P. Micropower system modeling with homer. Integr. Altern. Sources Energy 2006, 1, 379–418. [Google Scholar]
- Cvetkovic, I.; Dong, D.; Zhang, W.; Jiang, L.; Boroyevich, D.; Lee, F.C.Y.; Mattavelli, P. A testbed for experimental validation of a low-voltage DC nanogrid for buildings. In Proceedings of the 2012 15th International Power Electronics and Motion Control Conference and Exposition, EPE-PEMC 2012 ECCE Europe, Novi Sad, Serbia, 4–6 September 2012. [Google Scholar]
- Shwehdi, M.H.; Mohamed, S.R. Proposed smart dc nano-grid for green buildings—A reflective view. In Proceedings of the 3rd International Conference on Renewable Energy Research and Applications, ICRERA 2014, Milwaukee, WI, USA, 19–22 October 2014. [Google Scholar]
- Wu, H.; Wong, S.C.; Tse, C.K.; Chen, Q. Control and modulation of a family of bidirectional ac-dc converters with active power compensation. In Proceedings of the 2015 IEEE Energy Conversion Congress and Exposition, ECCE 2015, Montreal, QC, Canada, 20–24 September 2015. [Google Scholar]
- Ganesan, S.I.; Pattabiraman, D.; Govindarajan, R.K.; Rajan, M.; Nagamani, C. Control scheme for a bidirectional converter in a self-sustaining low-voltage dc nanogrid. IEEE Trans. Ind. Electron. 2015, 62, 6317–6326. [Google Scholar] [CrossRef]
- Mittelsteadt, C.; Norman, T.; Rich, M.; Willey, J. PEM electrolyzers and pem regenerative fuel cells industrial view. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Barbir, F.; Molter, T.; Dalton, L. Efficiency and weight trade-off analysis of regenerative fuel cells as energy storage for aerospace applications. Int. J. Hydrogen Energy 2005, 30, 351–357. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Meishner, F.; Sauer, D.U. Wayside energy recovery systems in DC urban railway grids. eTransportation 2019, 1, 100001. [Google Scholar] [CrossRef]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209. [Google Scholar] [CrossRef]
- Mano, N.; Mao, F.; Heller, A. A miniature biofuel cell operating in a physiological buffer. J. Am. Chem. Soc. 2002, 124, 12962–12963. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Wang, L.; Pérez-Fortes, M.; Maréchal, F. A cogeneration system based on solid oxide and proton exchange membrane fuel cells with hybrid storage for off-grid applications. Front. Energy Res. 2019, 6, 139. [Google Scholar] [CrossRef]
- Bizon, N.; Oproescu, M.; Raceanu, M. Efficient energy control strategies for a standalone renewable/fuel cell hybrid power source. Energy Convers. Manag. 2015, 90, 93–110. [Google Scholar] [CrossRef]
- Mudaliyar, S.R.; Mishra, S.; Sharma, R.K. Load following capability of fuel cell-microturbine based hybrid energy system for microgrid operation. In Proceedings of the 6th International Conference on Computer Applications in Electrical Engineering—Recent Advances, CERA 2017, Roorkee, India, 5–7 October 2017. [Google Scholar]
- Wang, C.; Nehrir, M.H. Load transient mitigation for stand-alone fuel cell power generation systems. IEEE Trans. Energy Convers. 2007, 22, 864–872. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Wang, K.; Sun, Y.; Zeng, D.; Guo, S. Energy management of data centers powered by fuel cells and heterogeneous energy storage. In Proceedings of the 2018 IEEE International Conference on Communications, Kansas City, MO, USA, 20–24 May 2018. [Google Scholar]
- Asghari, M.; Brouwer, J. Integration of a solid oxide fuel cell with an organic rankine cycle and absorption chiller for dynamic generation of power and cooling for a residential application. Fuel Cells 2019, 19, 361–373. [Google Scholar] [CrossRef]
- Chang, H.; Xu, X.; Shen, J.; Shu, S.; Tu, Z. Performance analysis of a micro-combined heating and power system with PEM fuel cell as a prime mover for a typical household in North China. Int. J. Hydrogen Energy 2019, 44, 24965–24976. [Google Scholar] [CrossRef]
- Agrawal, S.; Chourasiya, S.; Palwalia, D.K. Hybrid Energy Management System design with Renewable Energy Sources (Fuel Cells, PV Cells and Wind Energy): A Review. IJSET 2018, 6, 174–177. [Google Scholar]
- Taleb, S.A.; Brown, D.; Dillet, J.; Guillement, P.; Mainka, J.; Crosnier, O.; Douard, C.; Athouel, L.; Brouusse, L.; Brouse, T.; et al. Direct hybridization of polymer exchange membrane surface fuel cell with small aqueous supercapacitors. Fuel Cells 2018, 18, 299–305. [Google Scholar] [CrossRef]
- Yang, J.; Qin, S.; Zhang, W.; Ding, T.; Zhou, B.; Li, X.; Jian, L. Improving the load-following capability of a solid oxide fuel cell system through the use of time delay control. Int. J. Hydrogen Energy 2017, 42, 1221–1236. [Google Scholar] [CrossRef]
- Wu, X.; Gao, D. Optimal fault-tolerant control strategy of a solid oxide fuel cell system. J. Power Sources 2017, 364, 163–181. [Google Scholar] [CrossRef]
- Barakat, M.; Tala-Ighil, B.; Chaoui, H.; Gualous, H.; Hissel, D. Energy management of a hybrid tidal turbine-hydrogen micro-grid: Losses minimization strategy. Fuel Cells 2020, 20, 342–350. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, G.; Li, Q.; You, Z.; Chen, W.; Liu, H. Hierarchical energy management for PV/hydrogen/battery island DC microgrid. Int. J. Hydrogen Energy 2019, 44, 5507–5516. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Li, Q.; Yang, H.; Zare, F.; Zheng, Y. Two-level energy management strategy for PV-Fuel cell-battery-based DC microgrid. Int. J. Hydrogen Energy 2019, 44, 19395–19404. [Google Scholar] [CrossRef]
- Zia, M.F.; Elbouchikhi, E.; Benbouzid, M. Microgrids energy management systems: A critical review on methods, solutions, and prospects. Appl. Energy 2018, 222, 1033–1055. [Google Scholar] [CrossRef]
- Borlea, I.; Kilyeni, S.; Barbulescu, C.; Cristian, D. Substation ancillary services fuel cell power supply. Part 1. Solution overview. In Proceedings of the ICCC-CONTI 2010—IEEE International Joint Conferences on Computational Cybernetics and Technical Informatics, Timisoara, Romania, 27–29 May 2010. [Google Scholar]
- Vuc, G.; Ardelean, I.; Lustrea, B.; Dusa, V.; Borlea, I.; Kilyeni, S. Fuel cells solution for auxiliary services backup supply in a power system substation. In Proceedings of the EUROCON 2007—The International Conference on Computer as a Tool, Warsaw, Poland, 9–12 September 2007. [Google Scholar]
- Ma, Z.; Eichman, J.; Kurtz, J. Fuel cell backup power system for grid service and microgrid in telecommunication applications. J. Energy Resour. Technol. 2019, 141, 062002. [Google Scholar] [CrossRef]
- Ma, Z.; Eichman, J.; Kurtz, J. Fuel cell backup power system for grid-service and micro-grid in telecommunication applications. In Proceedings of the ASME 2018 12th International Conference on Energy Sustainability, ES 2018, Collocated with the ASME 2018 Power Conference and the ASME 2018 Nuclear Forum, Lake Buena Vista, FL, USA, 24–28 June 2018. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).