1. Introduction
For centuries, plants have provided the basis for the development of medicines for treating a wide range of human and animal diseases [
1]. Although modern chemical and biological techniques such as combinatorial chemistry and library-based automated screening have, at some point, eclipsed the more down-to-earth hunt for exotic natural products in equally exotic places, the last decade has witnessed a renewed interest in plant-based natural products [
2,
3]. This renaissance of the natural product has been fuelled in part by the emergence of drug-resistant pathogens, but also by a need for “milder” drugs, to treat, for instance, an ageing population over longer time periods and also against less aggressive pathogens. At the same time, plants represent a “green” and renewable resource.
Whilst plants often provide an interesting alternative to the use of synthetic drugs, the conversion of a plant part, such as a fruit or bark into a useful medication is far from trivial. The extraction and isolation of active ingredients, followed by the formulation of a suitable delivery form requires sophisticated and often expensive technologies and considerable expertise [
4]. It is time consuming, results in considerable waste and, eventually, is also not easily carried out in developing countries, which despite their richness in medical plants, cannot use them, and hence rely on expensive, often unaffordable, imported drugs [
5]. Not surprisingly, a large swathe of the population in developing countries is still treated by traditional healers with methods and materials dating back to the Middle Ages.
We have recently explored a possible alternative to either traditional medicine or (imported) synthetic drugs. Using basic technology from the arsenal of nanotechnology, we “milled down” whole fruits and barks of medical plants, namely
Solanum incanum and
Pterocarpus erinocaeus, and demonstrated that the resulting nanosuspension retains a considerable antimicrobial and nematicidal activity comparable to the one of the more sophisticated extracts [
6]. Still, this preliminary study left some room for improvement, in particular because of composition (soft plants are difficult to mill and yield considerable cell debris) and the degeneration and degradation of constituents, especially chlorophyll, which is present in most plants.
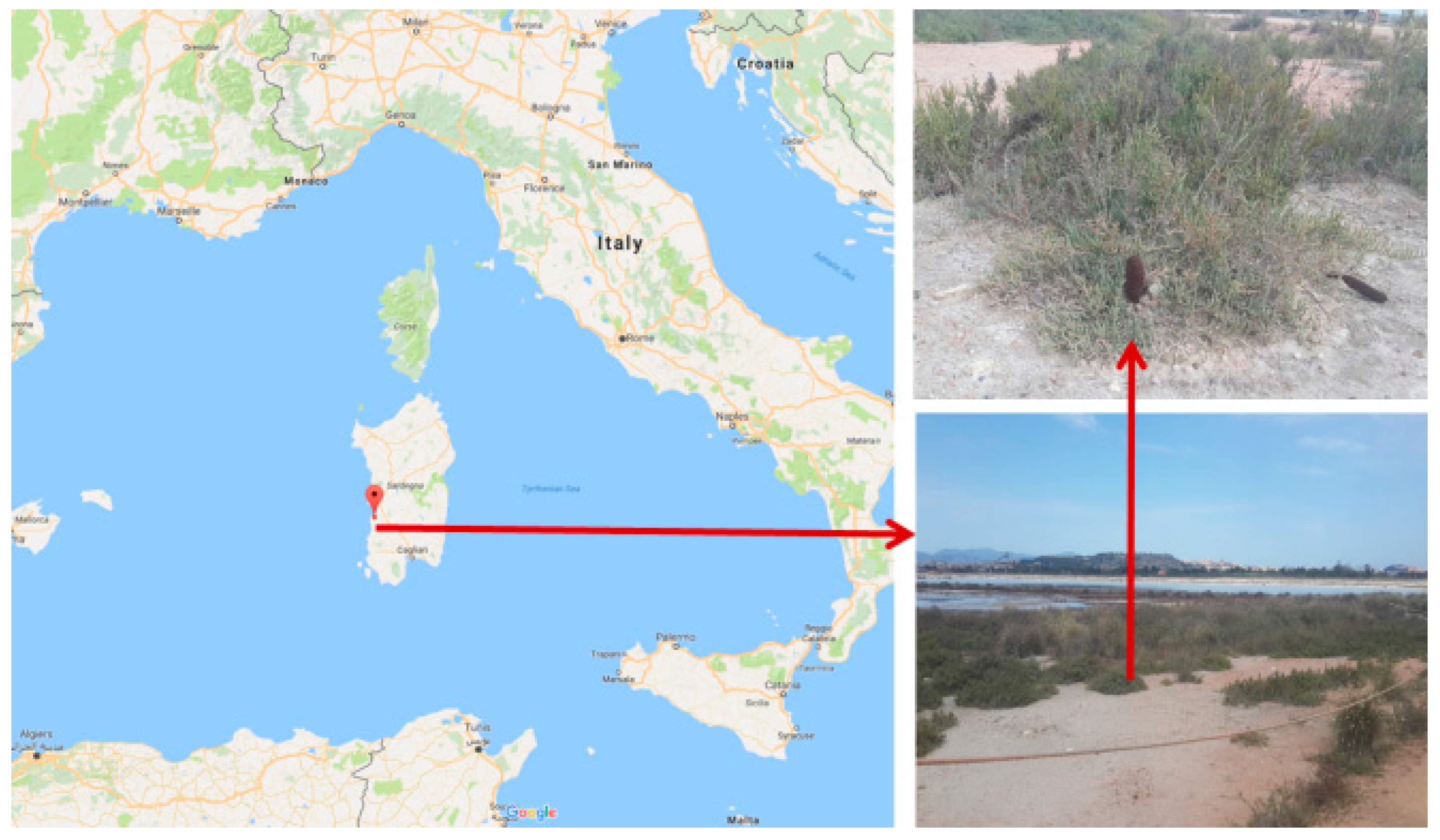
To circumvent some of these problems, we therefore decided to shift our focus to medical plants with specific physical properties that render them more amenable to nanosizing. Here,
Cynomorium, the Maltese Mushroom or Desert Thumb, as this parasitic non-photosynthetic plant is also known, seems to be a fine choice (
Figure 1).
Cynomorium is a renewable resource found readily and quite abundantly on islands such as Malta and Sardinia, is devoid of chlorophyll, yet rich in pharmacologically interesting anthocyanins and polyphenols, such as cyanidin 3-
O-glucoside and gallic acid, and its external layer is rather hard and brittle [
7]. Moreover, parts of the plant are edible, and hence not particularly toxic to humans. Yet at the same time, various products derived from it are biologically active, and have been used for centuries to treat common disorders, as they show certain antiemetic, aphrodisiac and hypotensive activities [
8,
9,
10]. Furthermore, we have recently reported some aspects of the active compounds found in this plant and possible mode(s) of action associated with them [
7]. Here, we provide initial evidence for the straightforward production of good quality nanoparticles of
Cynomorium—both its external layer and peeled interior—and for the significant activity of some of these preparations against a common pathogenic fungus,
Candida albicans.
3. Results
Overall, the results demonstrate that a combination of wet milling and high-pressure homogenization of Cynomorium results in rather uniformly sized and shaped particles with diameters in the hundred nanometre range, which exhibit some activity against C. albicans, whilst they are more or less inactive against other potential targets, such as nematodes (Steinernema feltiae) and certain bacteria. These results will now be presented and discussed in detail.
3.1. Nanosized Particles
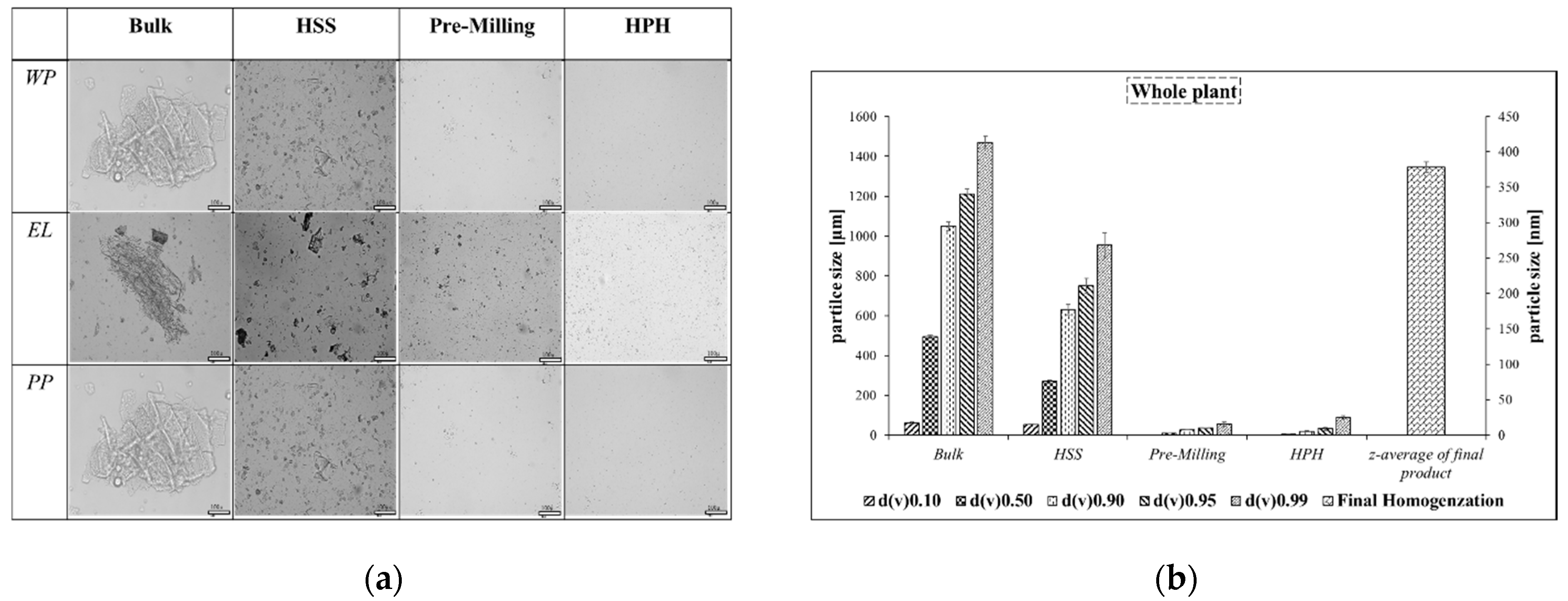
Considering the microscopy images of the nanosuspension, it is immediately apparent that the use of high-speed stirring and pre-milling results in a particular material which contains smaller particles but also still some larger objects. Further homogenization leads to a material which is more homogeneous, as indicated by the laser diffraction measurements [
16]. Interestingly, the whole plant seems to be the most amenable to milling and homogenization, as the particles obtained in this case are the most uniform with regard to size and shape. Processing the entire
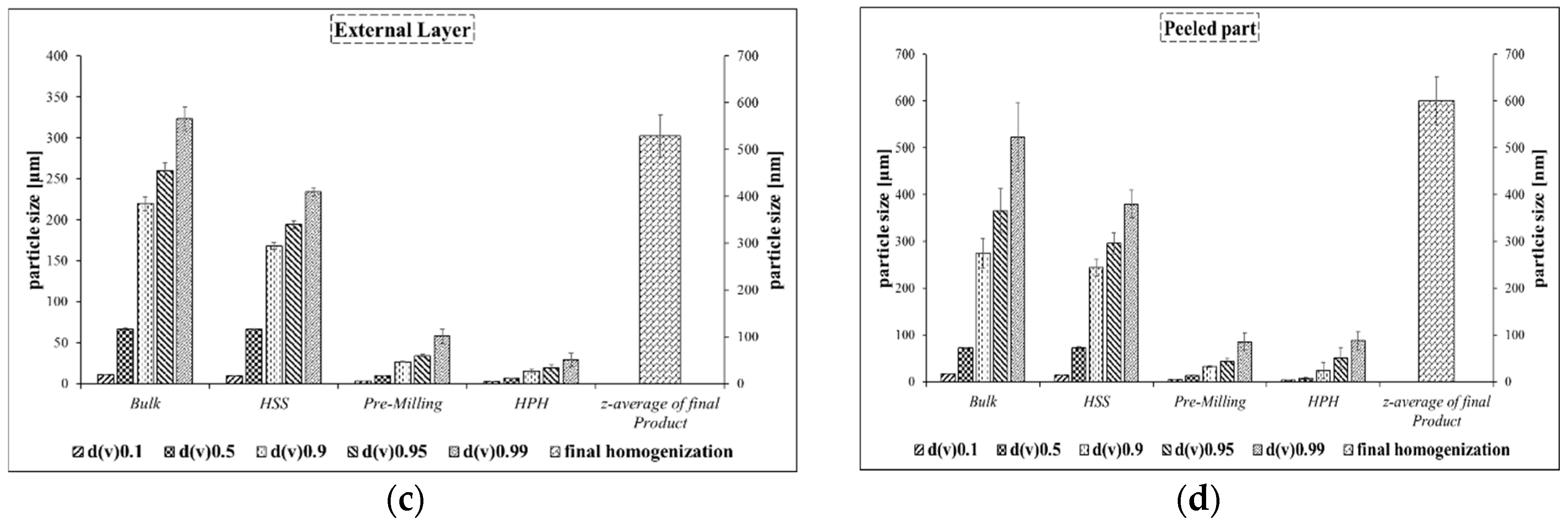
Cynomorium also yields the smallest set of particles, with an average diameter of around 400 nm (Panel A). In contrast, the particles obtained from the outer layer and inner section are slightly larger, with diameters of 500 and 600 nm, respectively.
It should be noted that the sequence of crushing the plant fabric, from simple pre-milling to high-pressure homogenization, successively leads to smaller and more uniform particles (
Figure 3). The small particles form a—stabilized—nanosuspension which is desirable for two main reasons. Firstly, it is, to some extent, bioavailable and can be administered; and secondly, the (rapid) release of biologically active substances is ensured by a large surface-to-volume ratio, which is paramount for such processes to take place. As the size of the particles is therefore crucial and essential to preserve, a short physical stability study was performed in order to rule out possible degradation or aggregation. The data obtained so far confirms a sufficient stability of the suspensions obtained, i.e., no significant changes of particle sizes in the nanosuspensions could be observed over a period of at least 2 weeks at 4 °C.
3.2. Activity against Microorganisms: C. albicans
Indeed, biological tests require either dissolved materials, as is usually the case for synthetic compounds, or fine suspensions, as is the case with nanosized Cynomorium. Whilst crude materials, such as the ones obtained initially by wet ball milling, could not be tested in our standard (micro-) biological assays including bacteria, yeasts and nematodes, the final homogenized products were of sufficient quality and stability to be tested. At this point, it was also possible to compare their activities with the ones of the corresponding extracts.
Eventually, the samples investigated showed no or only a low activity against
Steinernema feltiae, but a rather significant toxicity against
C. albicans, which is illustrated in
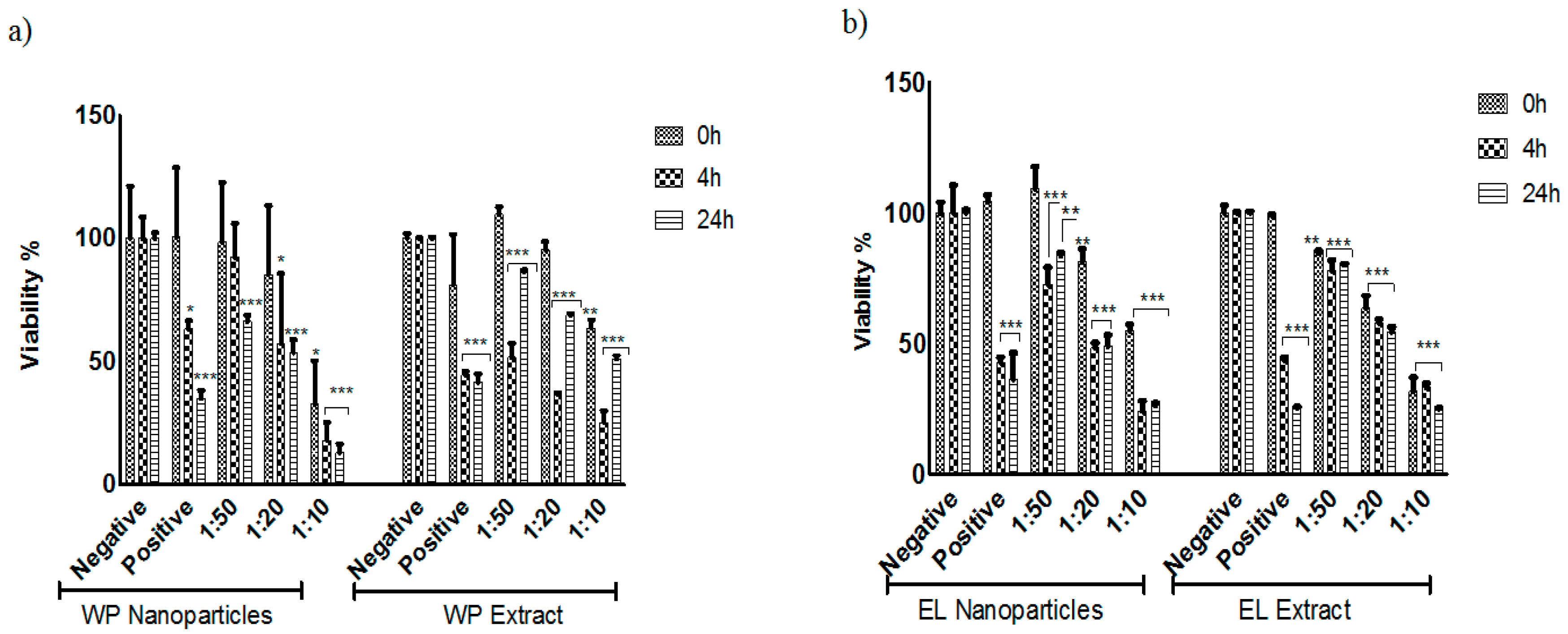
Figure 4. It is apparent that the particles of the entire plant exhibit a statistically significant activity even when employed at a 1:50 dilution of the nanosuspension, which in turn “only” contains 0.02% of particles per weight. This activity is apparent almost immediately, i.e., after addition, and becomes more pronounced, pointing towards a combination of cytotoxic and cytostatic action. It should be noted that this activity is not due to the presence of the natural surfactant Plantacare
®, which is commonly used to stabilize medical or cosmeceutical particles and, as mentioned already, is still essential to avoid aggregation of such particles [
17,
18,
19]. Furthermore, the activity observed for the nanosized whole plant corresponds well to the one of the corresponding extract: After 24 h and at a 1:50 dilution, the nanosized suspension results in a reduction of viability (compared to the untreated sample) by 40%, whilst the extract, at the same dilution, reduces viability by 10%. This is rather remarkable, as the extract is more concentrated and contains solely soluble substances, and hence also dissolves faster and almost completely. This somewhat intriguing finding will be discussed later on.
When comparing the activity of particles derived from the outer layer of
Cynomorium with those of the whole plant and the inner part, it is obvious that the outer layer is the more active part of the plant. At higher concentrations, i.e., at lower dilutions, the nanosuspension and extract based on this coat are both able to reduce viability and/or suppress growth considerably, i.e., to less than 25% viability (
Figure 4b). In contrast, the suspension and extract produced from the inner part of the plant are both somewhat less active, although not entirely inactive, either. The nanosized material from the inner part shows a growth inhibition of 50%, while the extracts and nanosized samples from the external reveal an inhibition of 75%.
It should also be mentioned that we have routinely tested the nanosuspensions against other targets, such as the model nematode S. feltiae, the Gram-negative bacterium Escherichia coli and the Gram-positive Bacterium Staphylococcus aureus. Whilst no significant activity could be found against the model nematode at the highest concentration used (i.e., 1:10 dilution of stock), a clear result for potential antibacterial activity was difficult to obtain due to the inherent antibacterial activity of the surfactant Plantacare®.
4. Discussion
In essence, the results obtained confirm the general notion that milling of entire—medicinal—plants lead to crude but biologically active materials based on a sustainable, environmentally friendly resource. The latter possess an activity comparable to the one of extracts, and may be applied directly, thus circumventing time-consuming extraction, purification, evaporation and formulation steps (
Figure 2). Still, this rather crude approach has both advantages and disadvantages, some of which will now be discussed in more detail.
First of all, it appears that dry, brittle parts of plants devoid of readily degrading compounds such as chlorophyll are more amenable to nanosizing when compared to fruitier or fibrous materials, such as fruits, leaves and probably also roots and barks. Whilst this may seem obvious and fairly trivial, one should remember that the outer layer of
Cynomorium in fact contains some flower tissue, which in other plants poses a serious challenge for milling. Indeed, the ease of ball milling and high pressure homogenization, together with the good quality of the particles—uniform, small size and spherical shape—are rather promising, even when compared to our own previous studies in this field with
S. incanum and
P. erinaceus [
6].
The biological activity of the nanosuspensions obtained is rather competitive, for instance when compared to other nanosized materials. Indeed, in our own hands, the nanoparticles obtained for
Cynomorium, and here in particular for the outer layer, were significantly more active against
C. albicans when compared to nanoparticles of the chalcogens sulphur, selenium and tellurium, as well as particles of
S. incanum and
P. erinaceus, whose formation and activity we have reported previously [
6,
15,
20,
21].
At the same time, the activities of the more active nanosuspensions also compare well with those of the respective extracts. As mentioned previously, this is rather stimulating, as the extracts are generally superior to whole plants; extracts contain soluble substances in a concentrated form and dissolve, rather than release, their active ingredients, fully and almost instantaneously. In this regard, particles tend to be inferior, as they carry insoluble and inactive “ballast”, and also prevent a rapid release of the soluble, active ingredients captured within. One may contemplate that such particles are slowly but constantly releasing substances toxic to C. albicans, or that C. albicans is “nibbling” on those particles, eventually to its own detriment.
As
Cynomorium can easily be dissected in a more brittle outer layer and a softer, edible inner part, we have wondered if the activity observed for the whole plant may be assigned to either of them. Indeed, the outer, brittle and harder layer of
Cynomorium yields the particles with the best quality and highest activity, whilst the peeled, softer inner part may be milled, yet the resulting nanosuspension is less active. Hence, the substances responsible for activity seem to reside primarily—but not exclusively—in the outer layer, a finding in line with previous studies on this plant, which have revealed a high content of biologically active cyanidin 3-
O-glucoside in this layer [
11]. In contrast, the peeled inner part of the plant, which has in the past on occasion served as food, is less active, probably due to the lack of such substances.
Still, when comparing the activity of the processed or extracted whole plant with the one of the processed or extracted outer layer, it seems that there is not that much difference (
Figure 4). There may be reasons for this, ranging from the fact that the inner part of the plant is also to some degree active to a more fanciful view, for instance, that the outer and the inner parts may blend during nanosizing into a unique sample with mutual synergy. In any case, the activity of products derived from the whole plant somewhat removes the need for dissection, an aspect that may become important as part of any future product development (e.g., less steps, higher quantities and yield, less waste).
As far as such product development is concerned, it may be premature at this stage to speculate about any practical applications of such nanosuspensions, as stability, storage, safety and mode(s) of application still need to be addressed. It may also be essential to find alternatives to the use of the detergent Plantacare®, either in the form of another detergent or of a method that leads to a self-stabilization of the plant particles, and hence a reduced need for any such additional stabilizer. Nonetheless, the ease of preparation, even with the methods available to date, the activity against a common human pathogenic fungus such as C. albicans, and the fact that such nanosuspensions may be prepared easily, are initially sterile, stable for several days (if not weeks) at 4 °C, and could probably also be freeze-dried, stored and resuspended as powders, renders them interesting as a possible remedy against this or related fungal infections, when more aggressive, synthetic drugs are not a first choice of treatment.
It should also be emphasized once more that plants such as Cynomorium represent a renewable and hence sustainable, environmentally friendly resource. Their production, processing and subsequent applications may—literally—open up new fields of ecological and economical farming.
5. Conclusions
In future, the approach to nanosizing entire medicinal plants or parts thereof needs to be investigated further. Simultaneously, the method needs to be refined, for instance, to produce smaller and more uniform particles. It may also be interesting to explore avenues for lyophilising and resuspending such particles in order to increase their stability and storage properties. Eventually, this line of investigation may lead to more applied research, i.e., to larger-scale methods for industrial production. On the other side, the more basic, chemical and pharmacological aspects need to be addressed further. Here, the question of the release of specific biologically active substances, their mode(s) of action, the issue of nanotoxicology, for instance due to physical damage caused by the fibrous nature of the particles, and of uptake, excretion and degradation may need to be addressed. Eventually, one may also see Cynomorium as just one example of an emerging class of “milling friendly” plants, and may branch out to other, similar renewable materials, such as barks, shells or even spikes of plants, i.e., of dry, hard and brittle materials that promise straight-forward nanosizing and, literally, fine particles.