A Novel Invention for Controlled Plant Cutting Growth: Chamber Design Enabling Data Collection for AI Tasks
Abstract
1. Introduction
2. Materials and Methods
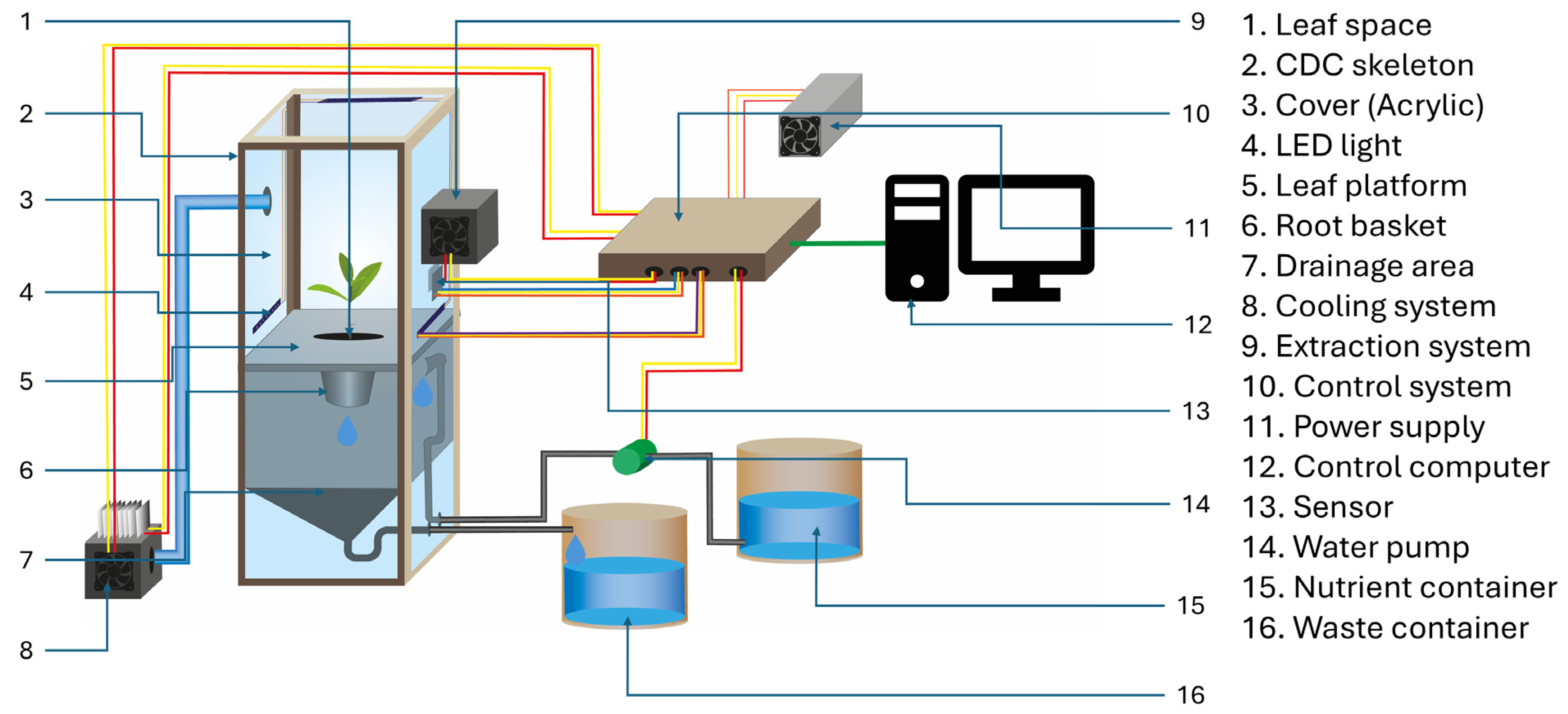
2.1. Development of the CDC
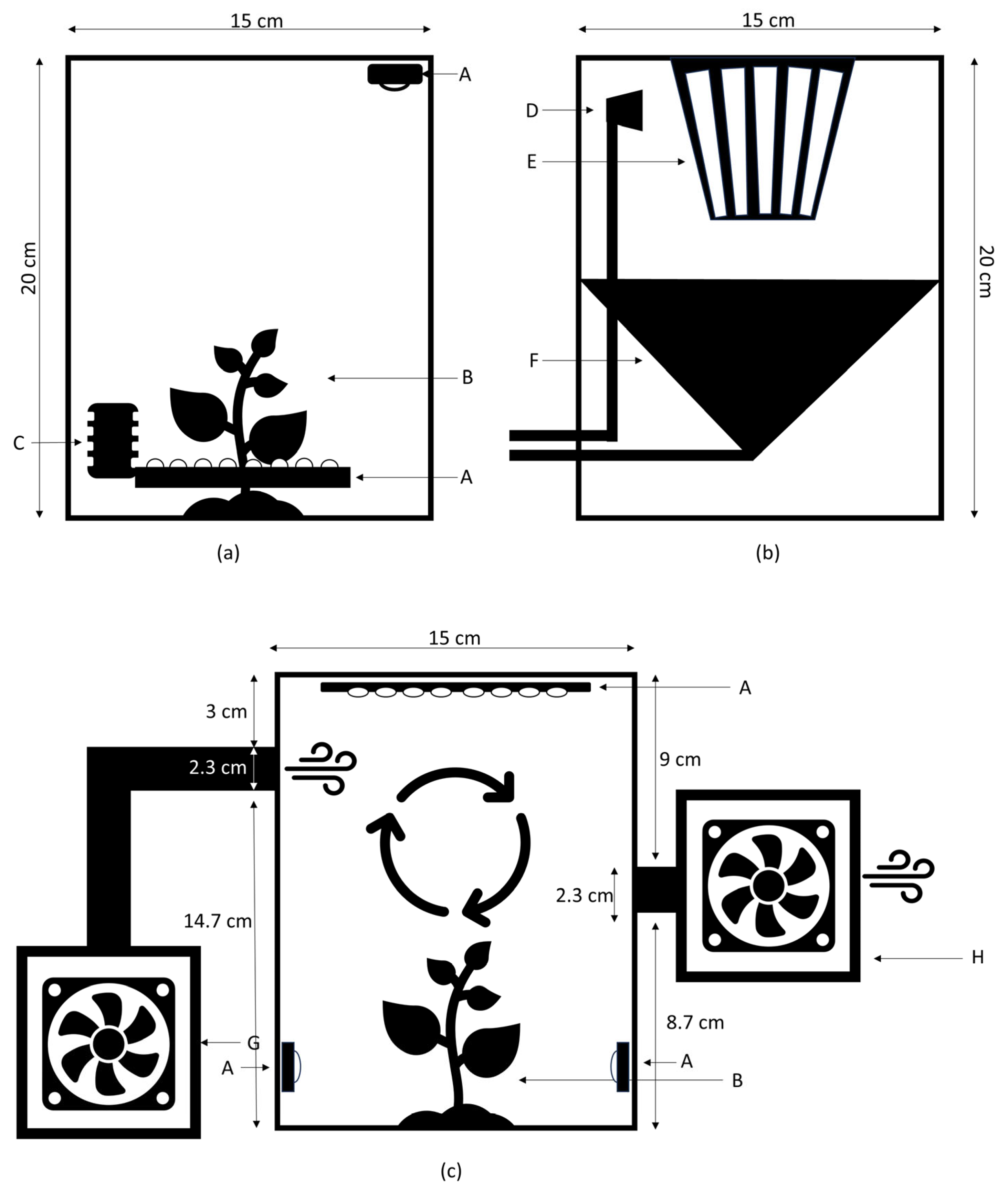
2.2. Conception and Structural Design
2.3. Development and Prototyping
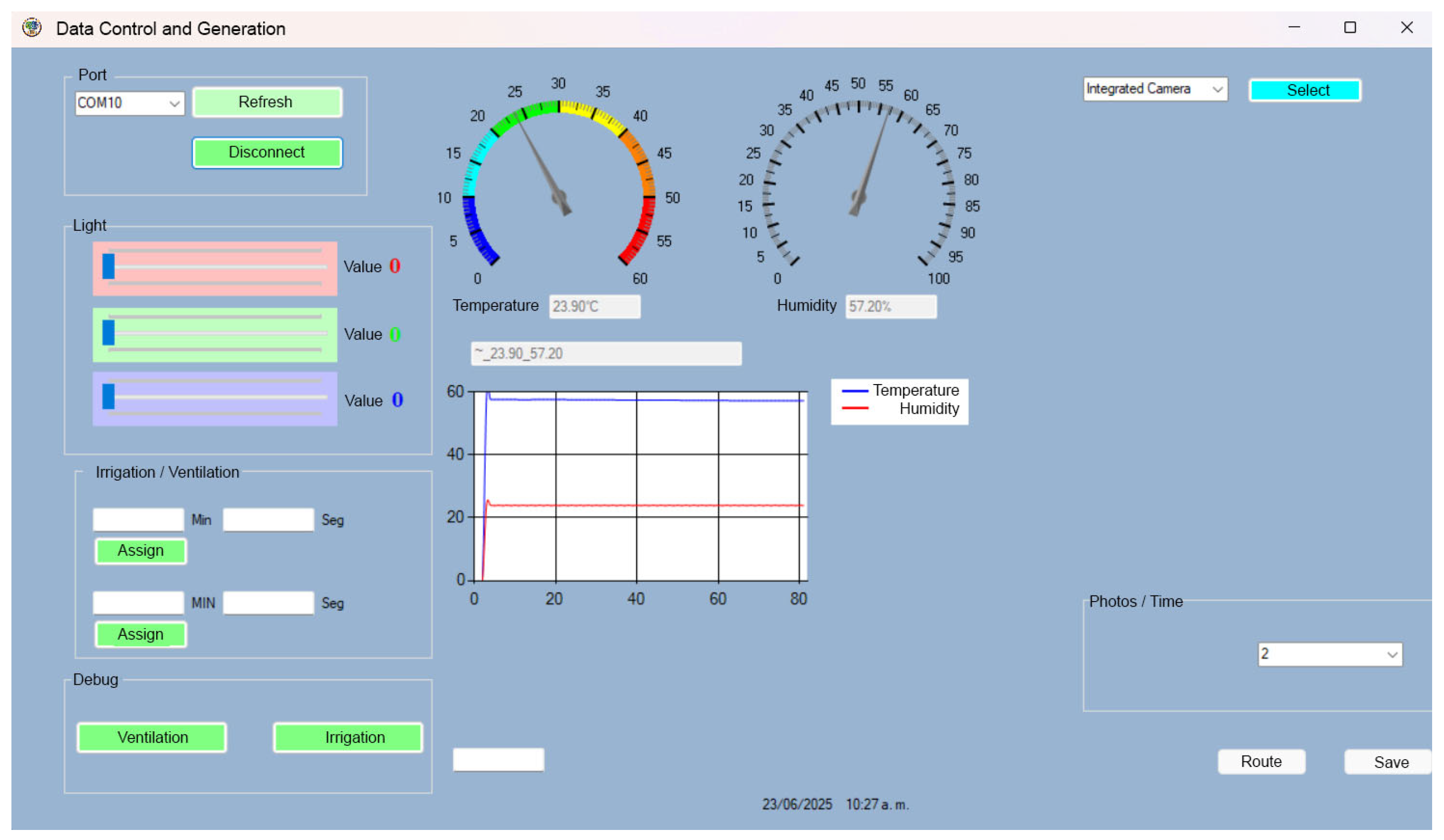
2.4. Development of Environmental Control Systems
3. Results
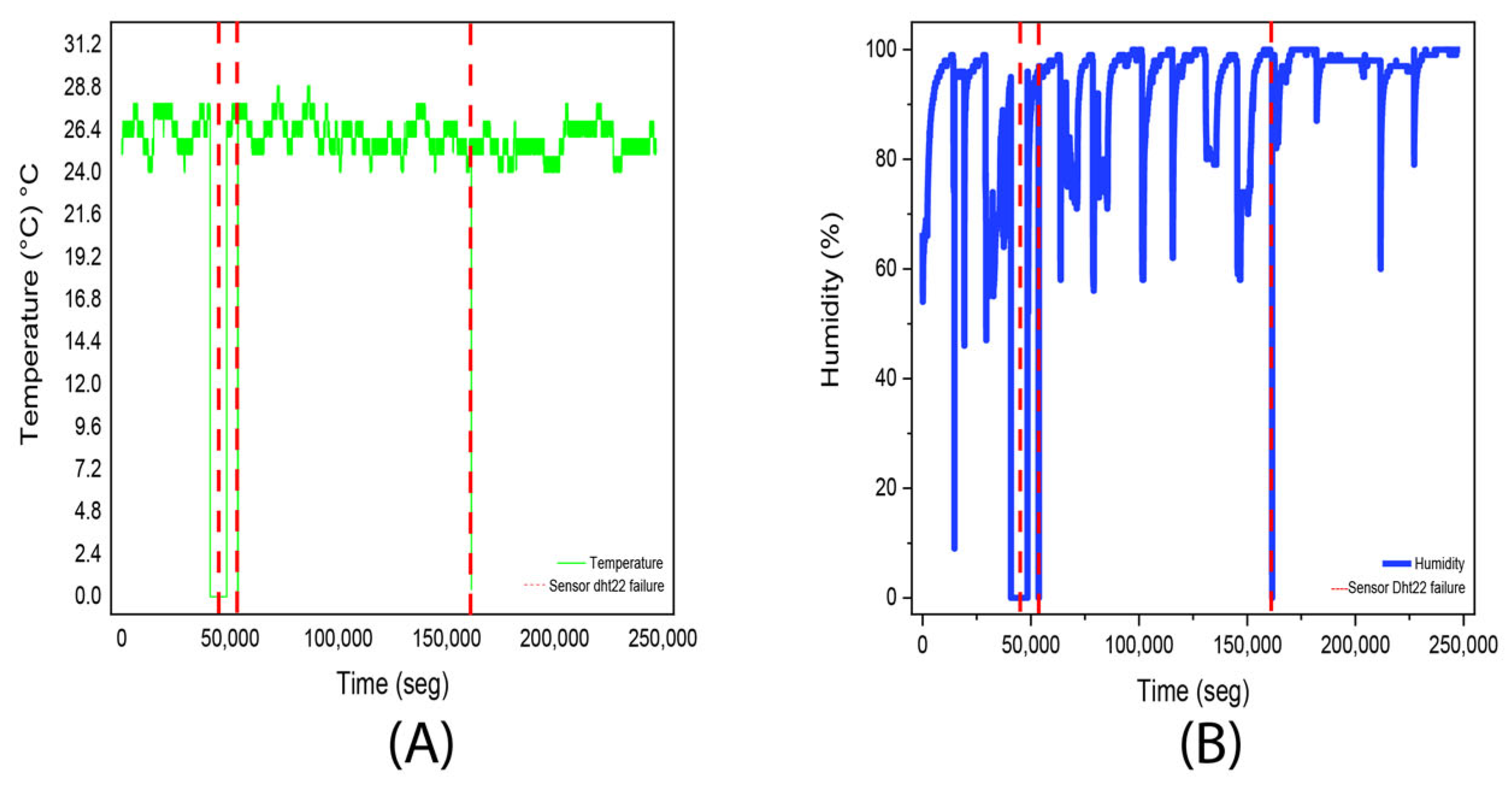
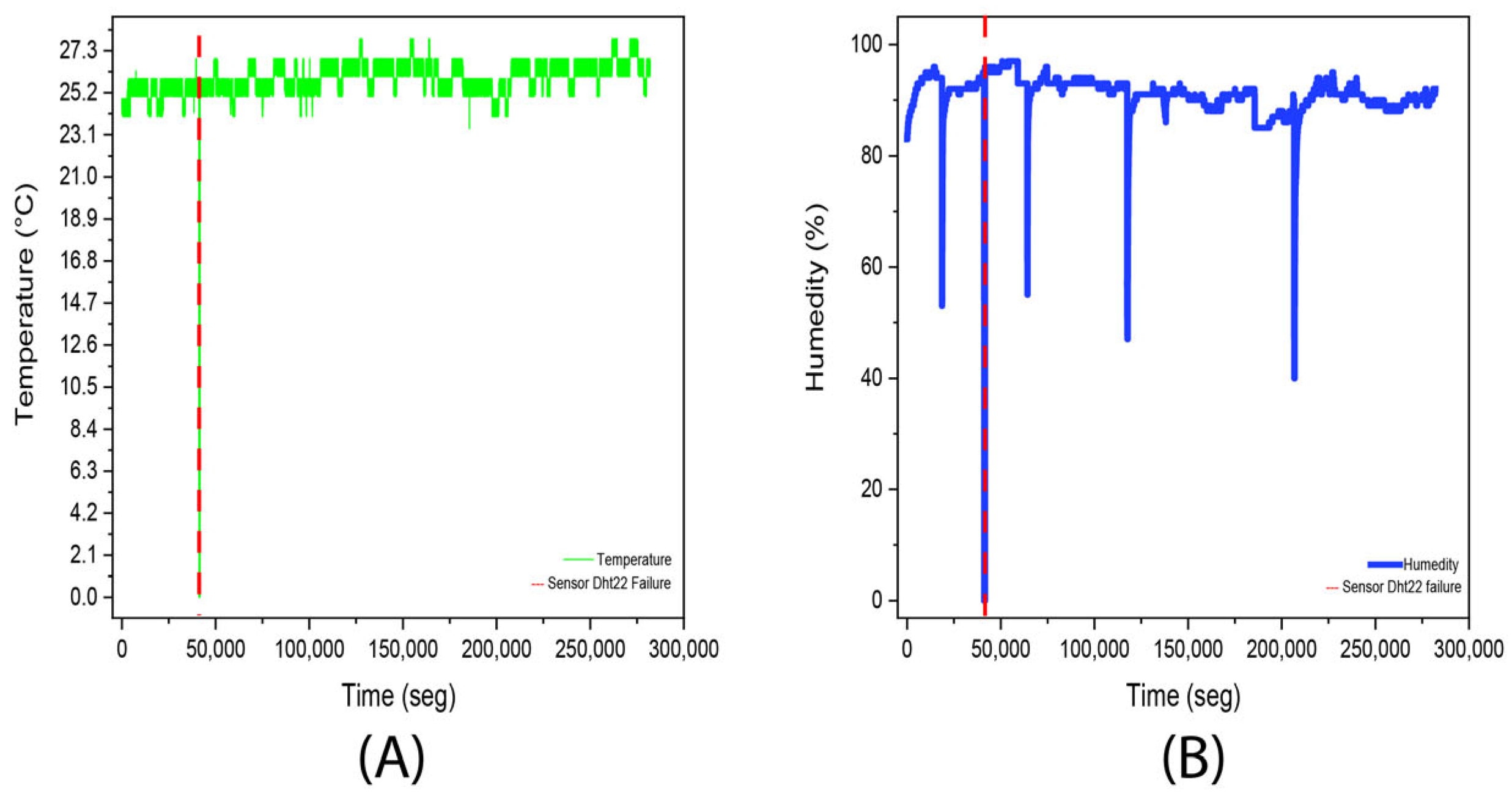
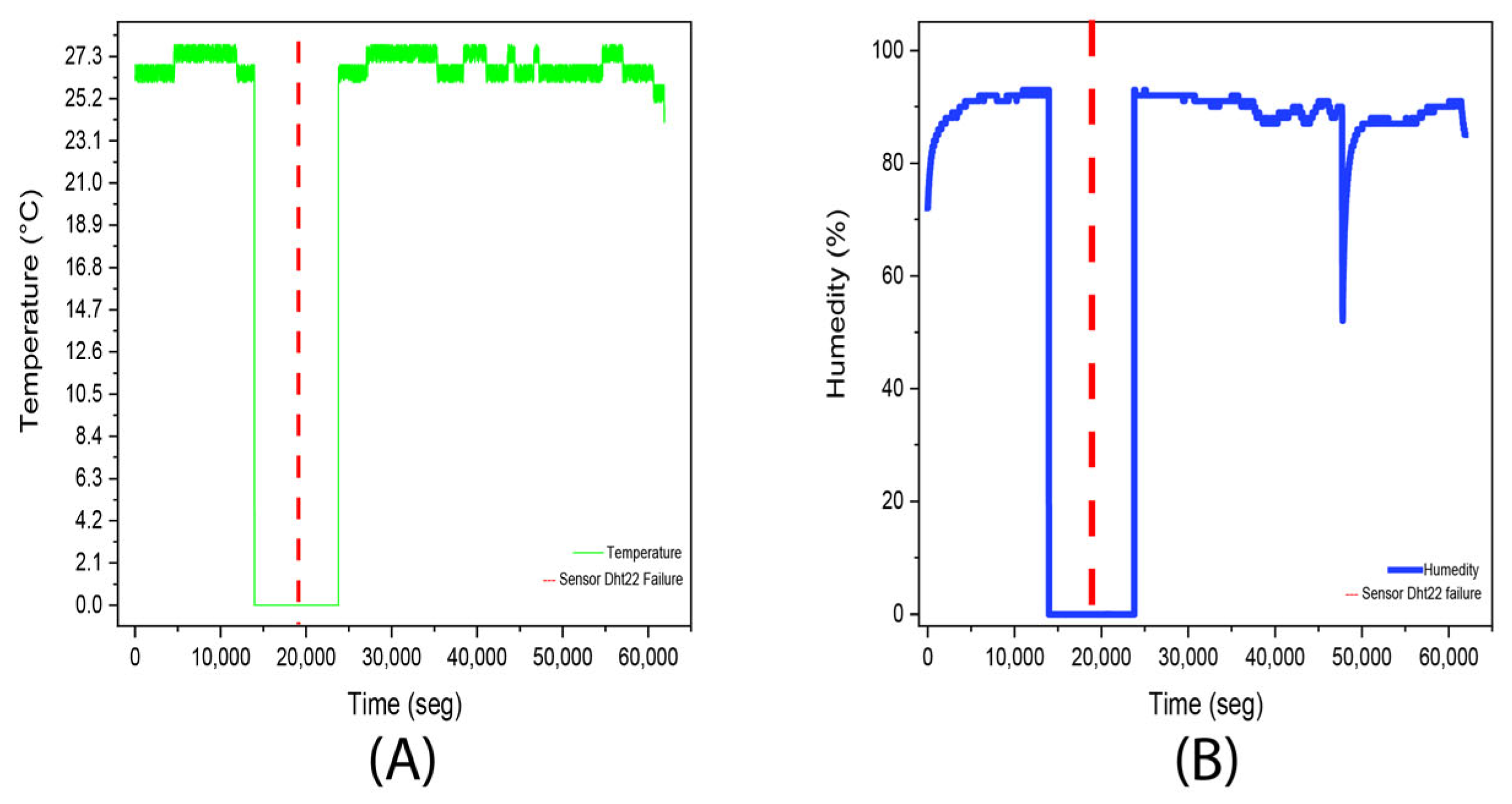
3.1. Experiment 1. (Aloysia citrodora)
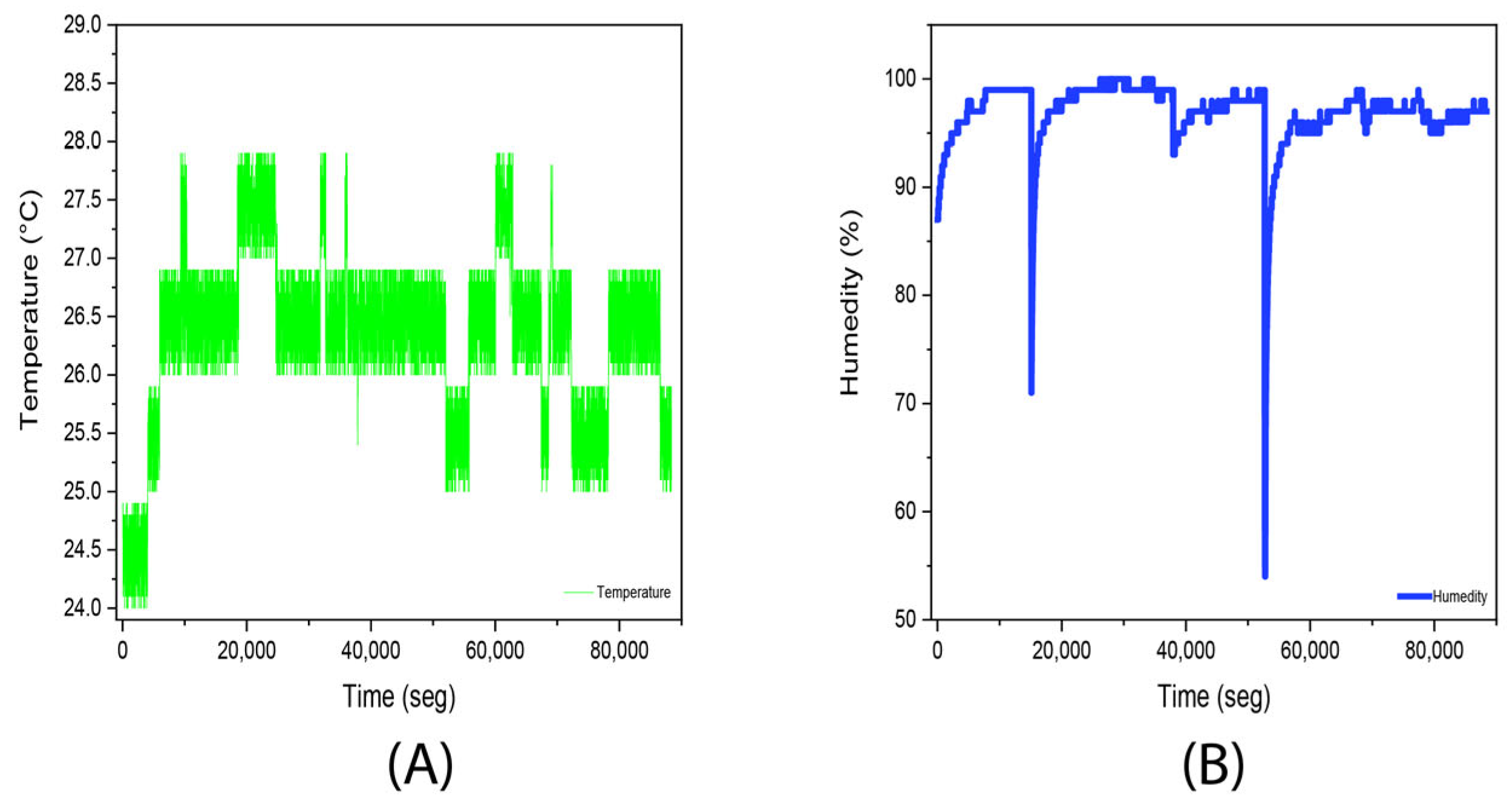
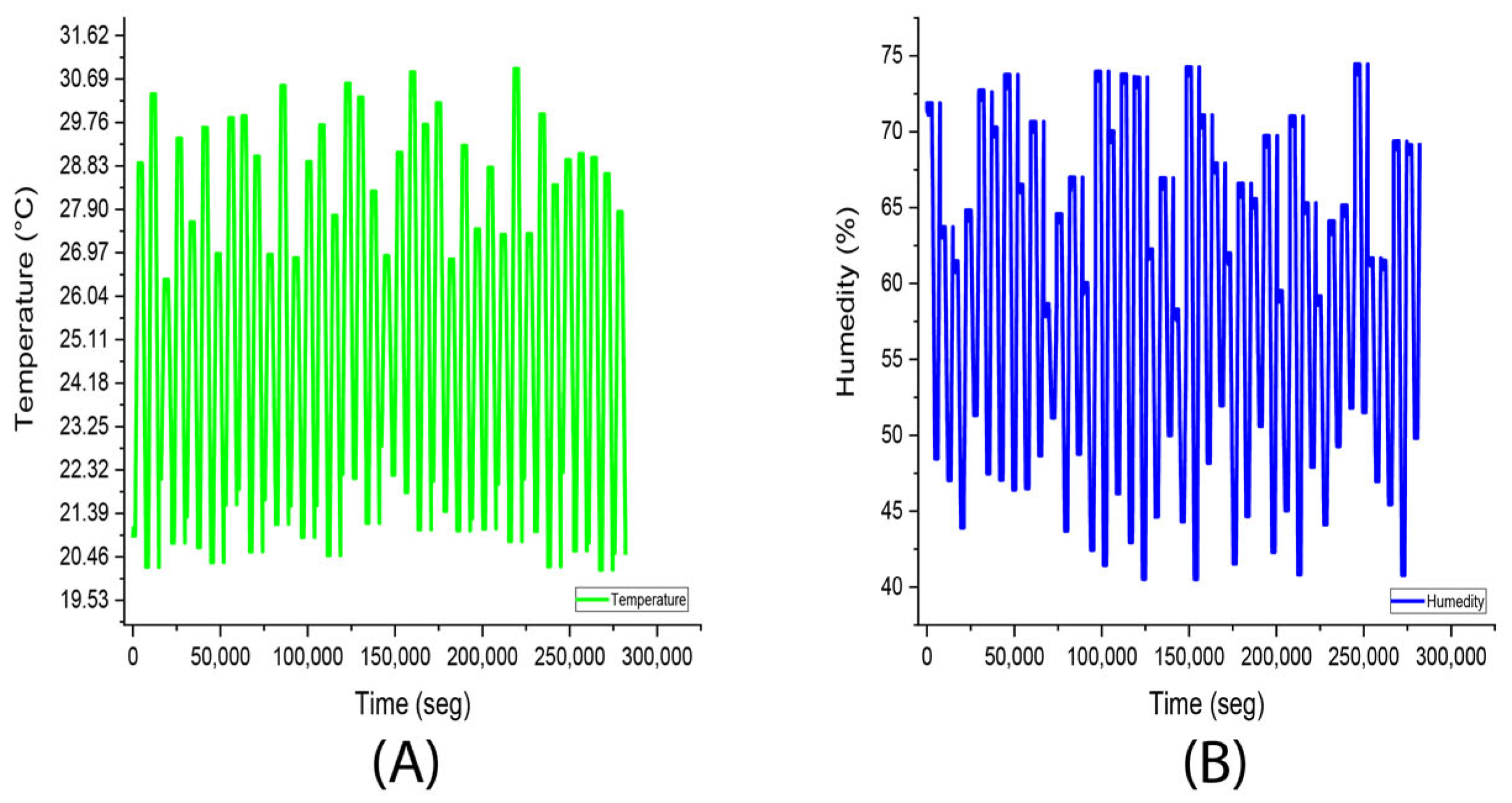
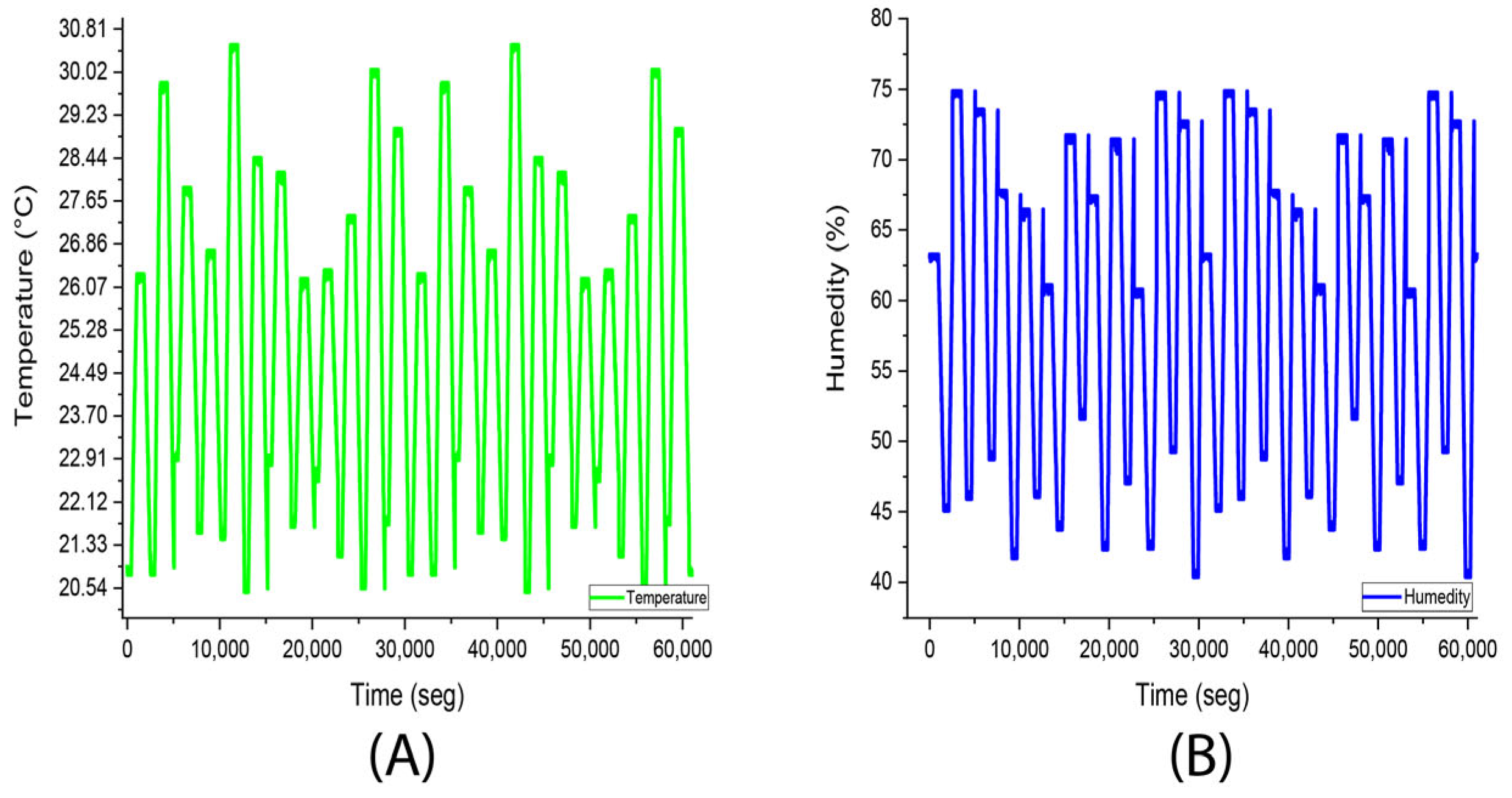
3.2. Experiment 2. (Stevia Rebaudiana Red Light)
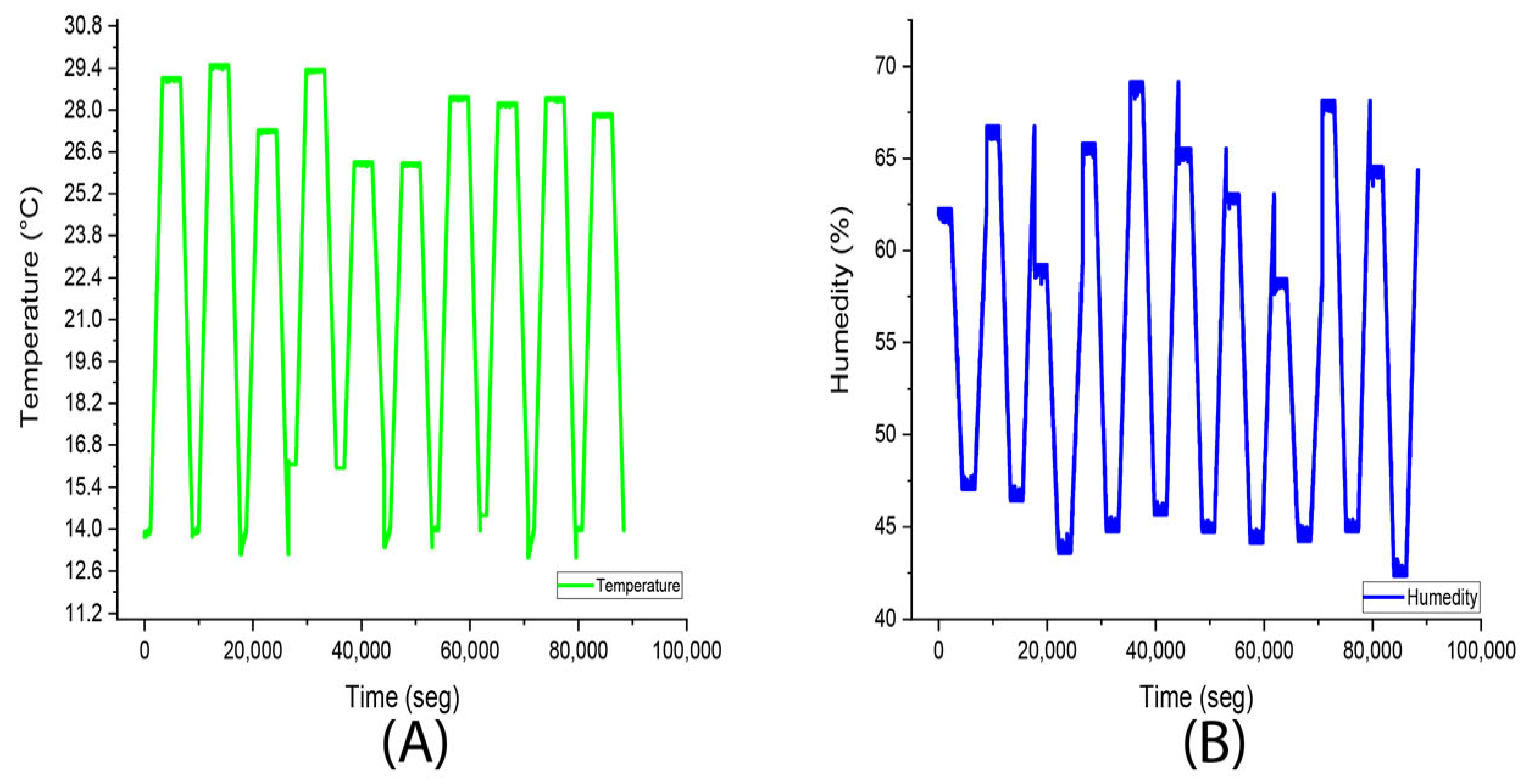
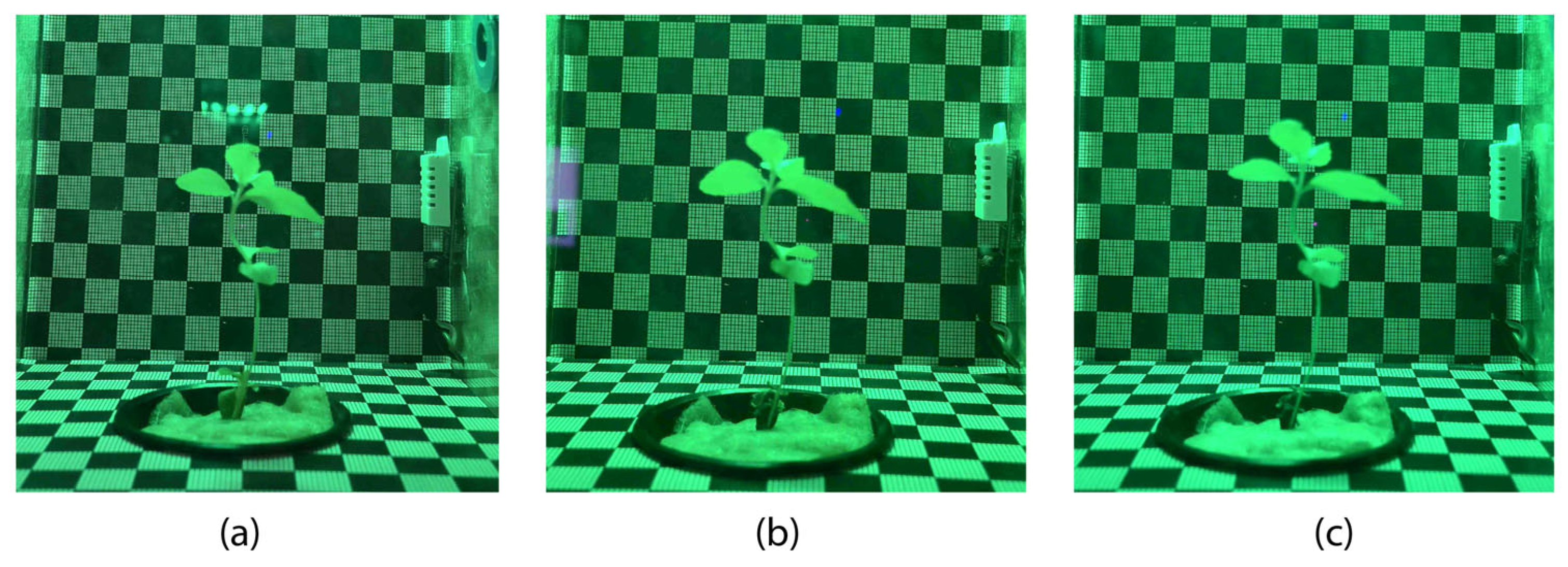
3.3. Experiment 3 (Stevia rebaudiana Green Light)
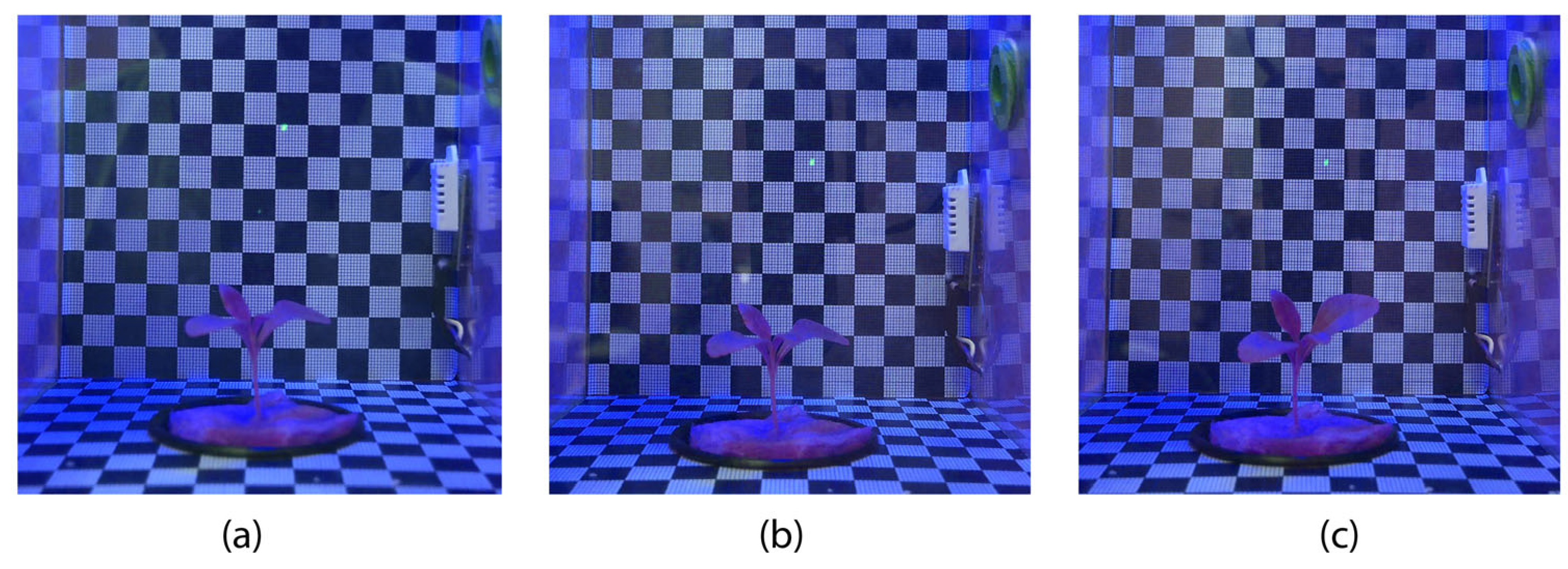
3.4. Experiment 4 (Stevia rebaudiana Blue Light)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Needs and Qualifications
| Need Category | No. | User Need (What the User Wants to Achieve or Avoid) | Average |
|---|---|---|---|
| Environmental control | 1 | Maintain a constant and precise temperature. | 5 |
| 2 | Ensure an optimal level of relative humidity. | 4.3 | |
| 3 | Provide adequate lighting (spectrum and photoperiod). | 5 | |
| 4 | Regulate CO2 levels. | 3.6 | |
| 5 | Ensure smooth, even air circulation. | 4.7 | |
| 6 | Control the temperature of the substrate/root. | 1.6 | |
| 7 | Eliminate excess heat generated by lighting. | 4.6 | |
| 8 | Minimize condensation inside the chamber. | 5 | |
| Monitoring and Data | 9 | Know the exact temperature in real time. | 4.8 |
| 10 | View the humidity level at all times. | 4.8 | |
| 11 | Measure light intensity (PAR). | 4.4 | |
| 12 | Record CO2 levels over time. | 3.9 | |
| 13 | Obtain periodic high-resolution images of each cutting. | 5 | |
| 14 | Automatically measure the height and leaf size of each cutting. | 4.8 | |
| 15 | Detect abnormalities or signs of stress/disease in cuttings. | 4.2 | |
| 16 | Access a complete history of environmental conditions. | 1.2 | |
| 17 | Collect data on the pH and EC of the culture medium. | 1.5 | |
| 18 | Receive alerts if parameters go out of range. | 0.3 | |
| 19 | Export data in compatible formats (CSV, Excel). | 5 | |
| 20 | View graphs and trends of collected data. | 4.8 | |
| Automation and Control | 21 | Automate the irrigation/misting cycle. | 5 |
| 22 | Program the photoperiod and light intensity. | 4.5 | |
| 23 | Adjust the fan speed. | 2.9 | |
| 24 | Control the camera remotely (via mobile/web app). | 3.2 | |
| 25 | Configure different “recipes” or growth profiles. | 1.2 | |
| Design and Ergonomics | 26 | That the camera is compact and does not take up much space. | 5 |
| 27 | Make it easy to clean and disinfect. | 4.7 | |
| 28 | Make access to the cuttings easy and unobstructed. | 5 | |
| 29 | That it has a robust and durable construction. | 4.8 | |
| 30 | That the internal materials are non-toxic to plants. | 4.9 | |
| 31 | That it is silent in operation. | 4.5 | |
| 32 | That the design is aesthetic and professional. | 5 | |
| Maintenance and Longevity | 33 | That the main components are easy to replace. | 5 |
| 34 | Requiring minimal maintenance. | 4.8 | |
| 35 | That it is energy efficient. | 4.7 | |
| 36 | That the light source has a long useful life. | 4.5 | |
| 37 | That the camera has readily available spare parts. | 4.7 |
Appendix B. Prototype Development and Testing
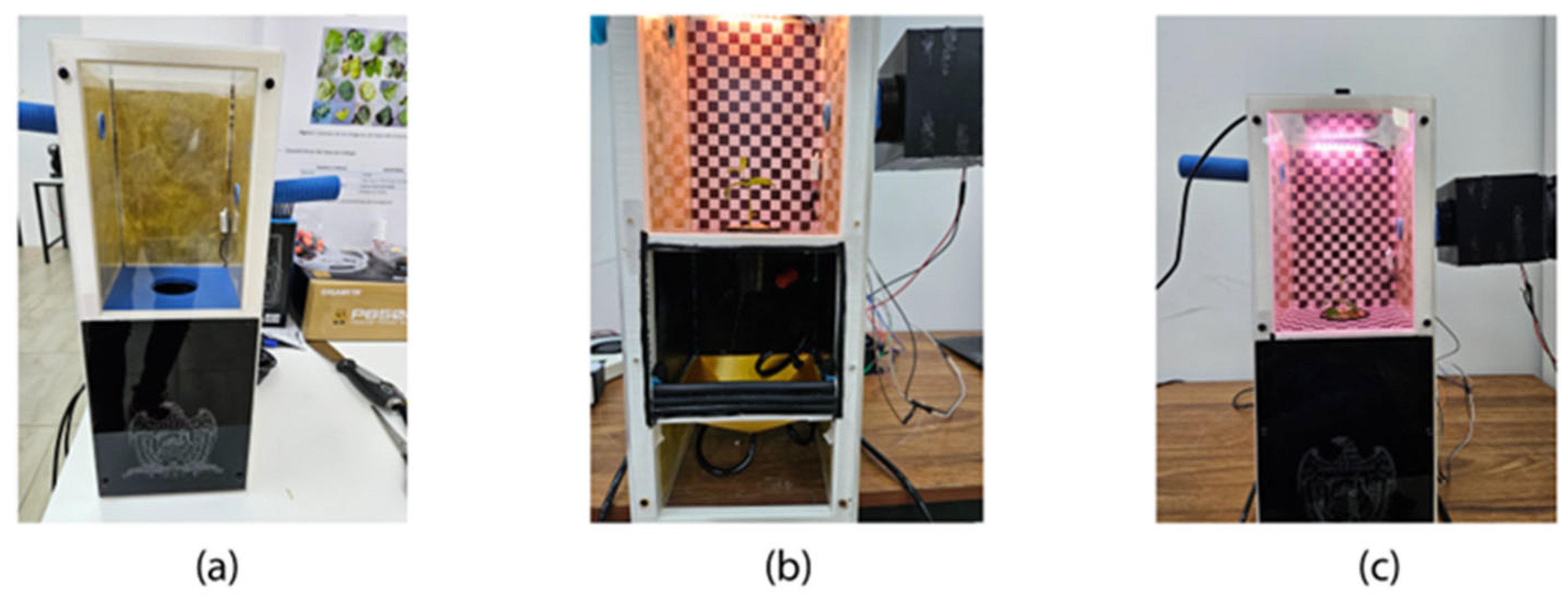
References
- Charles, H.; Beddington, J.R.; Crute, I.R.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Sandy, M.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Available online: www.sciencemag.org (accessed on 3 November 2025).
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium- and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Lin, B.B.; Philpott, S.M.; Jha, S. The future of urban agriculture and biodiversity-ecosystem services: Challenges and next steps. Basic Appl. Ecol. 2015, 16, 189–201. [Google Scholar] [CrossRef]
- Armanda, D.T.; Guinée, J.B.; Tukker, A. The second green revolution: Innovative urban agriculture’s contribution to food security and sustainability—A review. Glob. Food Secur. 2019, 22, 13–24. [Google Scholar] [CrossRef]
- Dennison, M.S.; Kumar, P.S.; Wamyil, F.; Meji, M.A.; Ganapathy, T. The role of automation and robotics in transforming hydroponics and aquaponics to large scale. Discov. Sustain. 2025, 6, 1–35. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, T.; Gan, R.; Wang, Z.; Peng, J.; Hu, J.; Guo, J.; Zhang, Y.; Li, Q.; et al. Plant factory technology lights up urban horticulture in the post-coronavirus world. Hortic. Res. 2022, 9, uhac018. [Google Scholar] [CrossRef]
- Mun, H.-S.; Lagua, E.B.; Hong, S.-K.; Ryu, S.-B.; Sharifuzzaman; Hasan, K.; Kim, Y.-H.; Yang, C.-J. Energy-Efficient Technologies and Strategies for Feasible and Sustainable Plant Factory Systems. Sustainability 2025, 17, 3259. [Google Scholar] [CrossRef]
- Garcillanosa, M.M.; Batino, Z.M., Jr.; Gabuyo, E.A.; Mojica, T.L.; Del Mundo, M.A.; Impelido, D.G.; Sison, M.P.A.A. Efficient and Low-Cost Method of Smart Indoor Vertical Farming for Lactuca sativa L. with Machine Learning. Chem. Eng. Trans. 2023, 106, 535–540. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, B.; Zhou, H.; Zhou, L.; Niu, K.; Jin, X.; Li, R.; Yuan, Y.; Zheng, Y. Digital Twins in Plant Factory: A Five-Dimensional Modeling Method for Plant Factory Transplanter Digital Twins. Agriculture 2023, 13, 1336. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep learning in agriculture: A survey. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Hughes, D.P.; Salathé, M. Using deep learning for image-based plant disease detection. Front. Plant Sci. 2016, 7, 1419. [Google Scholar] [CrossRef]
- Ayan, E.; Erbay, H.; Varçın, F. Crop pest classification with a genetic algorithm-based weighted ensemble of deep convolutional neural networks. Comput. Electron. Agric. 2020, 179, 105809. [Google Scholar] [CrossRef]
- Liu, W.; Han, T.; Wang, C.; Zhang, F.; Xu, Z. Predicting indoor temperature of solar green house by machine learning algorithms: A comparative analysis and a practical approach. Smart Agric. Technol. 2025, 12, 101096. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. High-productive agricultural technologies to fulfill future food demands: Hydroponics, aquaponics, and precision/smart agriculture. In Global Trends, Opportunities, and Sustainability Challenges; Academic Press: London, UK, 2022; pp. 555–567. ISBN 978-0-323-91001-9. [Google Scholar] [CrossRef]
- Dsouza, A.; Newman, L.; Graham, T.; Fraser, E.D. Exploring the landscape of controlled environment agriculture research: A systematic scoping review of trends and topics. Agric. Syst. 2023, 209, 103673. [Google Scholar] [CrossRef]
- Ampim, P.A.Y.; Obeng, E.; Olvera-Gonzalez, E. Indoor Vegetable Production: An Alternative Approach to Increasing Cultivation. Plants 2022, 11, 2843. [Google Scholar] [CrossRef]
- Lozano-Castellanos, L.F.; Navas-Gracia, L.M.; Lozano-Castellanos, I.C.; Correa-Guimaraes, A. Technologies Applied to Artificial Lighting in Indoor Agriculture: A Review. Sustainability 2025, 17, 3196. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2014, 49, 139–147. [Google Scholar] [CrossRef]
- AI Boosts Indoor Food Production’s Energy Sustainability|Cornell Chronicle. Available online: https://news.cornell.edu/stories/2024/09/ai-boosts-indoor-food-productions-energy-sustainability?utm_source=chatgpt.com (accessed on 11 August 2025).
- Cai, W.; Bu, K.; Zha, L.; Zhang, J.; Lai, D.; Bao, H. Energy consumption of plant factory with artificial light: Challenges and opportunities. Renew. Sustain. Energy Rev. 2024, 210, 115235. [Google Scholar] [CrossRef]
- Comprar Propagador Con Calefacción Para Germinación Y Esquejes Elcogollo.es. Available online: https://www.elcogollo.es/accesorios/2111-propagador-electrico-pequeno-38x24x19-cms.html (accessed on 11 August 2025).
- Comprar Armario Para Esquejes Garden High Pro 60 × 40 × 2,00 Metros Elcogollo.es. Available online: https://www.elcogollo.es/propagadores-invernaderos-cannabis/5532-armario-propagador-l-garden-highpro.html (accessed on 11 August 2025).
- Comprar Armario Propagador Esquejes XL High Pro Elcogollo.es. Available online: https://www.elcogollo.es/propagadores-invernaderos-cannabis/5533-armario-propagador-xl-garden-highpro.html (accessed on 11 August 2025).
- Cámaras Climáticas Tipo Fitotrones Para Cultivo E Investigación de Plantas. Available online: https://www.mpcontrol.es/equipos/camaras-climaticas-fitotrones-para-cultivo-crecimiento-e-investigacion-de-plantas-e-insectos (accessed on 11 August 2025).
- Greenhouse Sensor Data-10 Minute Interval. Available online: https://www.kaggle.com/datasets/marcelboonman/greenhouse-sensor-data-10-minute-interval?utm_source=chatgpt.com (accessed on 11 August 2025).
- Ihoume, I.; Tadili, R.; Arbaoui, N.; Benchrifa, M.; Idrissi, A.; Daoudi, M. Developing a TinyML-Oriented Deep Learning Model for an Intelligent Greenhouse Microclimate Control from Multivariate Sensed Data. Lect. Notes Netw. Syst. 2023, 579, 283–291. [Google Scholar] [CrossRef]
- Ećim-Đurić, O.; Milanović, M.; Dimitrijević-Petrović, A.; Mileusnić, Z.; Dragičević, A.; Miodragović, R. Prediction of Greenhouse Microclimatic Parameters Using Building Transient Simulation and Artificial Neural Networks. Agronomy 2024, 14, 1147. [Google Scholar] [CrossRef]
- Rasheed, A.; Kwak, C.S.; Kim, H.T.; Lee, H.W. Building Energy an Simulation Model for Analyzing Energy Saving Options of Multi-Span Greenhouses. Appl. Sci. 2020, 10, 6884. [Google Scholar] [CrossRef]
- Ahamed, M.S.; Guo, H.; Tanino, K. Energy-efficient design of greenhouse for Canadian Prairies using a heating simulation model. Int. J. Energy Res. 2018, 42, 2263–2272. [Google Scholar] [CrossRef]
- Boersma, S.; Cheng, X. A Bayesian Neural ODE for a Lettuce Greenhouse. In Proceedings of the 2024 IEEE Conference on Control Technology and Applications, CCTA 2024, Newcastle upon Tyne, UK, 21–23 August 2024; pp. 782–786. [Google Scholar] [CrossRef]
- Jara-Villagrana, M.F.; Olvera-Olvera, C.A.; Villagrana-Barraza, S.; Castro-Tapia, S.; Ibarra-Delgado, S.; Gómez-Rodríguez, J.R.; Sandoval-Aréchiga, R.; Rodríguez-Abdalá, V.I.; Díaz-Flórez, G. Conceptual Design Based on Modular Platforms for a Prototype of a Functional Growth Chamber for Cuttings in Controlled Agriculture. Designs 2025, 9, 86. [Google Scholar] [CrossRef]
- Guerrero-Sánchez, J.; Olvera-Olvera, C.A.; Solis-Sánchez, L.O.; Martínez-Blanco, M.D.R.; López-Martínez, M.d.J.; Castañeda-Miranda, C.L.; Soto-Zarazúa, G.M.; Díaz-Flórez, G. Design and Implementation of a Low-Cost Controlled-Environment Growth Chamber for Vegetative Propagation of Mother Plants. Agriengineering 2025, 7, 177. [Google Scholar] [CrossRef]
- van der Borg, G.; Warner, H.; Ioannidis, M.; Bogaart, G.v.D.; Roos, W.H. PLA 3D Printing as a Straightforward and Versatile Fabrication Method for PDMS Molds. Polymers 2023, 15, 1498. [Google Scholar] [CrossRef]
- Shukla, M.R.; Singh, A.S.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods 2017, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Compute Heat Index with Arduino and DHT Sensor One Transistor. Available online: https://www.onetransistor.eu/2018/01/compute-heat-index-arduino-dht.html?utm_source=chatgpt.com (accessed on 11 August 2025).
- Full Spectrum, Vs. Red/Blue LED Grow Lights|P.L. Light Systems. Available online: https://pllight.com/articles/2020/09/01/full-spectrum-vs-red-blue-leds/?utm_source=chatgpt.com (accessed on 11 August 2025).
- LED Grow Light (WS2812*10)—Smarthon Documentation 1.0 Documentation. Available online: https://smarthon-docs-en.readthedocs.io/en/latest/Sensors_and_actuators/LED_Grow_Light.html?utm_source=chatgpt.com (accessed on 11 August 2025).
- Złotkowska, E.; Wlazło, A.; Kiełkiewicz, M.; Misztal, K.; Dziosa, P.; Soja, K.; Barczak-Brzyżek, A.; Filipecki, M. Automated imaging coupled with AI-powered analysis accelerates the assessment of plant resistance to Tetranychus urticae. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Yasrab, R.; Zhang, J.; Smyth, P.; Pound, M.P. Predicting Plant Growth from Time-Series Data Using Deep Learning. Remote. Sens. 2021, 13, 331. [Google Scholar] [CrossRef]
- Xu, J.; Liu, H.; Shen, Y. Image and Point Cloud-Based Neural Network Models and Applications in Agricultural Nursery Plant Protection Tasks. Agronomy 2025, 15, 2147. [Google Scholar] [CrossRef]
- Tütüncü, M. Application of machine learning in in vitro propagation of endemic Lilium akkusianum R. Gämperle. PLoS ONE 2024, 19, e0307823. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.; Sunil, G.; Horvath, D.; Sun, X. Deep learning for plant stress detection: A comprehensive review of technologies, challenges, and future directions. Comput. Electron. Agric. 2025, 229, 109734. [Google Scholar] [CrossRef]
- Krishna, M.S.; Machado, P.; Otuka, R.I.; Yahaya, S.W.; dos Santos, F.N.; Ihianle, I.K. Plant Leaf Disease Detection Using Deep Learning: A Multi-Dataset Approach. J 2025, 8, 4. [Google Scholar] [CrossRef]
- CN203433329U-Dispositivo Inteligente de Monitorización Remota de Invernaderos Mediante Internet de Las Cosas-Patentes de Google. Available online: https://patents.google.com/patent/CN203433329U/en (accessed on 3 November 2025).
- US7823328B2-Sistema de Cultivo Aeropónico de Plantas-Patentes de Google. Available online: https://patents.google.com/patent/US7823328B2/en (accessed on 3 November 2025).
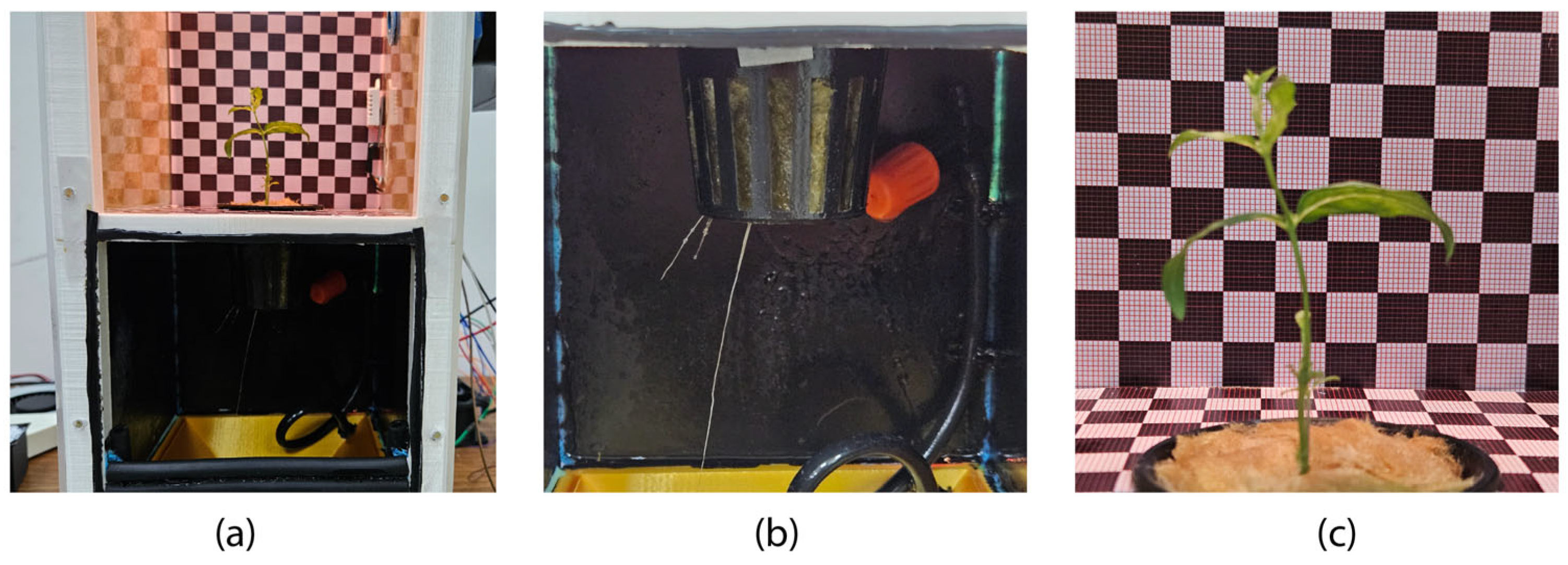



| System | Main Features | Dimensions (cm) | Estimated Cost USD |
|---|---|---|---|
| Electric propagator (Alicante, Spain) | “heated base, will maintain the interior of the greenhouse at approximately 20 °C” | 38 × 24 × 19 | 41.382 |
| L Garden HighPro Propagator Cabinet (Alicante, Spain) | reflective Nylon canvas | 40 × 40 × 200 | 134.52 |
| XL Garden HighPro Propagator Cabinet (Alicante, Spain) | “ultra-opaque and watertight 420D Nylon canvas, with 97% reflective Mylar interior” | 120 × 40 × 200 | 227.943 |
| PERCIVAL E-36L2 (Fremont County, IA, USA) | “metal and glass, temperature management (10–44 °C), Humidity management (20–95%), interior lighting” | 85.1 × 85.4 × 196.1 | 26,897.50 |
| Dataset Name | Description | Key Parameters | Format | Size (Approx.) |
|---|---|---|---|---|
| “Autonomous Greenhouse Challenge (AGC)—2nd Edition.” | Interior and exterior climate data, irrigation, actuators, consumption, harvest, cherry tomato parameters. | Temperature, humidity, CO2, light, irrigation, harvest, fruit quality. | CSV | Variable (multiple files) |
| “IoT Agriculture 2024.” | Interior and exterior sensor data. | Temperature, humidity, light, VOC, eCO2. | CSV | 1.5 MB |
| “Advanced IoT Agriculture.” | California agricultural data with environmental, soil, and crop parameters. | Nitrogen, Phosphorus, Potassium, temperature, humidity, pH, rain, crop type, soil moisture, etc. | CSV | Not specified |
| “Greenhouse Plant Growth.” | Plant metrics related to vegetative and root growth. | Plant growth metrics. | CSV | 1.8 MB |
| Simulator Name | Description | Key Features | Data Output Capabilities | License/Availability |
|---|---|---|---|---|
| TRNSYS | Transient systems simulation program for energy analysis in buildings, including greenhouses. | Component-based modeling, dynamic simulation of energy and thermal systems. | Indoor air temperature, energy requirements, evapotranspiration, ventilation. | Commercial |
| Modelica (Greenhouses Library) | Modeling language for physical systems, with a specific library for greenhouses. | Equation-based modeling, simulation of energy and mass flows. | Temperature, humidity, CO2, energy flows; text formats, potentially CSV. | Open Source (Modelica Standard Library) |
| Grinnell College Greenhouse Simulation | Online simulation for crop cultivation and data collection. | Interactive simulation of crop growth, data collection on yields and profits. | Tabular data on yields, profits, growth factors. | Free (online) |
| Number | Component Name | Description |
|---|---|---|
| 1 | DHT22 Sensor (Guangdong, China) | Temperature range: −40 to 80 °C. Humidity range: 0 to 100% RH. Accuracy: Temperature: ±0.5 °C, Humidity: ±2–5% RH. Resolution: Temperature: 0.1 °C, Humidity: 0.1%. Power: 3.3 V to 6 V DC. Communication: Digital |
| Wastewater collection system | 3D printed wastewater collection chamber with ABS material | |
| ABS exoskeleton | 3D printed exoskeleton with ABS material | |
| Acrylic walls | 4 3 mm thick acrylic walls measuring 15 cm × 40 cm 3 3 mm thick acrylic walls measuring 13 cm × 40 cm 2 3 mm thick acrylic walls measuring 15 cm × 15 cm | |
| 2 | NeoPixel WS2812b (Shenzhen, China) | Color Range: RGB. Pixel Count: 8. Power Supply: 5 V DC. Communication: Digital |
| 3 | Fan | Power supply: 12 V 1.0 A (Generic). |
| 4 | Irrigation system | 12 V 6.0 A pump (Generic). Flow rate: 6 L/min. Pressure: 0.9 MPA. Hoses and sprinklers |
| Temperature | Humidity | ||
|---|---|---|---|
| Average | 24.8 °C | Average | 89.8% |
| Median | 25 °C | Median | 97% |
| Fashion | 25 °C | Fashion | 98% |
| Standard deviation | 4.6 °C | Standard deviation | 19.1% |
| Minimum | 0 °C | Minimum | 0% |
| Maximum | 28 °C | Maximum | 100% |
| Account | 24,691 | Account | 24,691 |
| Data Set Name | Format | Number of Files | Data Set Size | Tags |
|---|---|---|---|---|
| ALCD | JPG | 1388 | 1.11 GB | Date, Time, Humidity and Temperature |
| ALCD | CSV | 1 | 837 KB | Date, Time, Humidity and Temperature |
| Temperature | Humidity | ||
|---|---|---|---|
| Average | 25.9 °C | Average | 96.8% |
| Median | 26 °C | Median | 97% |
| Fashion | 26 °C | Fashion | 97% |
| Standard deviation | 0.7 °C | Standard deviation | 3.2% |
| Minimum | 24 °C | Minimum | 54% |
| Maximum | 27 °C | Maximum | 100% |
| Account | 8839 | Account | 8839 |
| Data Set Name | Format | Number of files | Data Set Size | Tags |
|---|---|---|---|---|
| STVR | JPG | 885 | 307 MB | Date, Time, Humidity and Temperature |
| STVR | CSV | 1 | 557 KB | Date, Time, Humidity and Temperature |
| Temperature | Humidity | ||
|---|---|---|---|
| Average | 25.4 °C | Average | 90.8% |
| Median | 25 °C | Median | 91% |
| Fashion | 25 °C | Fashion | 93% |
| Standard deviation | 1.2 °C | Standard deviation | 5.1% |
| Minimum | 0 °C | Minimum | 0% |
| Maximum | 27 °C | Maximum | 97% |
| Account | 28,199 | Account | 28,199 |
| Data Set Name | Format | Number of Files | Data Set Size | Tags |
|---|---|---|---|---|
| STVG | JPG | 2153 | 1.23 GB | Date, Time, Humidity and Temperature |
| STVG | CSV | 1 | 1.35 MB | Date, Time, Humidity and Temperature |
| Temperature | Humidity | ||
|---|---|---|---|
| Average | 26.3 °C | Average | 88.3% |
| Median | 26 °C | Median | 89% |
| Fashion | 26 °C | Fashion | 87% |
| Standard deviation | 0.5 °C | Standard deviation | 3.8% |
| Minimum | 24 °C | Minimum | 52% |
| Maximum | 27 °C | Maximum | 92% |
| Account | 3035 | Account | 3035 |
| Data Set Name | Format | Number of Files | Data Set Size | Tags |
|---|---|---|---|---|
| STVB | JPG | 2153 | 1.23 GB | Date, Time, Humidity and Temperature |
| STVB | CSV | 1 | 1.35 MB | Date, Time, Humidity and Temperature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Sánchez, J.G.; López-Martínez, M.d.J.; Maeda-Gutiérrez, V.; López-Monteagudo, F.E.; Castañeda-Miranda, C.L.; Rivera-Escobedo, M.; Verlienden, S.; Soto-Zarazua, G.M.; Olvera-Olvera, C.A. A Novel Invention for Controlled Plant Cutting Growth: Chamber Design Enabling Data Collection for AI Tasks. Inventions 2025, 10, 108. https://doi.org/10.3390/inventions10060108
Ávila-Sánchez JG, López-Martínez MdJ, Maeda-Gutiérrez V, López-Monteagudo FE, Castañeda-Miranda CL, Rivera-Escobedo M, Verlienden S, Soto-Zarazua GM, Olvera-Olvera CA. A Novel Invention for Controlled Plant Cutting Growth: Chamber Design Enabling Data Collection for AI Tasks. Inventions. 2025; 10(6):108. https://doi.org/10.3390/inventions10060108
Chicago/Turabian StyleÁvila-Sánchez, Jesús Gerardo, Manuel de Jesús López-Martínez, Valeria Maeda-Gutiérrez, Francisco E. López-Monteagudo, Celina L. Castañeda-Miranda, Manuel Rivera-Escobedo, Sven Verlienden, Genaro M. Soto-Zarazua, and Carlos A. Olvera-Olvera. 2025. "A Novel Invention for Controlled Plant Cutting Growth: Chamber Design Enabling Data Collection for AI Tasks" Inventions 10, no. 6: 108. https://doi.org/10.3390/inventions10060108
APA StyleÁvila-Sánchez, J. G., López-Martínez, M. d. J., Maeda-Gutiérrez, V., López-Monteagudo, F. E., Castañeda-Miranda, C. L., Rivera-Escobedo, M., Verlienden, S., Soto-Zarazua, G. M., & Olvera-Olvera, C. A. (2025). A Novel Invention for Controlled Plant Cutting Growth: Chamber Design Enabling Data Collection for AI Tasks. Inventions, 10(6), 108. https://doi.org/10.3390/inventions10060108







