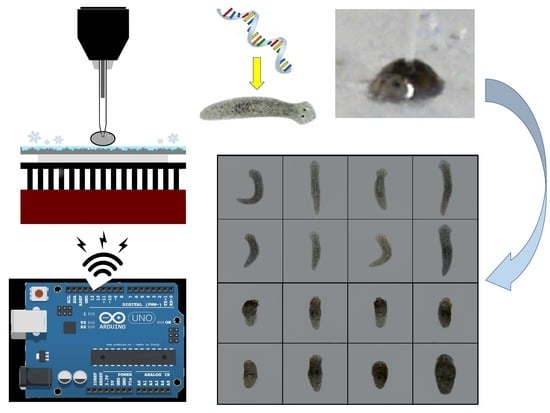
Gene Delivery System Using Droplet Injector and Temperature-Controlled Planarian Holder
Abstract
:1. Introduction
2. Materials and Methods
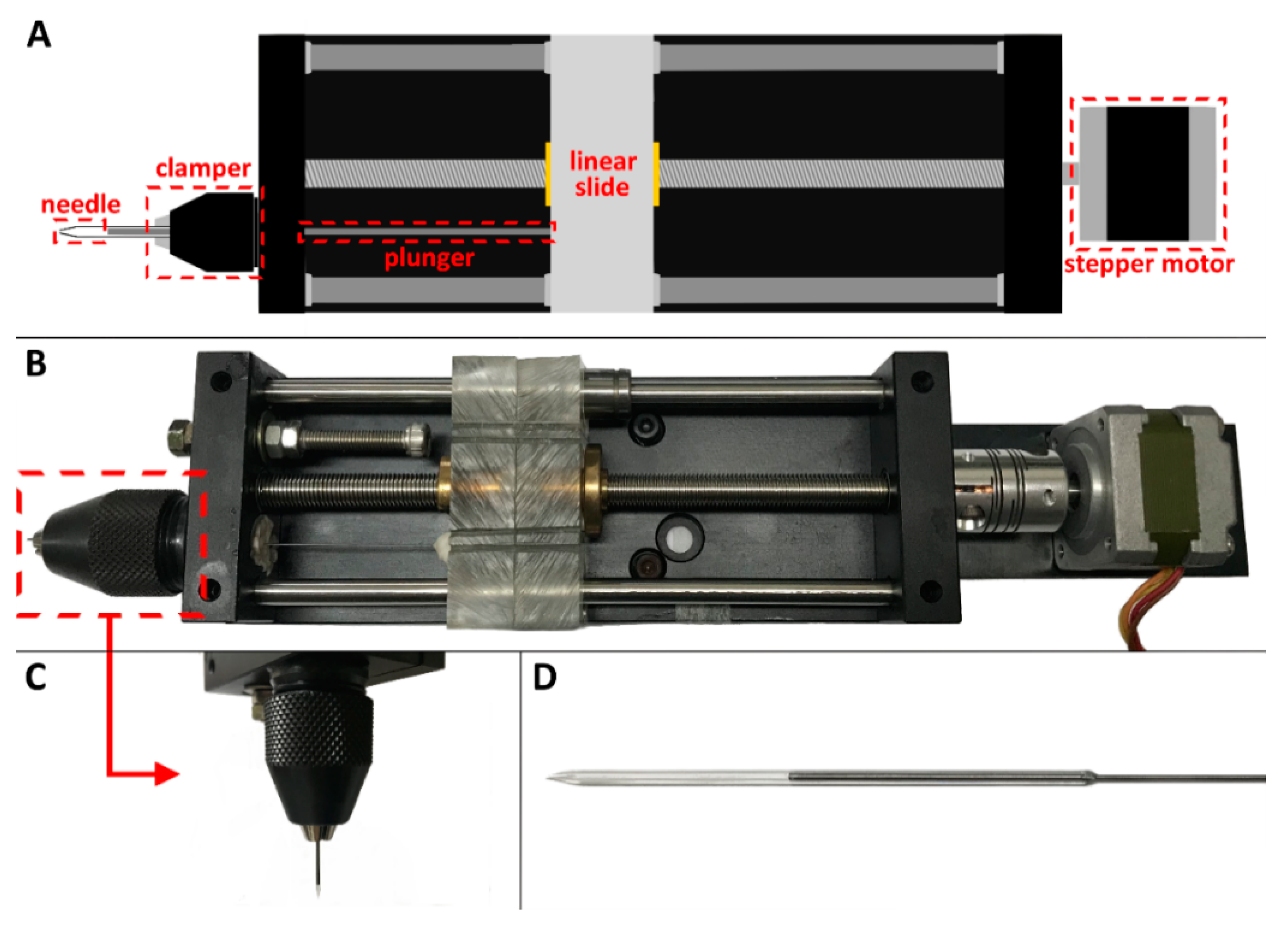
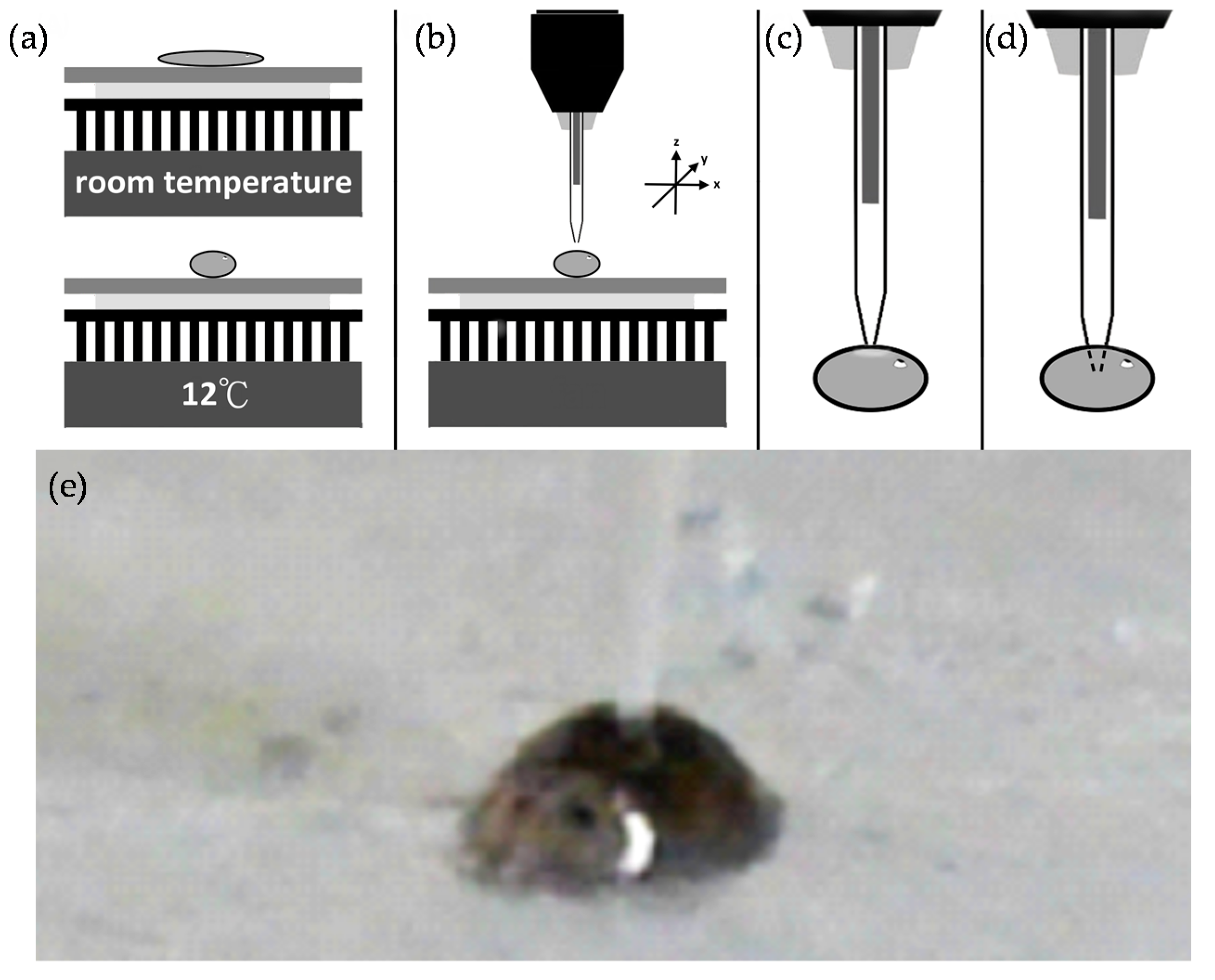
2.1. Droplet Generator/Injector
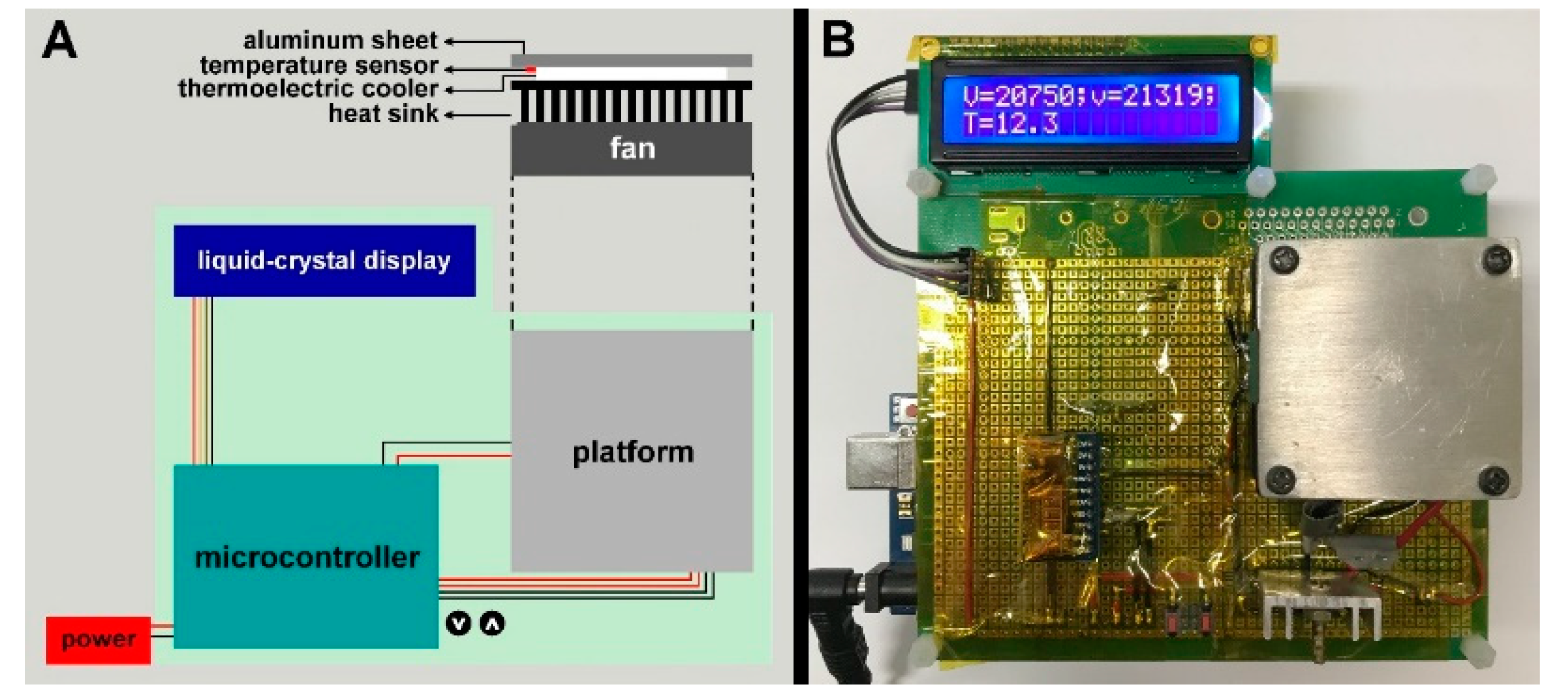
2.2. Planarian Holder
2.3. Characterization and Evaluation of Our Microinjection System
2.3.1. Double-Stranded RNA (dsRNA) Preparation
- Ago2 forward primer: 5′-GCCTAATACGAGACACTATAGAAGGTGTTTGGGATATGAGAGG-3′
- Ago2 reverse primer: 5′-GCCTAATACGACTCACTATAGGGTAACAATGCCGAAATTTGAT-3′
- YFP forward primer: 5′-GCCTAATACGAGACACTATAGGGATGGTGAGCAAGGGC-3′
- YFP reverse primer: 5′-GCCTAATACGAGACACTATAGGGACTTGTACAGCTCGTCC-3′
2.3.2. Microinjection of dsRNA into Planarians
2.3.3. dsRNA Delivery by Feeding
2.3.4. Expression Analysis
- Djβ-tubulin forward: 5′-TGTCAGTGAACAATTTACTGCC-3′
- Djβ-tubulin reverse: 5′-GTTGATATTCACTAACCAAATCATTC-3′
- DjAgo2 forward: 5′-CCTGTAATATTTCTCGGTGCTGA-3′
- DjAgo2 reverse: 5′-CATCCATACTGCCTACAACAGC-3′
- DjpiwiA forward: 5′-GGAGCCATAGGAGAAATCTCATTTG-3′
- DjpiwiA reverse: 5′-CGCTAATCCAAATCCGGGAAC-3′
3. Results
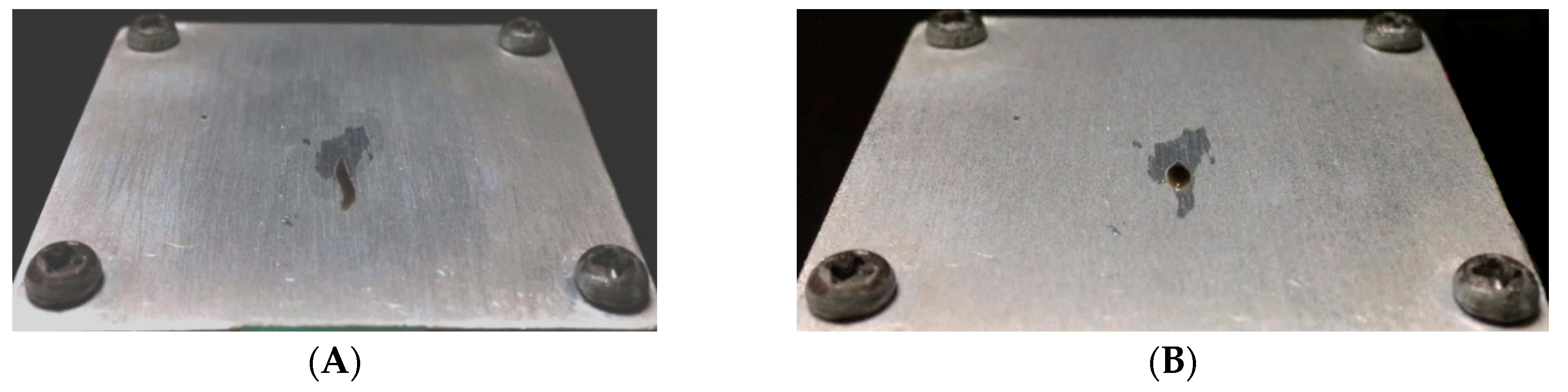
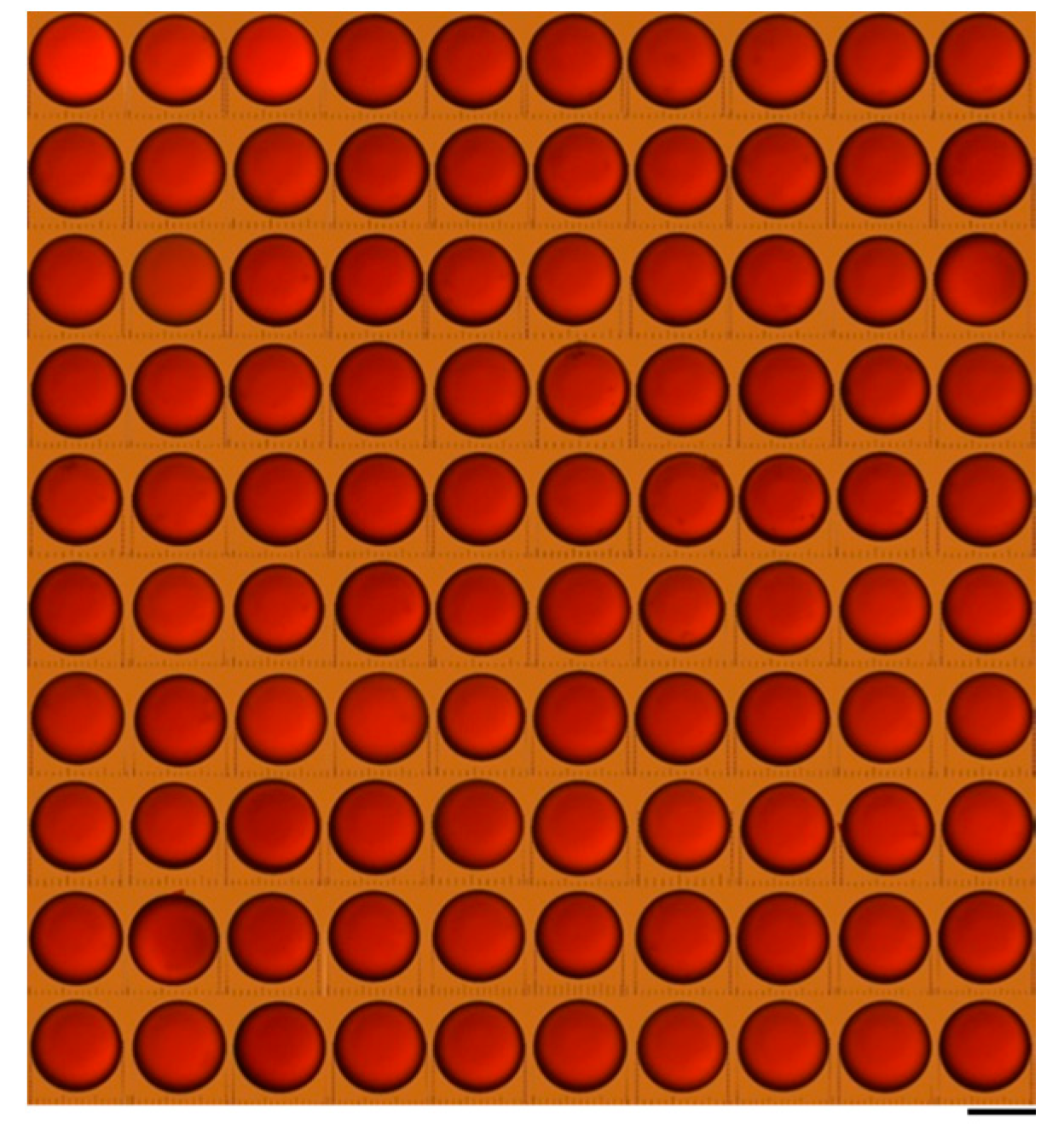
3.1. Microinjection Droplet Volume Calibriation
3.2. Evaluation on Microinjections
3.2.1. Microinjections with Beads
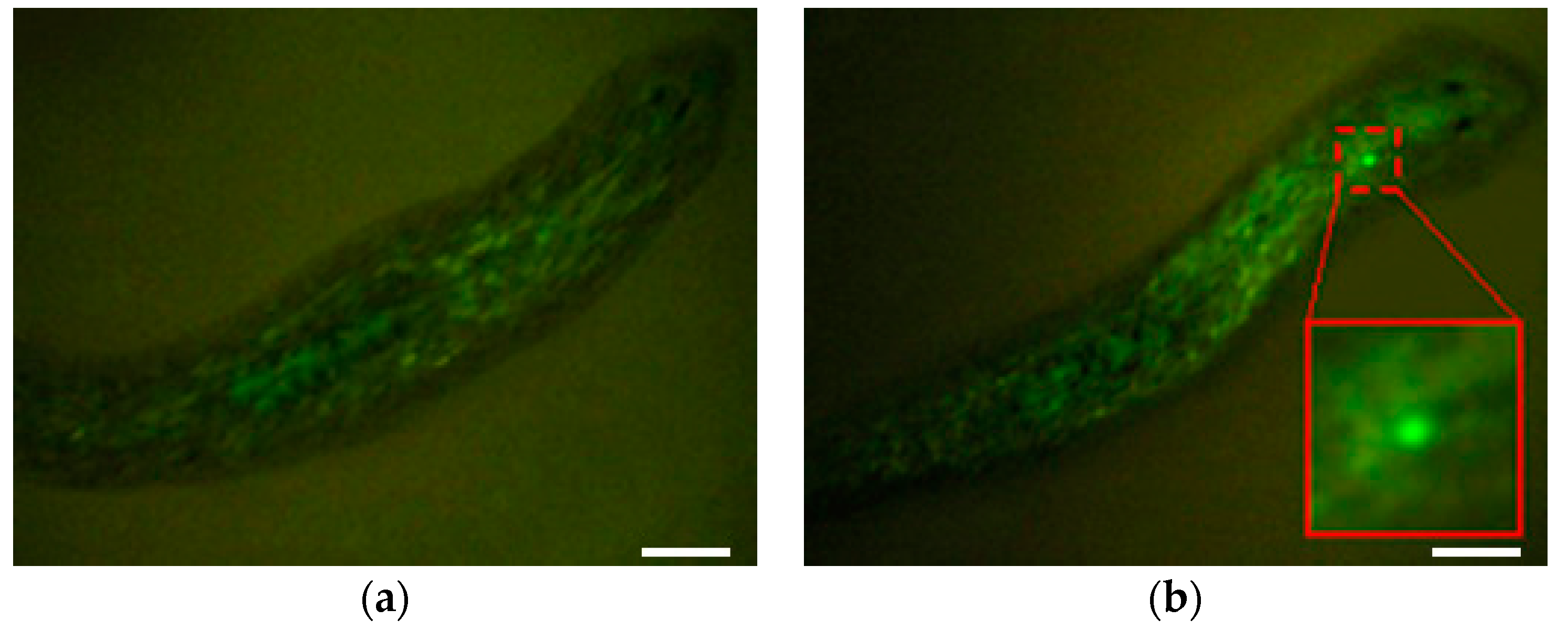
3.2.2. Microinjections with DjAgo2 dsRNA
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Manzotti, E. Regenerative medicine cell therapies: Numbers of units manufactured and patients treated between 1988 and 2010. Regener. Med. 2010, 5, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Alvarado, A.S. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 2004, 20, 725–757. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.J. Cytological studies on the planarian neoblast. Cell Tissue Res. 1959, 50, 799–817. [Google Scholar] [CrossRef]
- Baguna, J. The planarian neoblast: The rambling history of its origin and some current black boxes. Int. J. Dev. Biol. 2012, 56, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Rouhana, L.; Weiss, J.A.; Forsthoefel, D.J.; Lee, H.; King, R.S.; Inoue, T.; Shibata, N.; Agata, K.; Newmark, P.A. RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: Methodology and dynamics. Dev. Dyn. 2013, 242, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Newmark, P.A.; Reddien, P.W.; Cebrià, F.; Alvarado, A.S. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc. Natl. Acad. Sci. USA 2003, 100, 11861–11865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feramisco, J.; Perona, R.; Lacal, J.C. Needle Microinjection: A Brief History. In Microinjection; Lacal, J.C., Feramisco, J., Perona, R., Eds.; Birkhäuser: Basel, Switzerland, 1999; pp. 9–15. [Google Scholar]
- Capecchi, M.R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell 1980, 22, 479–488. [Google Scholar] [CrossRef]
- Li, S.; Zheng, D.; Li, N.; Wang, X.; Liu, Y.; Sun, M.; Zhao, X. Size-adjustable microdroplets generation based on microinjection. Micromachines 2017, 8, 88. [Google Scholar] [CrossRef]
- Rosen, J.N.; Sweeney, M.F.; Mably, J.D. Microinjection of zebrafish embryos to analyze gene function. J. Visual. Exp. 2009, 25. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Scangos, G.A.; Plotkin, D.J.; Barbosa, J.A.; Ruddle, F.H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 7380–7384. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Selvaganapathy, P.R.; Wilson, J. Microinjection in a microfluidic format using flexible and compliant channels and electroosmotic dosage control. Lab Chip 2009, 9, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, W.; Pepperkok, R. Performance of an automated system for capillary microinjection into living cells. J. Biochem. Biophys. Methods 1988, 16, 283–292. [Google Scholar] [CrossRef]
- Song, P.; Dong, X.; Liu, X. A microfluidic device for automated, high-speed microinjection of Caenorhabditis elegans. Biomicrofluidics 2016, 10, 011912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, F.; Tang, L.; Du, W.; Feng, X.; Liu, B.-F. Microfluidic chip-based C. elegans microinjection system for investigating cell–cell communication in vivo. Biosens. Bioelectron. 2013, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Agata, K. RNA interference in planarians: Feeding and injection of synthetic dsRNA. Methods Mol. Biol. 2018, 1774, 455–466. [Google Scholar] [PubMed]
- Rossi, L.; Salvetti, A.; Lena, A.; Batistoni, R.; Deri, P.; Pugliesi, C.; Loreti, E.; Gremigni, V. DjPiwi-1, a member of the PAZ-Piwi gene family, defines a subpopulation of planarian stem cells. Dev. Genes Evol. 2006, 216, 335. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Zeng, A.; Han, X.-S.; Wang, C.; Li, G.; Zhang, Z.-C.; Wang, J.-Y.; Qin, Y.-W.; Jing, Q. Argonaute-2 regulates the proliferation of adult stem cells in planarian. Cell Res. 2012, 22, 275. [Google Scholar] [CrossRef]

| HEAT | VEL | PULL | I.D. | Photos |
|---|---|---|---|---|
| 617 | 10 | 10 | 10 μm |  |
| 615 | 10 | 10 | 12 μm |  |
| 620 | 0 | 10 | 15 μm |  |
| 600 | 20 | 10 | 20 μm |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Syu, J.-J.; Chu, C.-Y.; Lu, Y.-W. Gene Delivery System Using Droplet Injector and Temperature-Controlled Planarian Holder. Inventions 2018, 3, 57. https://doi.org/10.3390/inventions3030057
Lee M, Syu J-J, Chu C-Y, Lu Y-W. Gene Delivery System Using Droplet Injector and Temperature-Controlled Planarian Holder. Inventions. 2018; 3(3):57. https://doi.org/10.3390/inventions3030057
Chicago/Turabian StyleLee, Michael, Jing-Jie Syu, Chia-Ying Chu, and Yen-Wen Lu. 2018. "Gene Delivery System Using Droplet Injector and Temperature-Controlled Planarian Holder" Inventions 3, no. 3: 57. https://doi.org/10.3390/inventions3030057