A Review of Heat and Energy Recovery Possibilities Within CO2 Refrigeration Systems
Abstract
1. Introduction
2. CO2-Based Refrigeration Systems
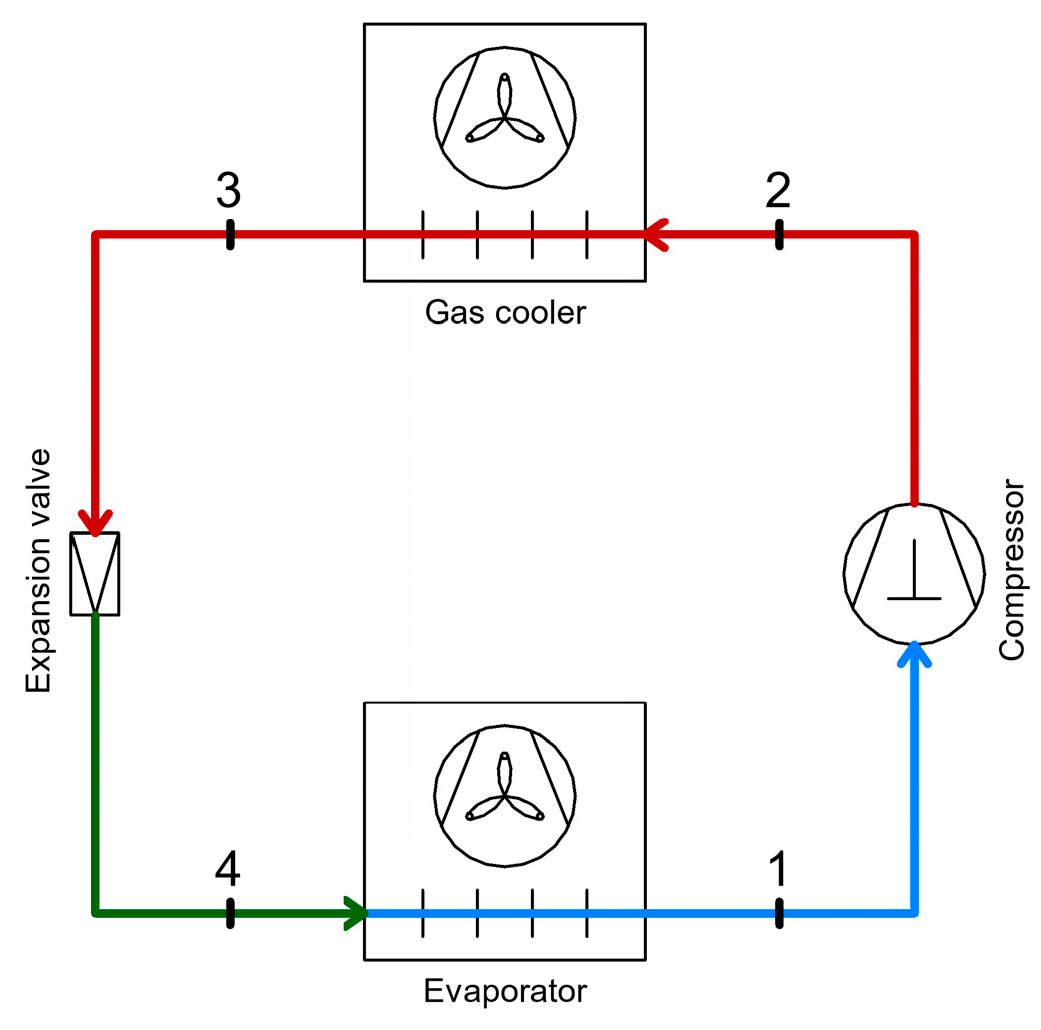
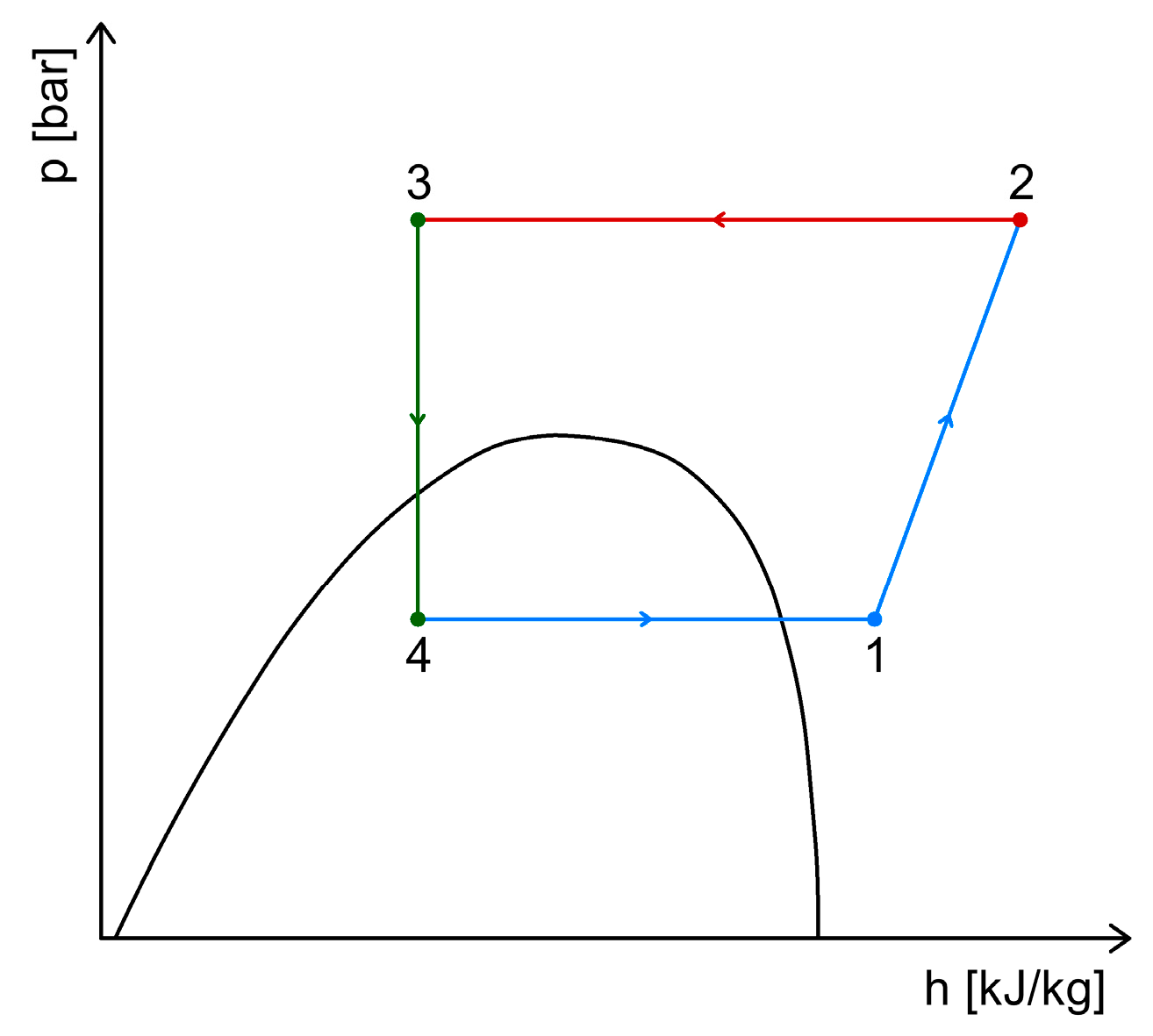
2.1. Simple CO2 Refrigeration System Operating in Transcritical Mode
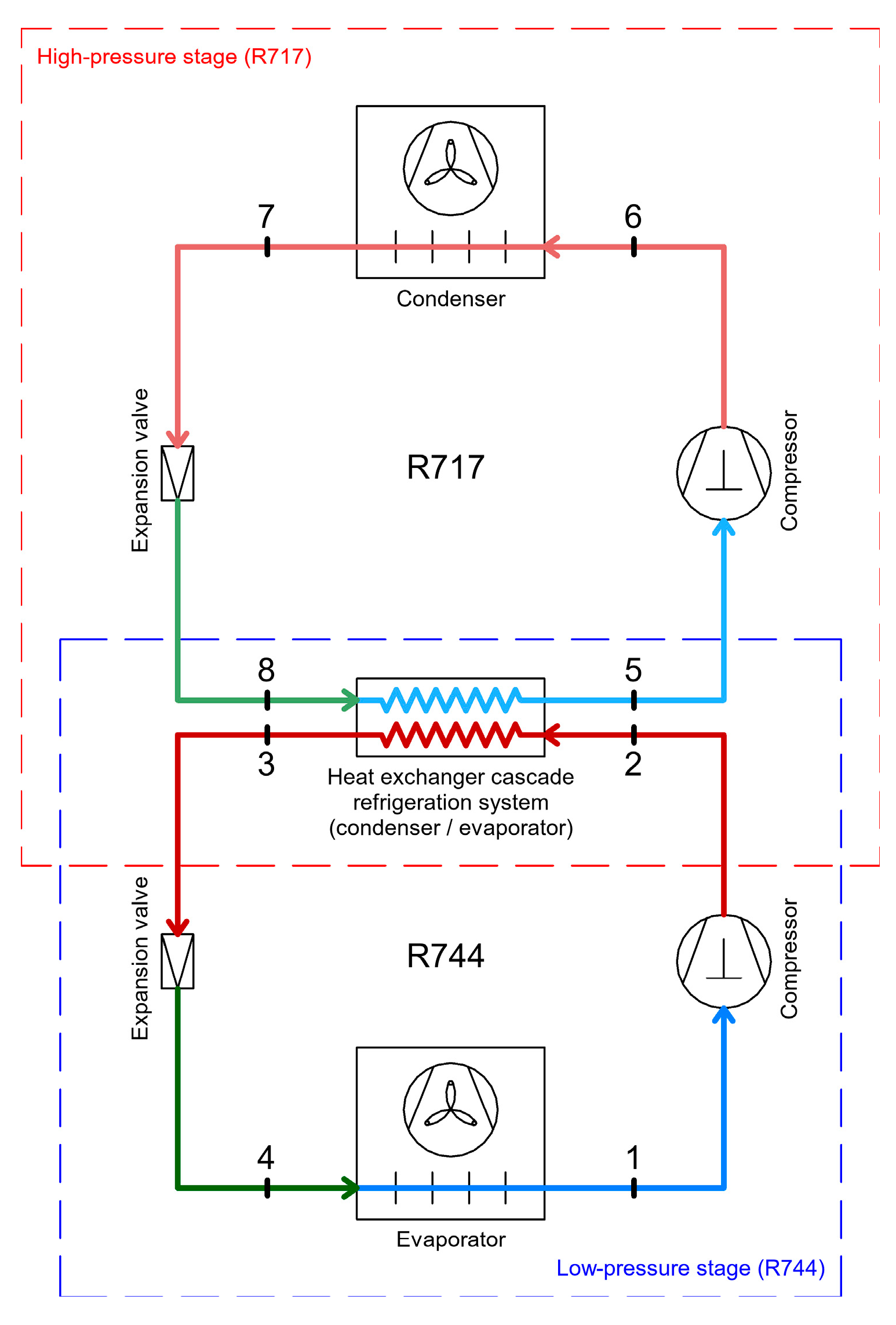
2.2. Cascade Refrigeration System Using CO2 and Ammonia
2.3. Single-Stage CO2 Refrigeration System
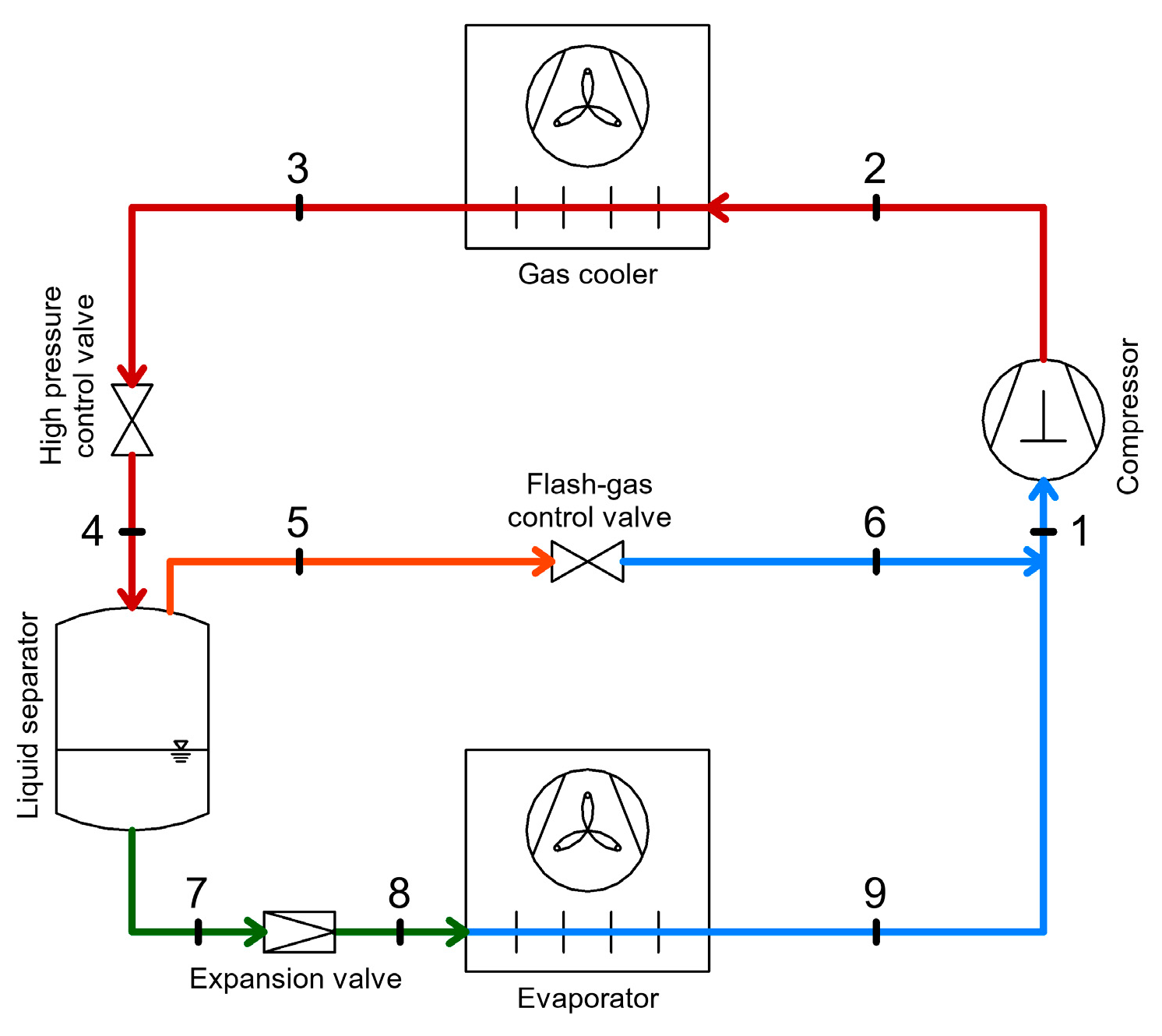
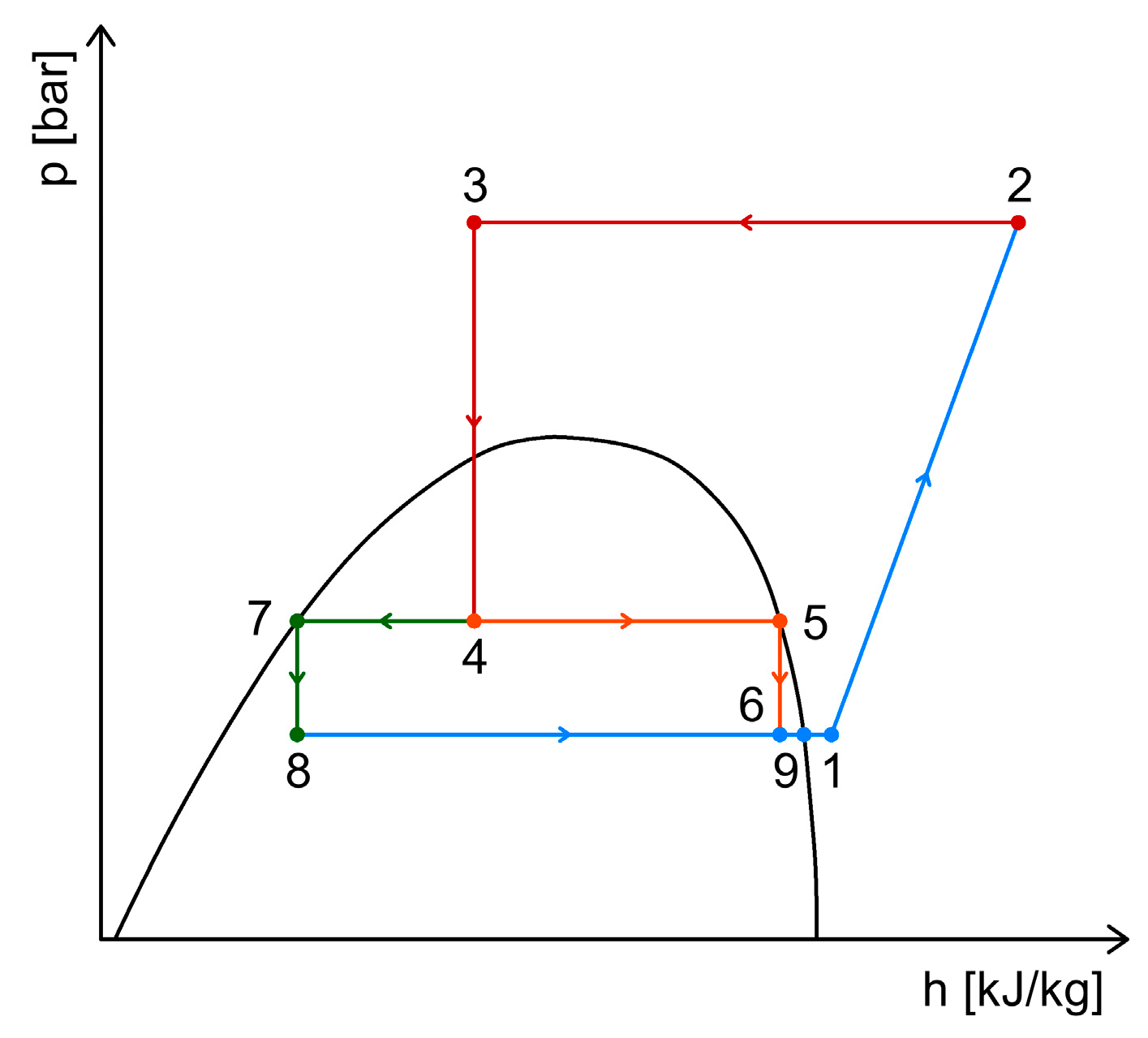
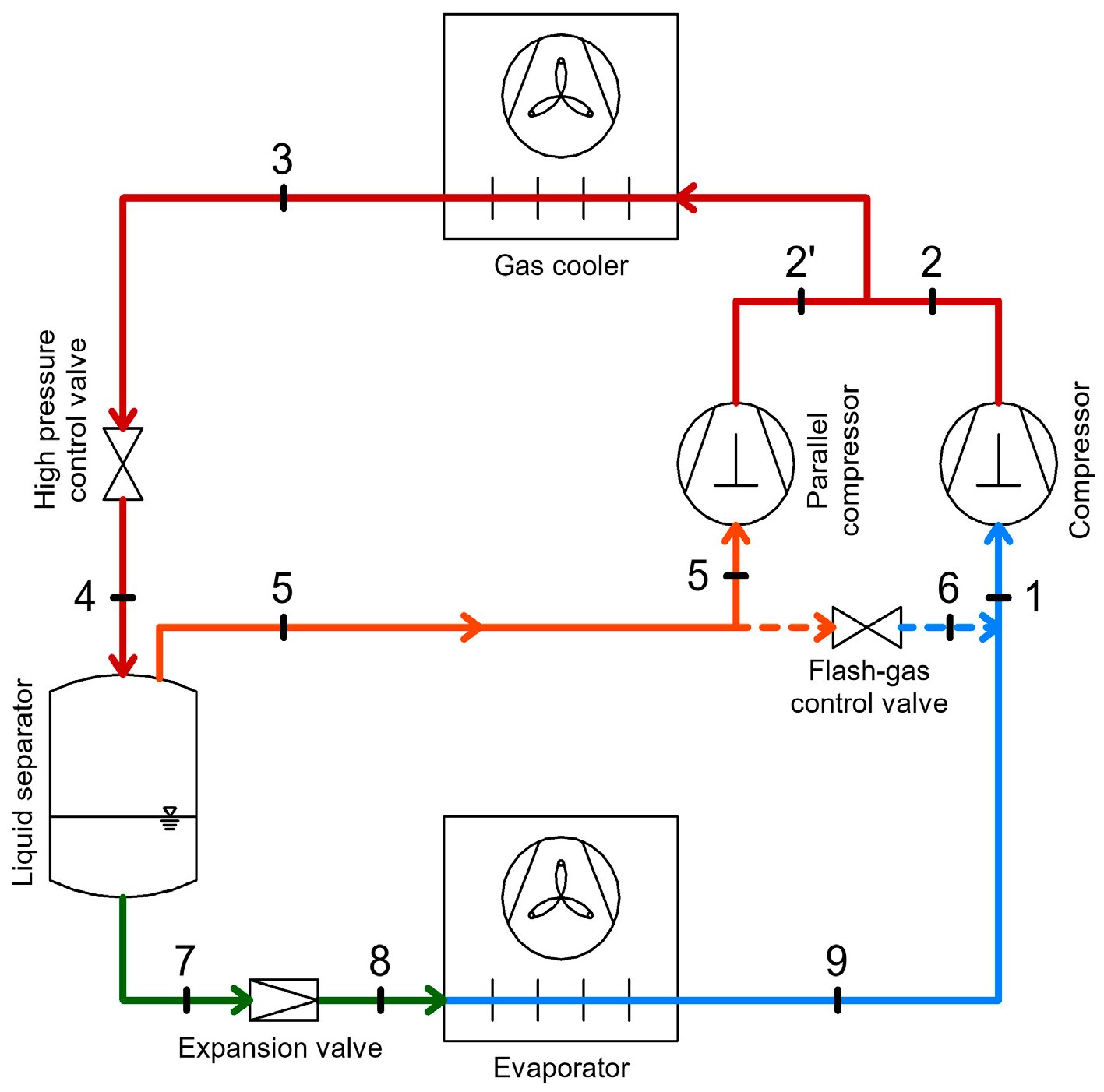
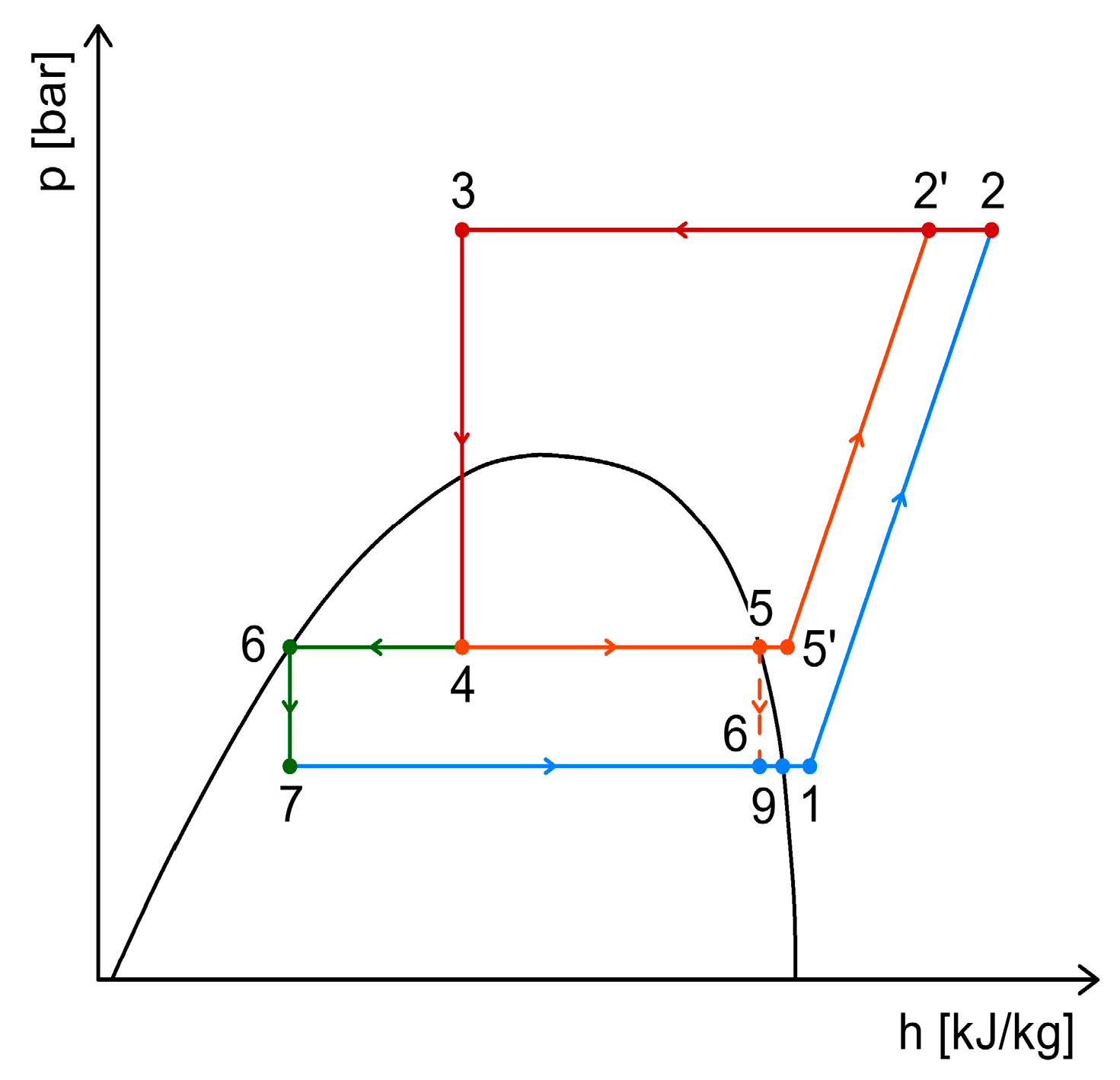
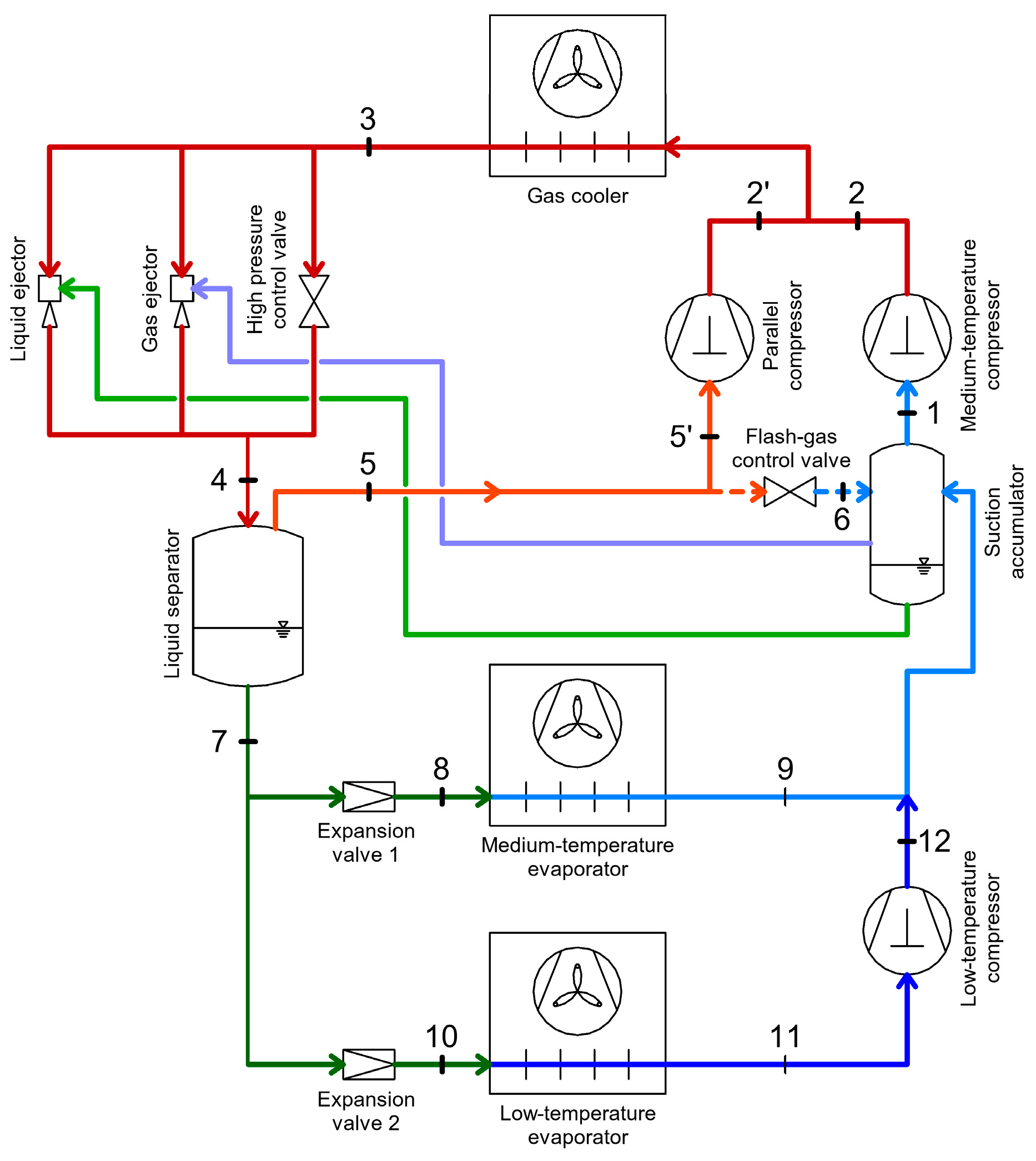
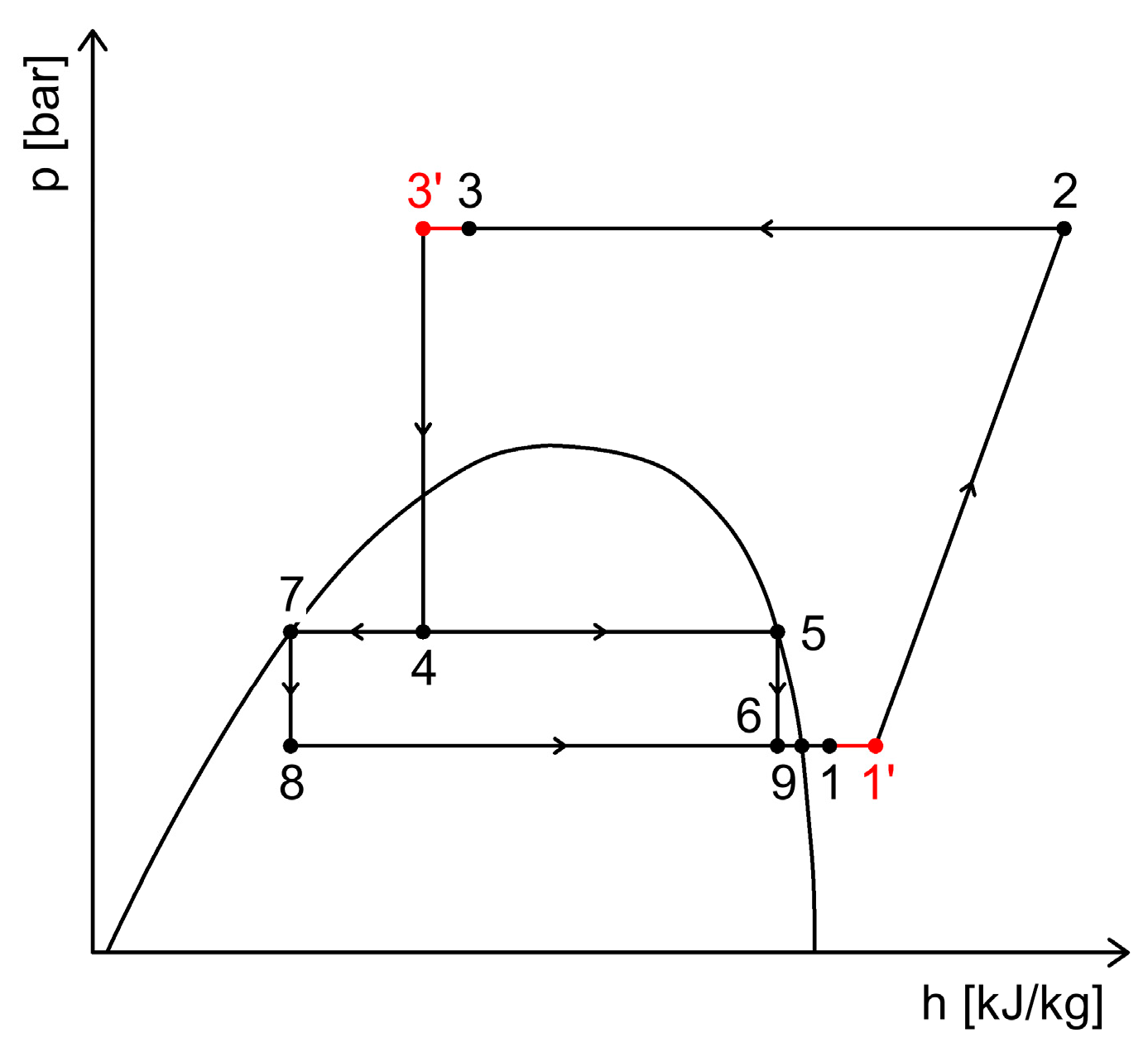
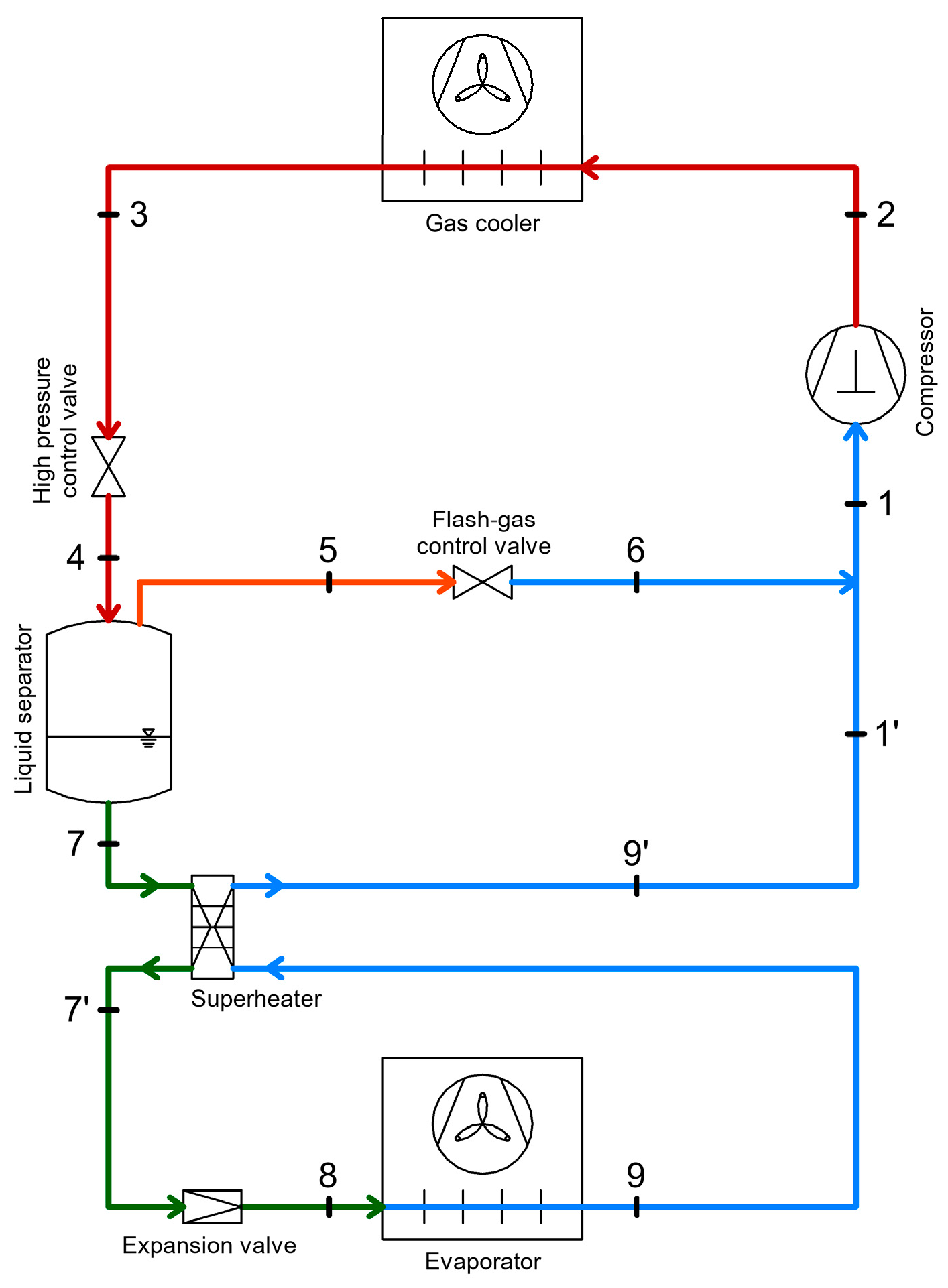
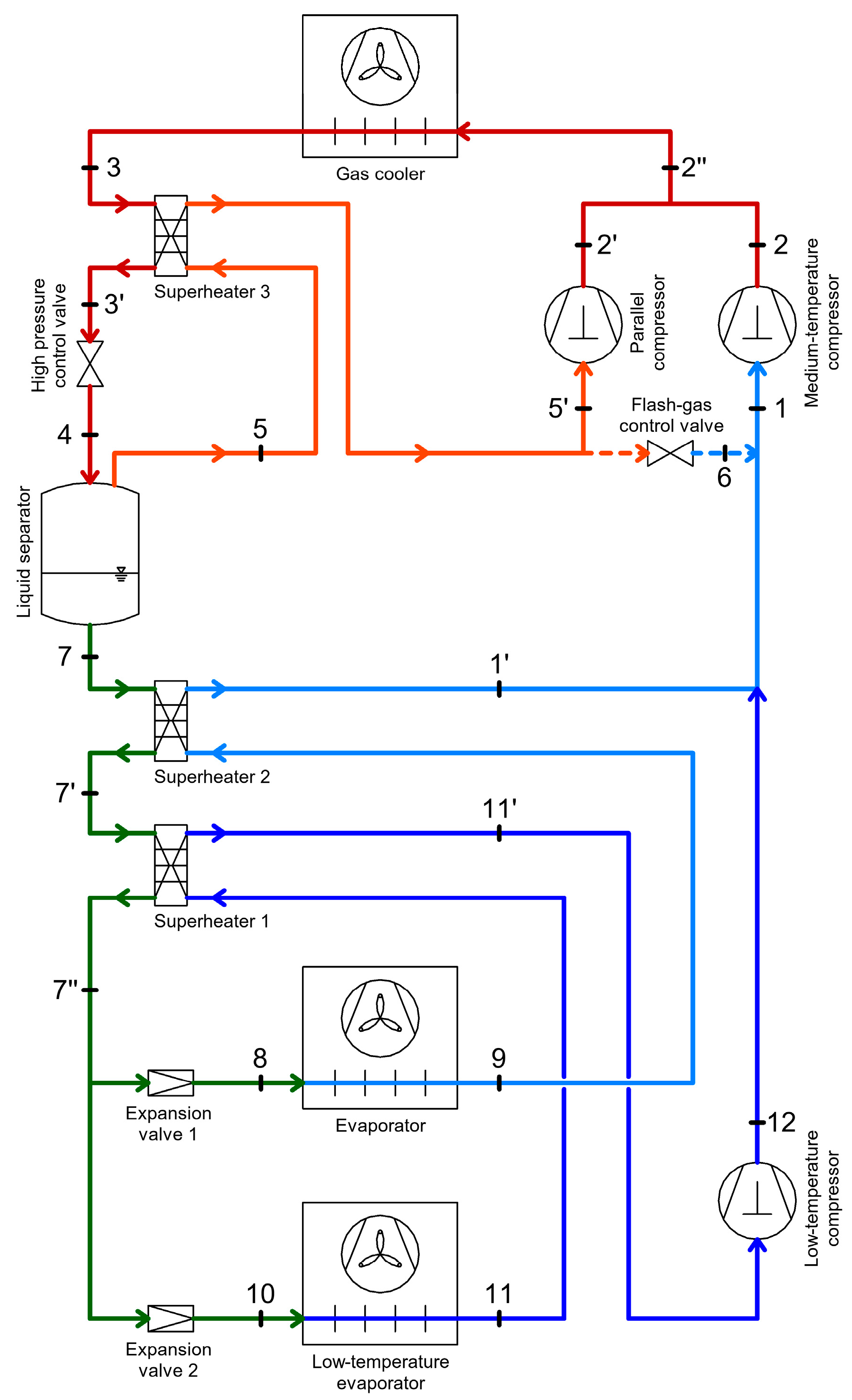
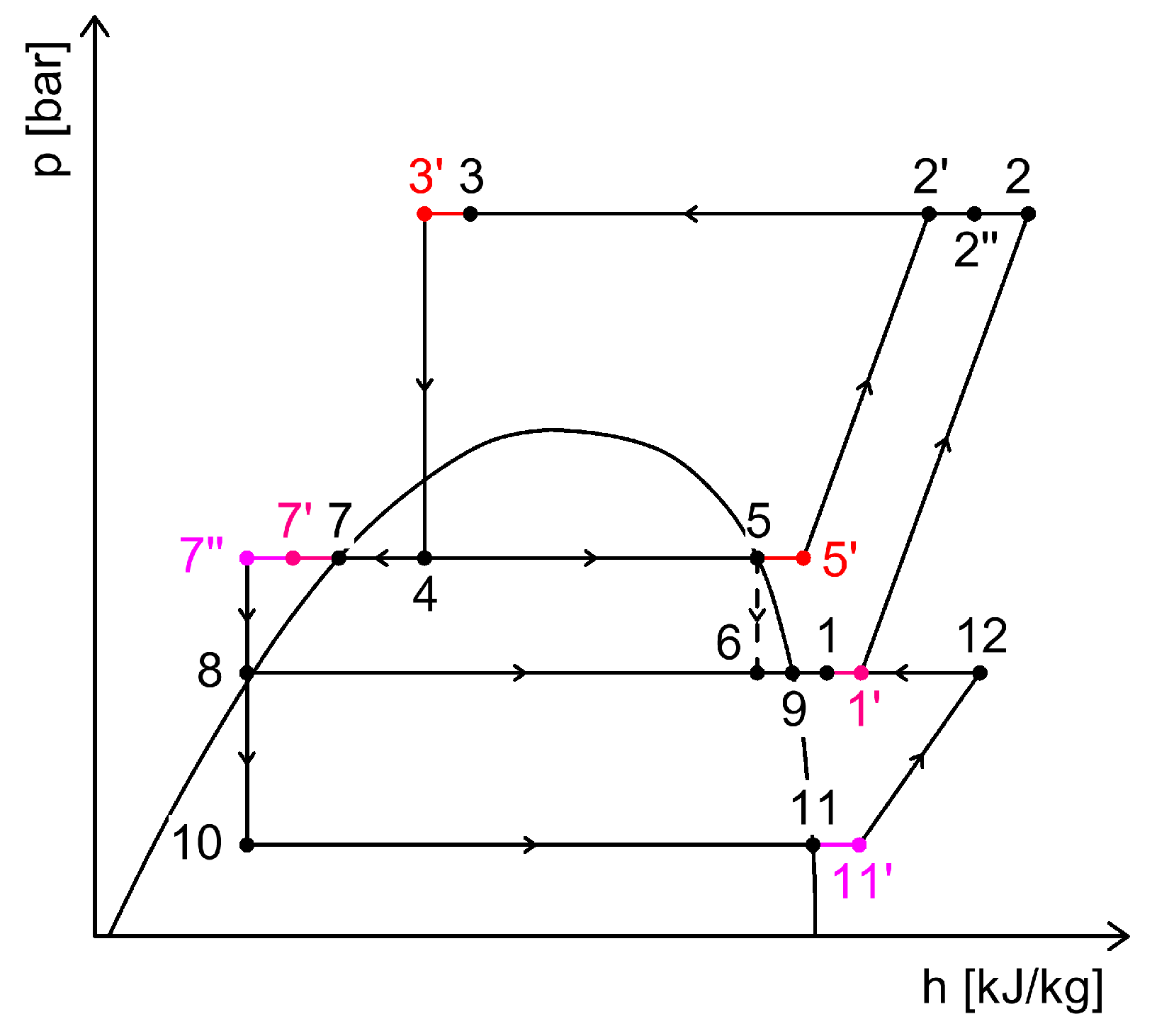
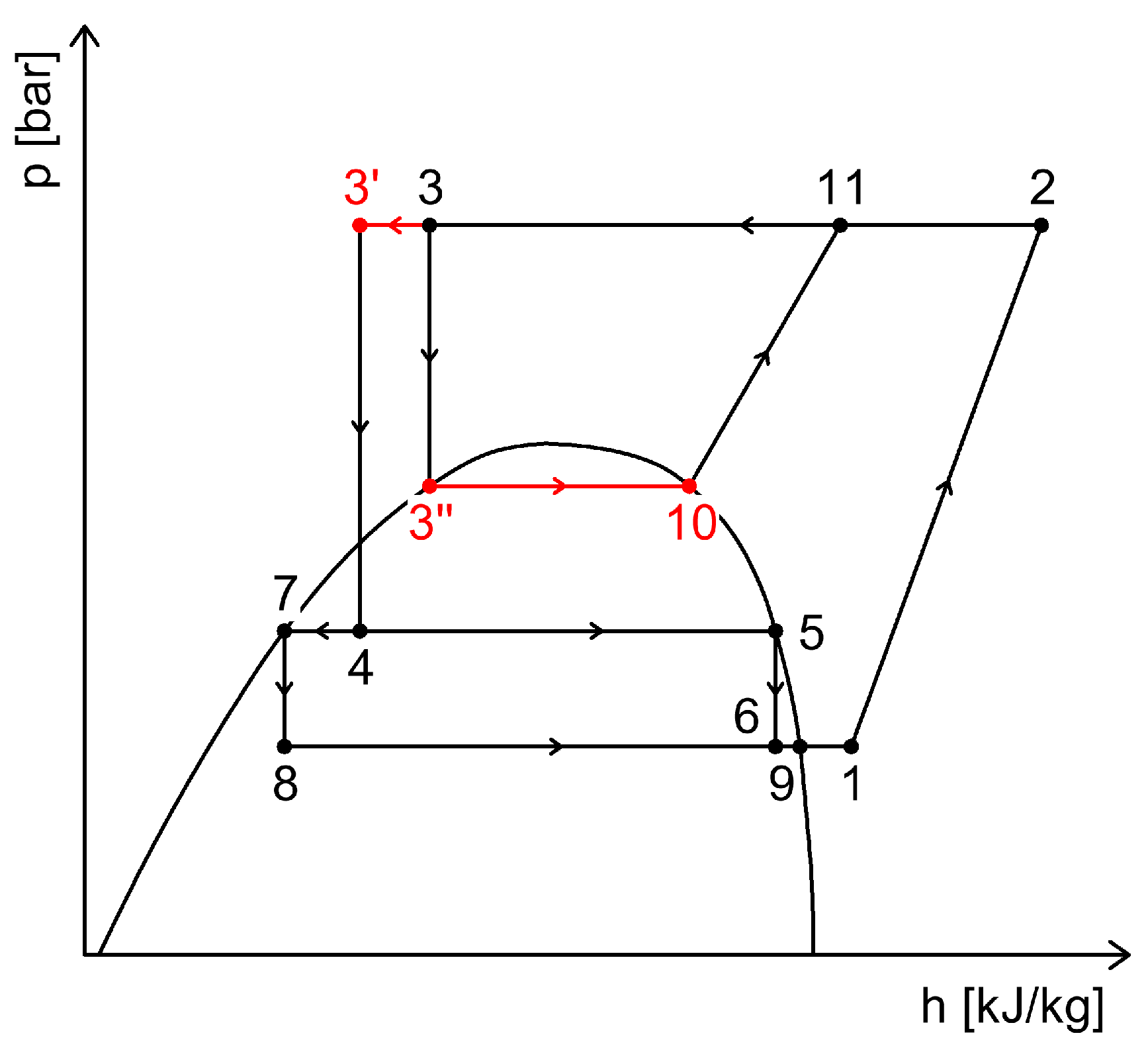
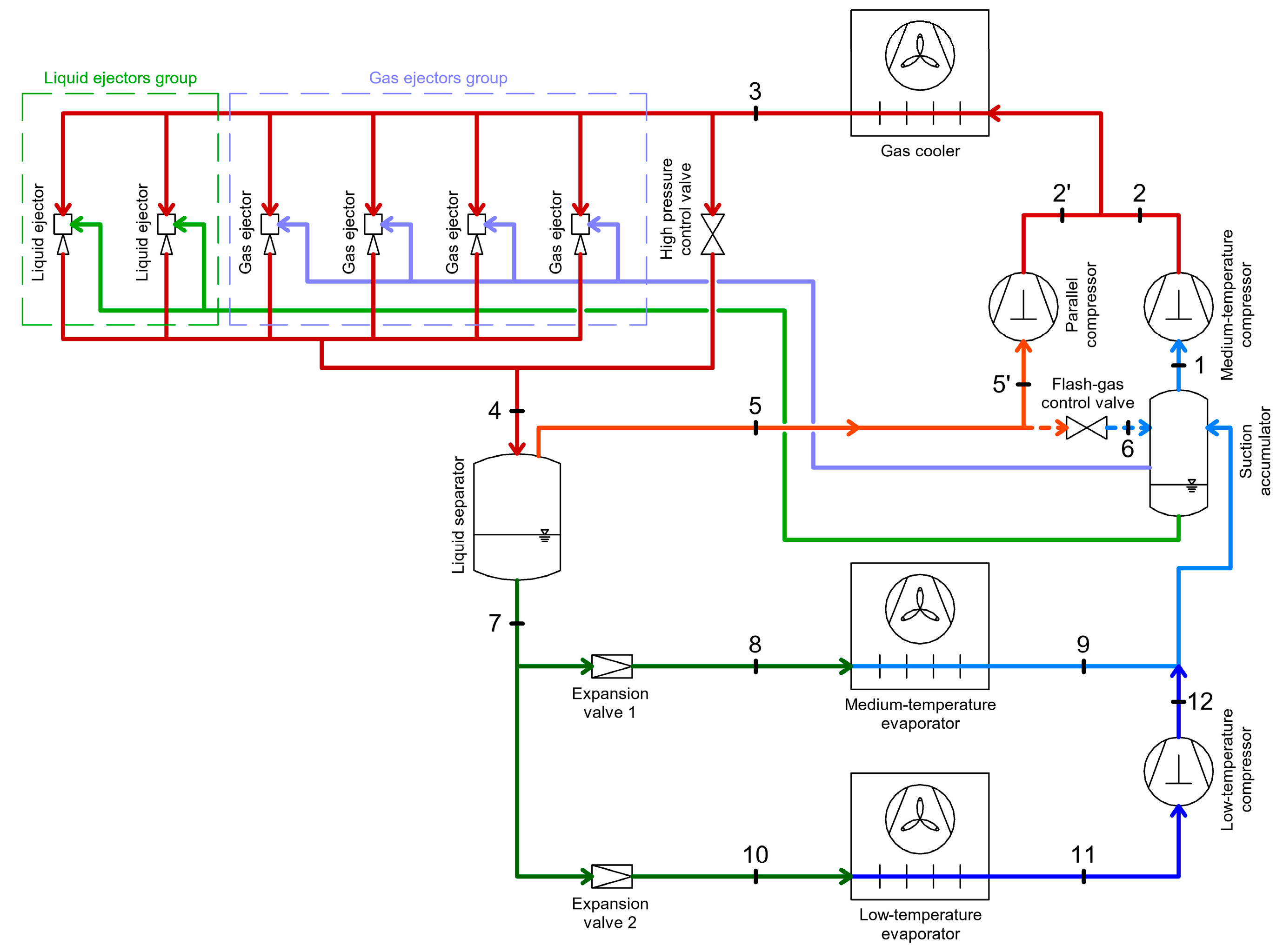
2.4. CO2 Booster Refrigeration System
3. Heat Recovery Possibilities Within CO2 Refrigeration Systems
- In air conditioning systems for reheating dehumidified air;
- In butcheries, dairy factories, hotels, etc., where on one hand refrigerated rooms are operated, and on the other hand, there is always a high demand for domestic hot water;
- In stores, where besides cooling food products, there is also a demand for heat;
- In cold storage areas, for heating and domestic hot water;
- In industrial processes (e.g., drying processes) [29].
3.1. Heat Recovery from the High-Pressure Compressor Discharge
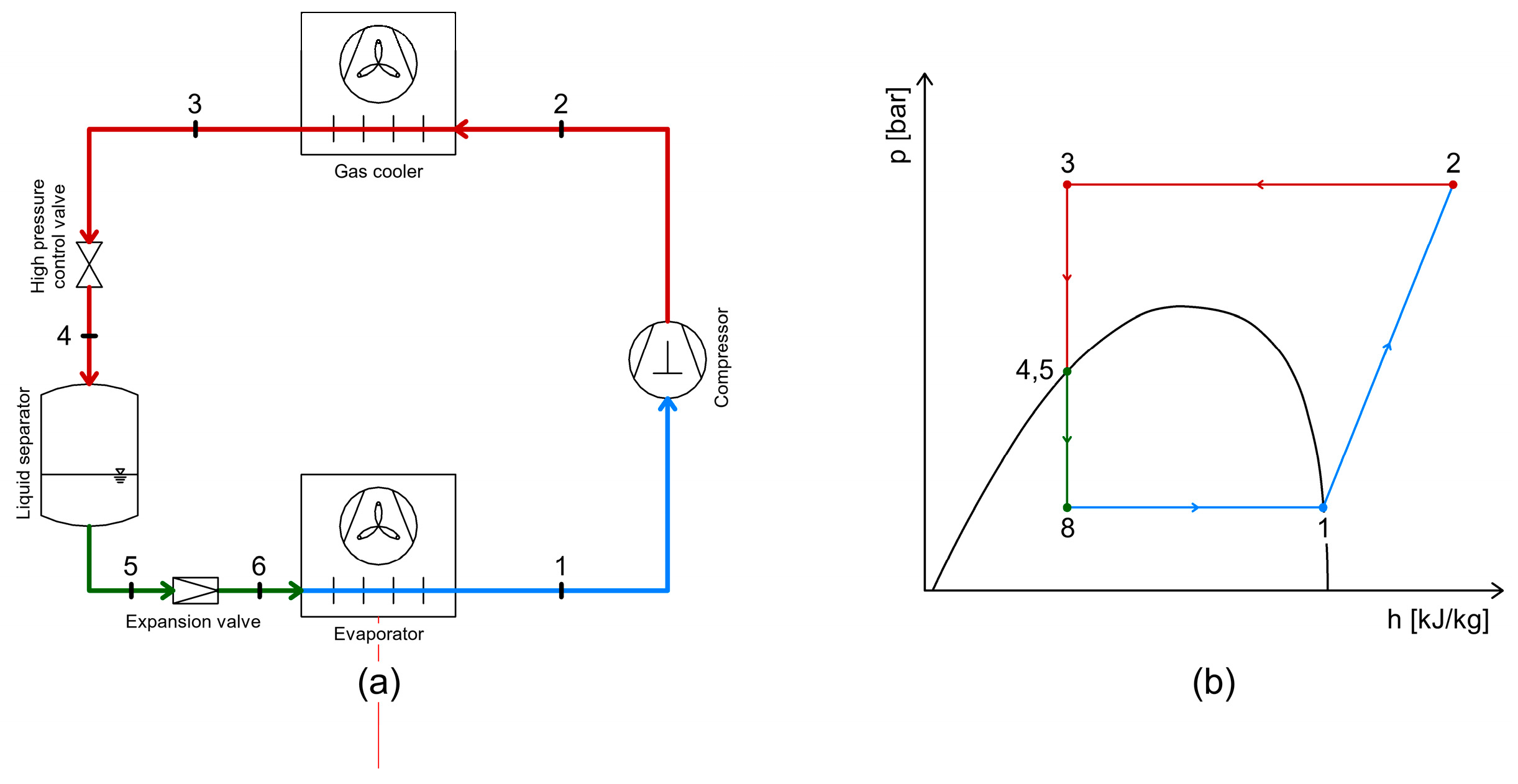
3.2. Heat Recovery Between the Gas Cooler and the Intermediate Separator Receiver
3.3. Heat Recovery for Defrosting Evaporators
3.3.1. Heat Recovery Through Defrosting Evaporators with Hot Gases
3.3.2. Heat Recovery Through Defrosting of Evaporators with Hot Glycol
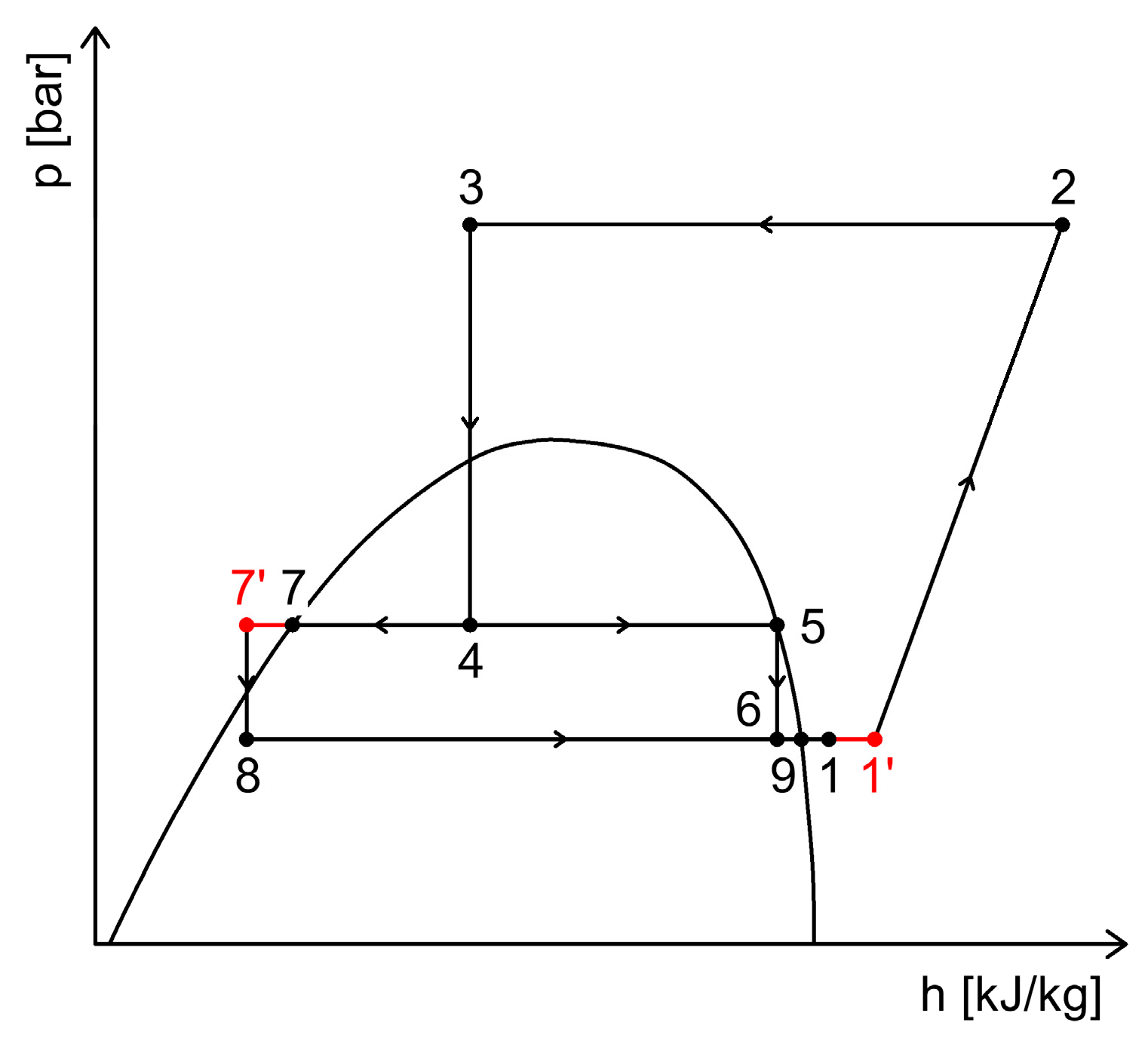
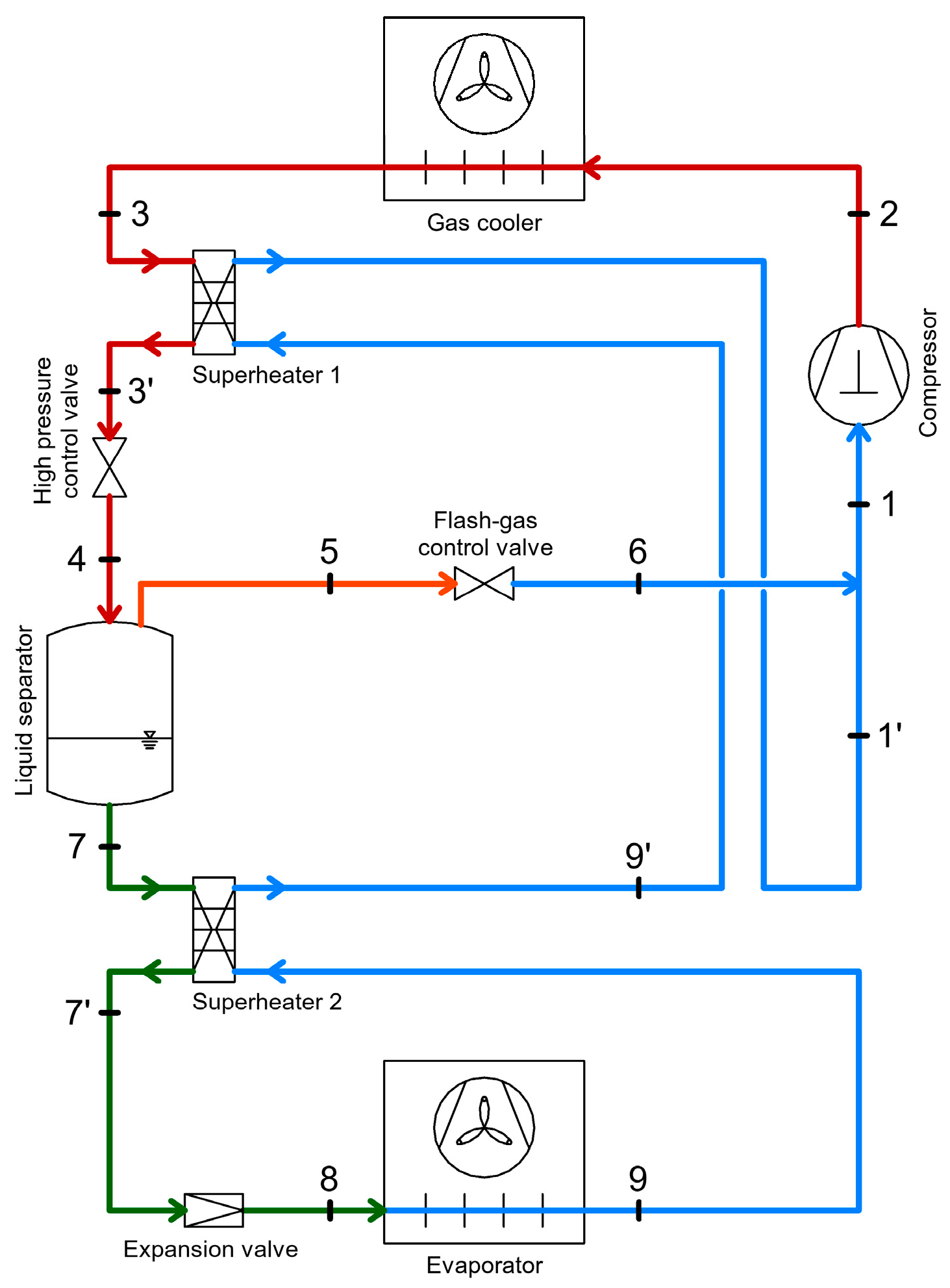
3.4. Heat Recovery Through Internal Heat Exchangers in Refrigeration Systems
3.4.1. Heat Recovery Through Internal Heat Exchangers Acting as Superheaters
3.4.2. Heat Recovery via Internal Heat Exchangers with Subcooling Function
- Dedicated mechanical subcooling: This involves integrating an additional vapor compression refrigeration system using a different refrigerant. The evaporator of this auxiliary system is connected to the outlet of the gas cooler to achieve optimal subcooling. Using such a setup boost both the cooling capacity and performance of the main CO2 system [50];
- Thermoelectric subcooling: Based on the Peltier effect, this method creates a temperature difference between two semiconductors when a direct current is applied. The system extracts heat from the refrigerant (subcooling) and rejects it into the surrounding environment [51];
| Heat Recovery Possibilities | COP Improvement | Exergy Efficiency Improvement | System Integration/Complexity | Advantages | Limitations | Cost (Estimated) | |
|---|---|---|---|---|---|---|---|
| Heat Recovery for DHW, SH, AC | HR from the high-pressure compressor discharge | +20–40% [61,62] | +10–45% [62,63] | Medium |
|
| +5–25% investment cost, payback period 2–5 years |
| HR between the gas cooler and the intermediate separator receiver | +10–25% [64,65] | +5–20% [64] | Medium |
|
| +5–20% investment cost, payback period 2–5 years | |
| Heat Recovery for Defrosting of Evaporators | HR through defrosting evaporators with hot gases | +5–10% estimated | +5–15% estimated | Medium |
| +5–10% investment cost | |
| HR through defrosting of evaporators with warm glycol | +3–10% estimated | +5–15% estimated | Medium |
|
| +5–15% investment cost, payback period 2–4 years | |
| Heat recovery through Internal Heat Exchangers | HR through internal heat exchangers acting as superheaters | +3–17% [67,68] | +5–20% [69] | Low |
| +2–10% investment cost, payback period 2–4 years | |
| HR via Internal Heat Exchangers with Subcooling Function | +10–22% [70,71] | +5–15% [71] | Low |
| +2–8% investment cost, payback period 2–4 years | ||
4. Energy Recovery Within CO2 Refrigeration Systems
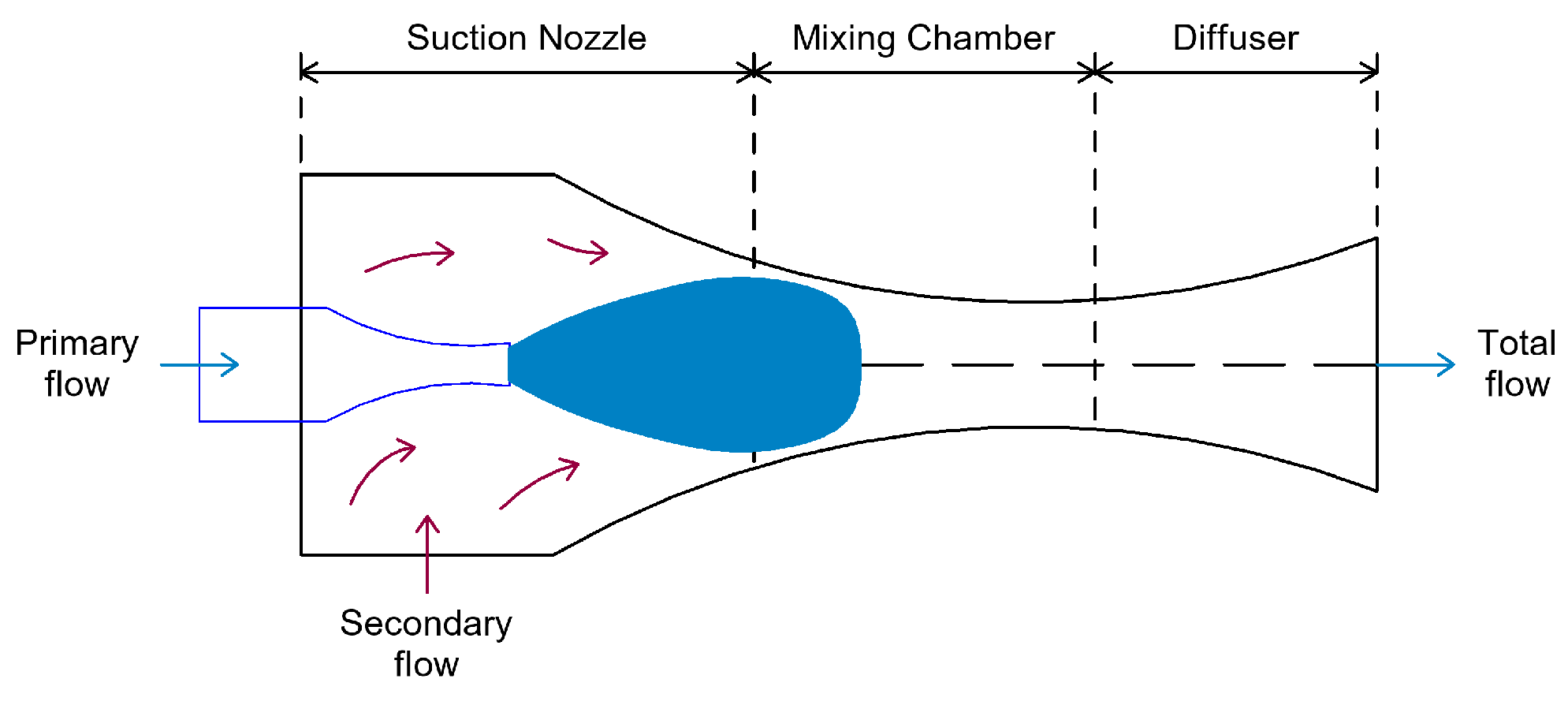
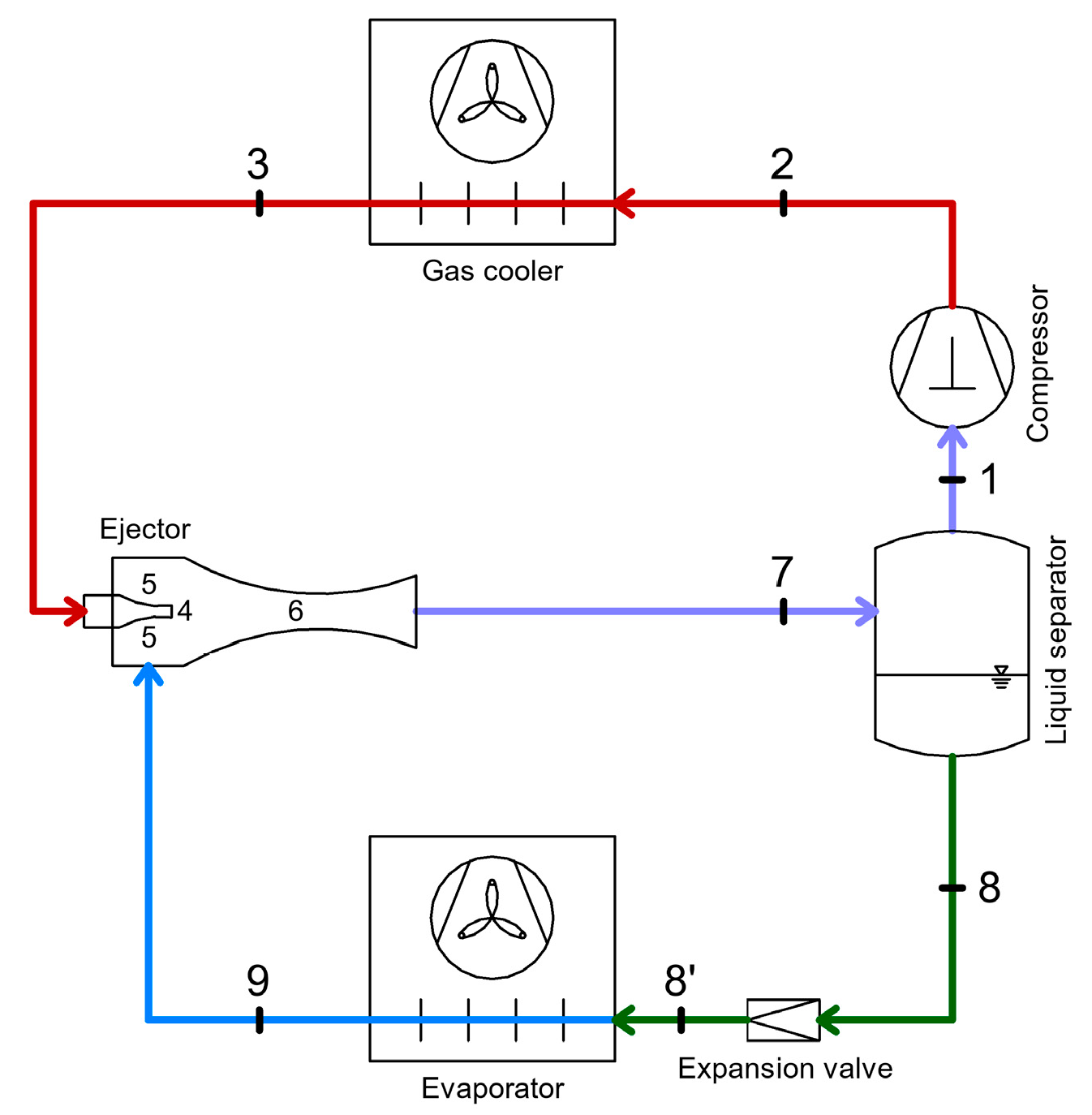
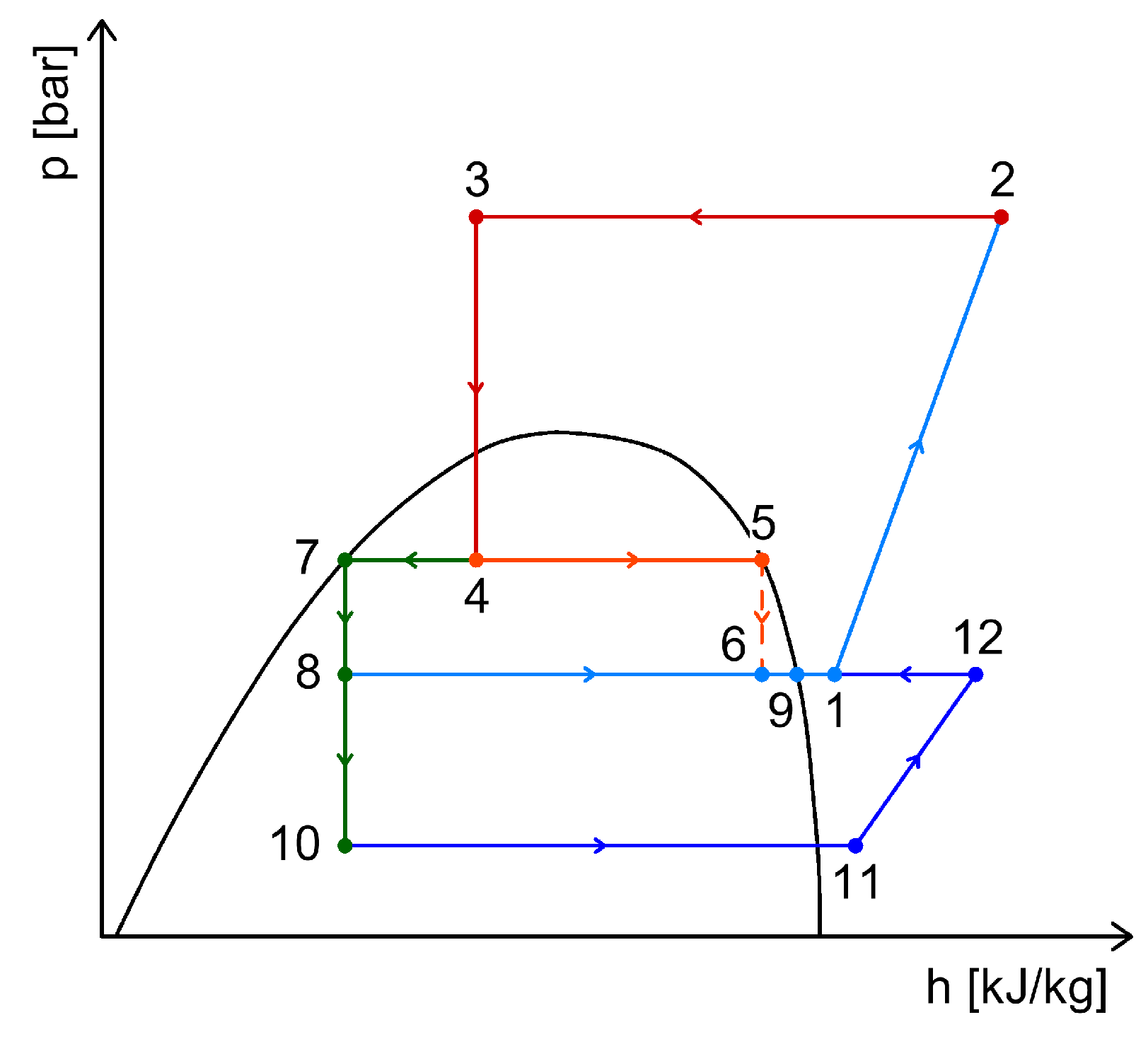
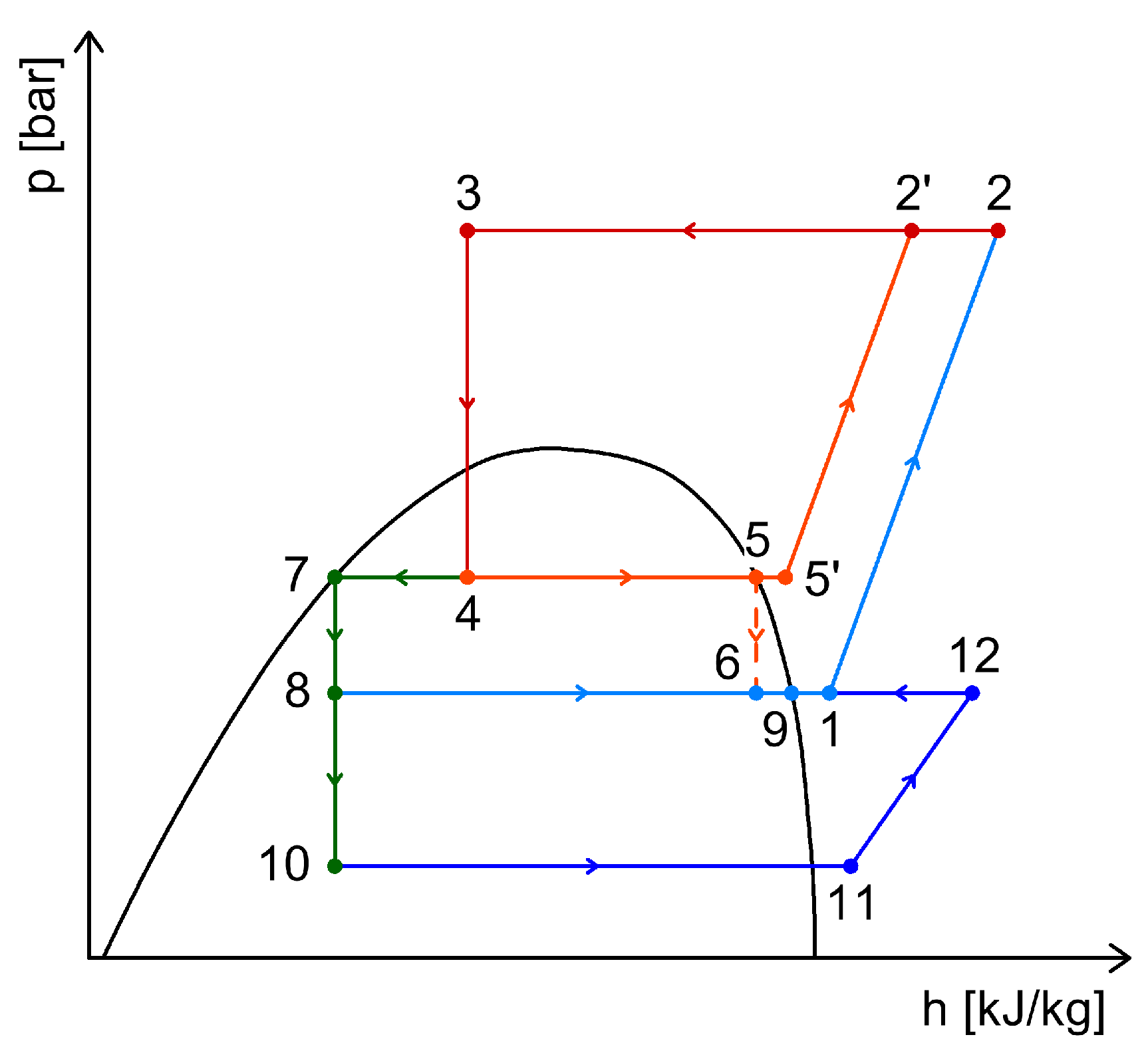
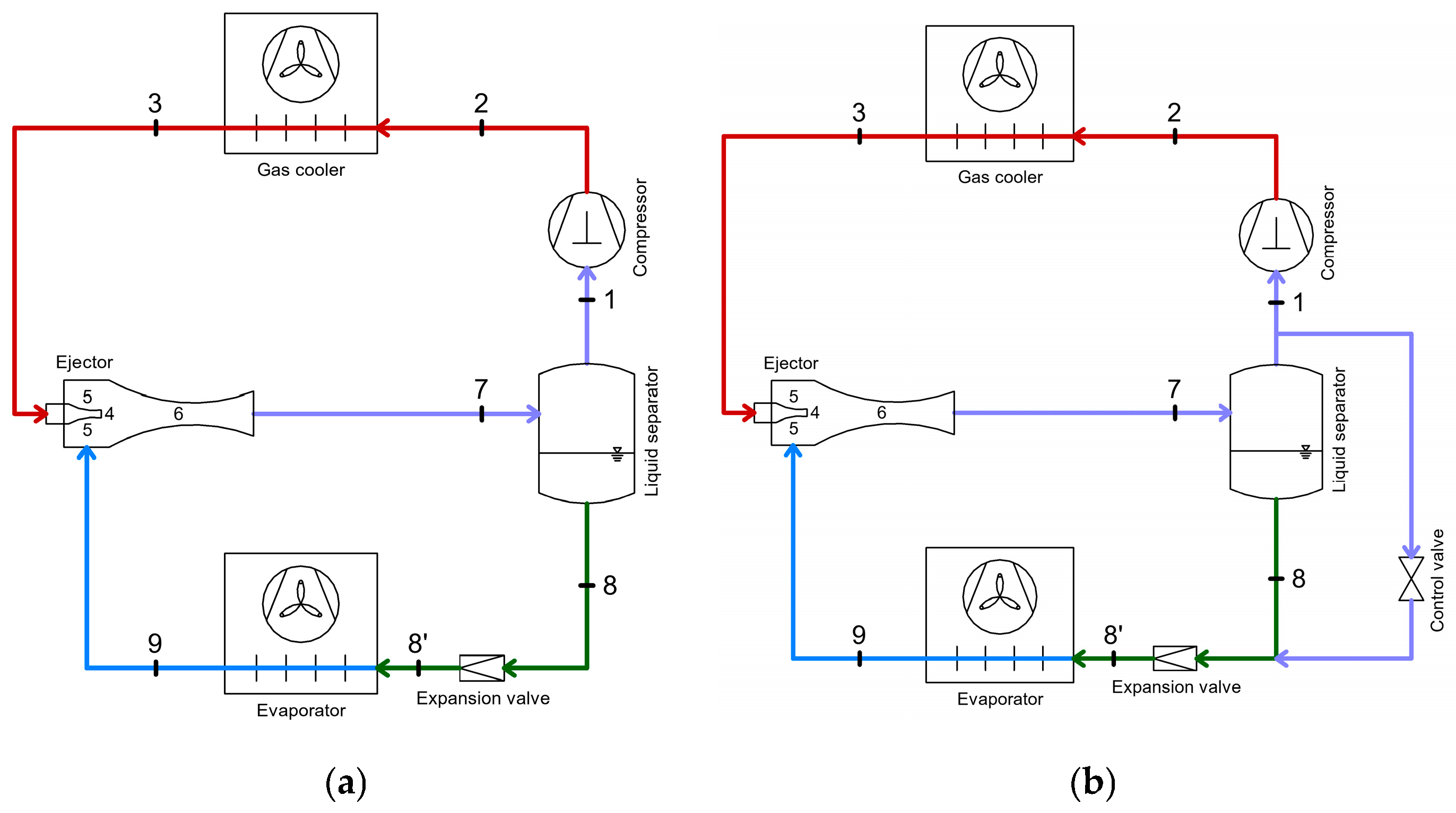
4.1. Energy Recovery Using Ejectors
- According to nozzle position, two configurations are distinguished:
- CPM (Constant Pressure Mixing)–the ejector with constant pressure mixing, where the nozzle outlet is located in the mixing chamber;
- CAM (Constant Area Mixing)—the ejector with constant area mixing, where the nozzle outlet is placed in a constant-area section.
- According to nozzle geometry, the ejector operation is influenced in two ways:
- Convergent nozzle: the ejector operates in a subsonic regime, and the working fluid can reach at most sonic conditions at the nozzle outlet;
- Convergent-divergent nozzle: the flow through the ejector can reach supersonic speeds.
- According to the number of phases:
- Single-phase ejectors: both primary and secondary fluid flows are in the same phase (gas–gas or liquid–liquid);
- Two-phase ejectors: include condensing ejectors (where the primary fluid condenses inside the ejector—a highly complex modeling process) or ejectors where the flow at the outlet is in two phases [74].
- As a replacement for the compressor;
- Integration between the compressor and condenser;
- Substitution of conventional expansion devices, aiming to reduce interstage losses [75].
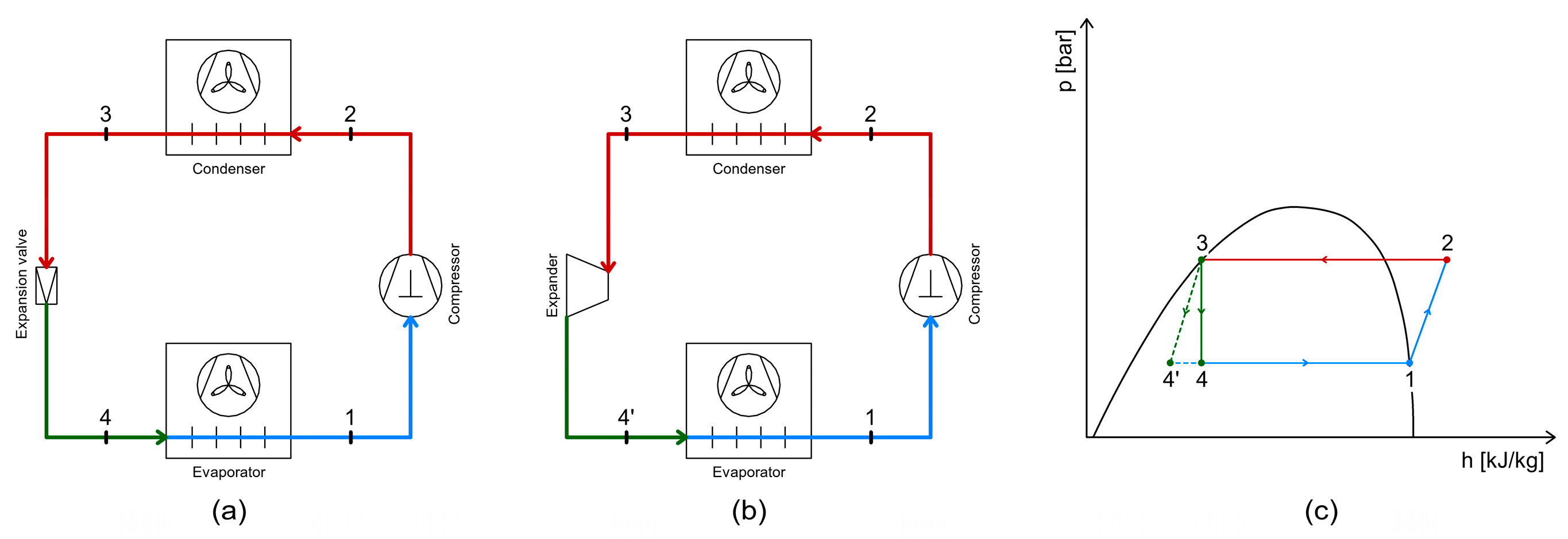
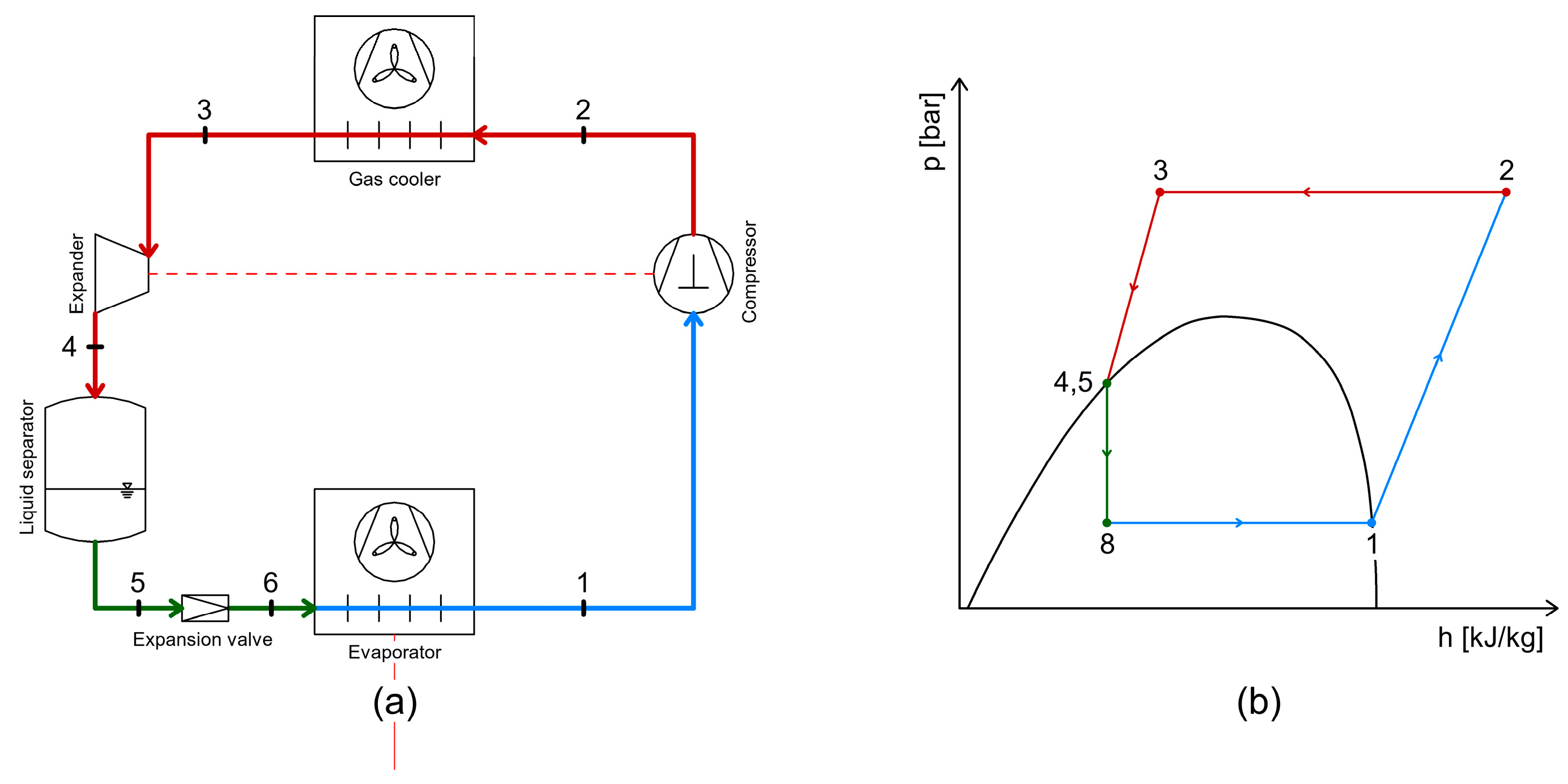
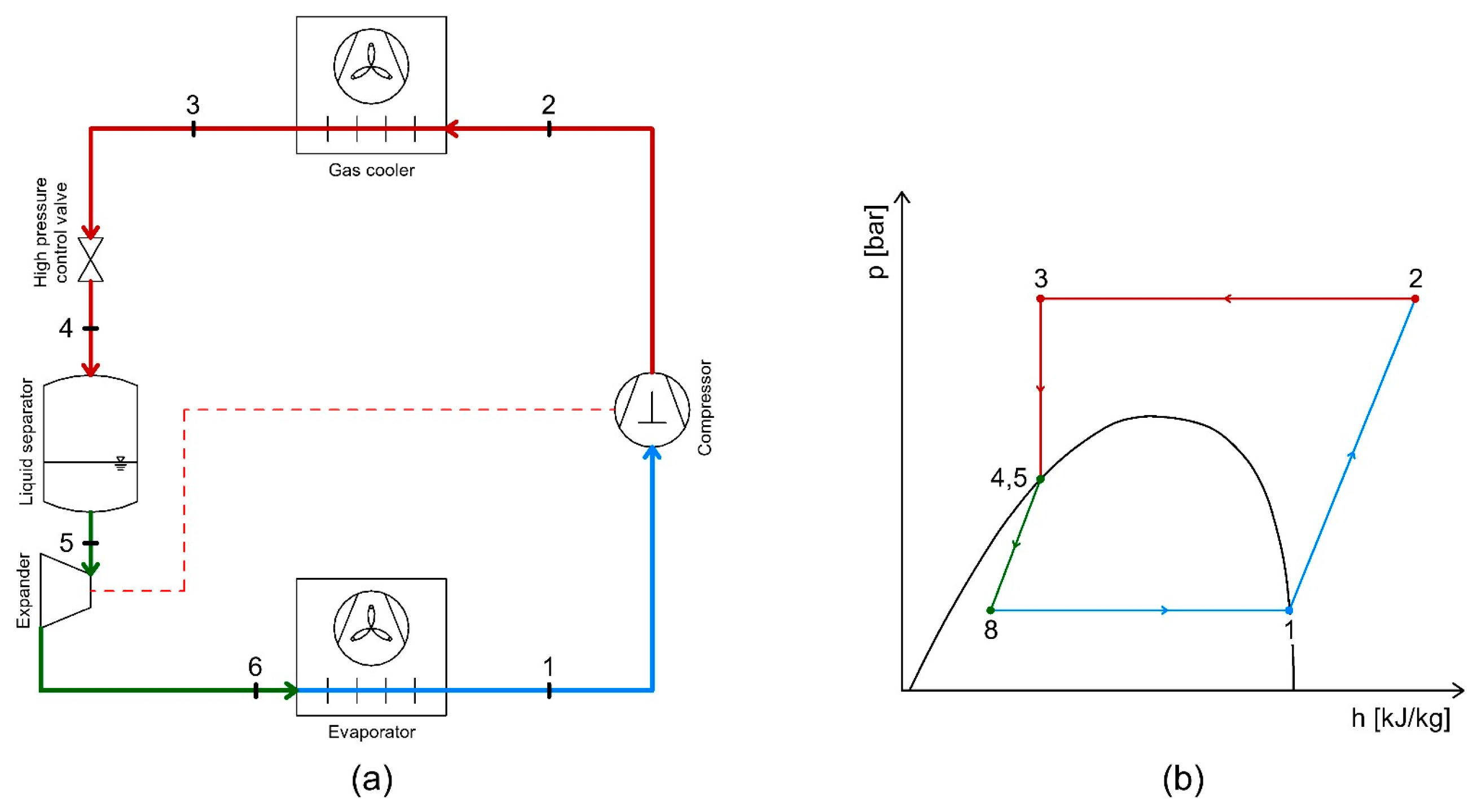
4.2. Energy Recovery Using Expanders
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruno, F.; Belusko, M.; Halawa, E. CO2 Refrigeration and Heat Pump Systems—A Comprehensive Review. Energies 2019, 12, 29–59. [Google Scholar] [CrossRef]
- Minetto, S.; Marinetti, S.; Saglia, P.; Masson, N.; Rossetti, A. Non-technological barriers to the diffusion of energy-efficient HVAC&R solutions in the food retail sector. Int. J. Refrig. 2018, 86, 422–434. [Google Scholar] [CrossRef]
- Galvez-Martos, J.-L.; Styles, D.; Schoenberger, H. Identified best environmental management practises to improve the energy performance of the retail trade sector in Europe. Energy Policy 2013, 63, 982–994. [Google Scholar] [CrossRef]
- Tassou, S.A.; Ge, Y.; Hadawey, A.; Marriott, D. Energy consumption and conservation in food retailing. Appl. Therm. Eng. 2011, 31, 147–156. [Google Scholar] [CrossRef]
- Metz, B.; Kuijpers, L.; Solomon, S.; Andersen, S.O.; Davidson, O.; Pons, J.; de Jager, D.; Kestin, T.; Manning, M.; Meyer, L. (Eds.) Safeguarding the Ozone Layer and the Global Climate System: Issues Related to Hydrofluorocarbons and Perfluorocarbons; IPCC/TEAP; Cambridge University Press: Cambridge, UK, 2005; p. 478. [Google Scholar]
- (EC) No 842/2006; Regulation (Eu) No 517/2014 of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation. European Parliament and Council: Strasbourg, France, 2014.
- Lorentzen, G. Revival of carbon dioxide as a refrigerant. Int. J. Refrig. 1994, 17, 292–301. [Google Scholar] [CrossRef]
- Sooben, D.; Purohit, N.; Mohee, R.; Meunier, F. R744 refrigeration as an alternative for the supermarket sector in small tropical island developing states: The case of Mauritius. Int. J. Refrig. 2019, 103, 264–273. [Google Scholar] [CrossRef]
- Messineo, A. R744-R717 Cascade Refrigeration System: Performance Evaluation compared with a HFC Two-Stage System. Energy Procedia 2012, 14, 56–65. [Google Scholar] [CrossRef]
- Lee, T.S.; Liu, C.H.; Chen, T.W. Thermodynamic analysis of optimal condensing temperature of cascade-condenser in CO2/NH3 cascade refrigeration systems. Int. J. Refrig. 2006, 29, 1100–1108. [Google Scholar] [CrossRef]
- Getu, H.M.; Bansal, P.K. Modeling and performance analysis of evaporators in frozen food supermarket display cabinets at low temperatures. Int. J. Refrig. 2007, 30, 1227–1243. [Google Scholar] [CrossRef]
- Getu, H.M.; Bansal, P.K. Thermodynamic analysis of an R744/R717 cascade refrigeration system. Int. J. Refrig. 2008, 31, 45–54. [Google Scholar] [CrossRef]
- Emerson Climate Technologies. Commercial CO2 Refrigeration Systems—Guide for Subcritical and Transcritical CO2 Applications. 2016. Available online: https://www.emerson.com/documents/commercial-residential/commercial-co%E2%82%82-refrigeration-systems-en-us-181472.pdf (accessed on 20 June 2025).
- Rolfsman, L. Experiences from CO2 cascade plants. In Proceedings of the 21st IIR International Congress of Refrigeration, Washington, DC, USA, 17–22 August 2003. [Google Scholar]
- Vaishak, S.; Singha, P.; Dasgupta, M.S.; Hafner, A.; Widell, K.; Bhattacharyya, S.; Saini, S.K.; Arun, B.S.; Samuel, M.P.; Ninan, G. Performance analysis of a CO2/NH3 cascade refrigeration system with subcooling for low-temperature freezing applications. Int. J. Refrig. 2023, 153, 140–154. [Google Scholar] [CrossRef]
- Saini, S.K.; Dasgupta, M.S.; Widell, K.N.; Bhattacharyya, S. Comparative analysis of a few novel multi-evaporator CO2–NH3 cascade refrigeration system for seafood processing & storage. Int. J. Refrig. 2021, 131, 817–825. [Google Scholar] [CrossRef]
- Aleu, P. Makro Continues CO2 Installations in Latin America. 2019. Available online: https://archive.r744.com/articles/9267/makro_continues_co2_installations_in_latin_america?utm_source=chatgpt.com (accessed on 24 June 2025).
- Danfoss. Industrial Refrigeration—Ammonia and CO2 Applications (Application Handbook). 2010. Available online: www.danfoss.com/ir (accessed on 25 June 2025).
- Elbel, S.; Hrnjak, P. Ejector Refrigeration: An Overview of Historical and Present Developments with an Emphasis on Air-Conditioning Applications. 2008. Available online: https://docs.lib.purdue.edu/cgi/viewcontent.cgi?article=1883&context=iracc (accessed on 26 June 2025).
- Bai, T.; Shi, R.; Yu, J. Thermodynamic performance evaluation of an ejector-enhanced transcritical CO2 parallel compression refrigeration cycle. Int. J. Refrig. 2023, 149, 49–61. [Google Scholar] [CrossRef]
- Gholizadeh, T.; Rostami, S.; Arabkoohsar, A. Performance enhancement of transcritical CO2 compression cycles: Techno-environmental analysis and machine learning optimization. Appl. Therm. Eng. 2025, 266, 125591. [Google Scholar] [CrossRef]
- Zou, L.; Liu, Y.; Yu, J. Thermodynamic and environment analysis of a modified transcritical CO2 refrigeration cycle integrated with ejector and subcooler. Int. J. Refrig. 2025, 173, 123–138. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Liu, K.; Wu, Y.; Wang, X.; Liang, X.; Shu, G. Optimum pressure control with three controllable ejectors of a CO2 multi-ejector refrigeration system. Int. J. Refrig. 2024, 157, 172–185. [Google Scholar] [CrossRef]
- Ge, Y.T.; Tassou, S.A. Thermodynamic analysis of transcritical CO2 booster refrigeration systems in supermarket. Energy Convers. Manag. 2011, 52, 1868–1875. [Google Scholar] [CrossRef]
- Li, W.; Korolija, I.; Tang, R.; Mumovic, D. High-fidelity model development of CO2 booster refrigeration systems in supermarkets using field measurements. Int. J. Refrig. 2025, 169, 152–165. [Google Scholar] [CrossRef]
- Sacasas, D.; Vega, J.; Cuevas, C. An annual energetic evaluation of booster and parallel refrigeration systems with R744 in food retail supermarkets. A Chilean perspective. Int. J. Refrig. 2022, 133, 326–336. [Google Scholar] [CrossRef]
- Syngounas, E.; Tsimpoukis, D.; Bellos, E.; Koukou, M.K.; Tzivanidis, C.; Vrachopoulos, M.G. Energy and exergy performance investigation of a transcritical CO2 vapor ejector-based refrigeration system for marine provision plants. Appl. Therm. Eng. 2025, 269, 126036. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Y.; Dong, J.; Zhang, Y.; Hu, J.; Jiang, P. Dynamic simulation study on regulation characteristics of transcritical CO2 multi-ejector refrigeration system. Appl. Therm. Eng. 2024, 238, 121592. [Google Scholar] [CrossRef]
- Siemens Building Technologies. Heat Recovery in the Refrigeration Cycle. Brochure BO8RF Refrigeration Technology. Available online: http://www.bt.siemens.com.cn/partner/DownloadCenter/upload/20121217015059201.pdf (accessed on 27 June 2025).
- Sawalha, S. Investigation of heat recovery in CO2 trans-critical solution for supermarket refrigeration. Int. J. Refrig. 2013, 36, 145–156. [Google Scholar] [CrossRef]
- Sawalha, S.; Karampour, M. Supermarket Refrigeration and Heat Recovery Using CO2 as Refrigerant; Energimyndigheten: Eskilstuna, Sweden, 2014. [Google Scholar] [CrossRef]
- Polzot, A.; D’Agaro, P.; Cortella, G. Energy analysis of a transcritical CO2 supermarket refrigeration system with heat recovery. Energy Procedia 2017, 111, 648–657. [Google Scholar] [CrossRef]
- Danfoss. Heat Reclaim in Transcritical CO2 Systems (Application Guide). 2015. Available online: https://assets.danfoss.com/documents/latest/372092/AB167786419087en-000401.pdf?utm_source=chatgpt.com (accessed on 30 June 2025).
- Escriva, E.J.S.; Acha, S.; Le Brun, N.; Francés, V.S.; Ojer, J.M.P.; Markides, C.N.; Shah, N. Modelling of a real CO2 booster installation and evaluation of control strategies for heat recovery applications in supermarkets. Int. J. Refrig. 2019, 107, 288–300. [Google Scholar] [CrossRef]
- Thanasoulas, S. Investigation of heat recovery in CO2 refrigeration cycles for supermarkets: A mechanism for determining optimal discharge pressure. Appl. Therm. Eng. 2025, 274, 1358–4311. [Google Scholar] [CrossRef]
- Giunta, F.; Sawalha, S. Techno-economic analysis of heat recovery from supermarket’s CO2 refrigeration systems to district heating networks. Appl. Therm. Eng. 2021, 193, 239–257. [Google Scholar] [CrossRef]
- Steuer, D.; Arias, J.; Sawalha, S. Techno-economic evaluation of heat recovery from supermarket refrigeration systems: A case study of four real-world installations. Appl. Therm. Eng. 2025, 281, 798–811. [Google Scholar] [CrossRef]
- Aboaltabooq, M.H.K.; Prisecaru, T.; Pop, H.; Apostol, V.; Prisecaru, M.; Popescu, G.; Pop, E.; Alexandru, A.-M.; Petcu, C.; Ciobanu, C. Effect of variable heat input on the heat transfer characteristics in an Organic Rankine Cycle system. Renew. Energy Environ. Sustain. 2016, 1, 13. [Google Scholar] [CrossRef]
- Karampour, M.; Sawalha, S. Theoretical analysis of CO2 trans-critical system with parallel compression for heat recovery and air conditioning in supermarkets. In Proceedings of the 24th IIR International Congress of Refrigeration—ICR2015, Yokohama, Japan, 16–22 August 2015; Available online: https://www.researchgate.net/publication/281583937 (accessed on 1 July 2025).
- Azzolin, M.; Cattelan, G.; Dugaria, S.; Minetto, S.; Calabrese, L.; Del Col, D. Integrated CO2 systems for supermarkets: Field measurements and assessment for alternative solutions in hot climate. Appl. Therm. Eng. 2021, 187, 116560. [Google Scholar] [CrossRef]
- Hoffenbecker, N.; Klein, S.A.; Reindl, D.T. Hot gas defrost model development and validation. Int. J. Refrig. 2005, 28, 605–615. [Google Scholar] [CrossRef]
- Amer, M.; Wang, C.C. Review of defrosting methods. Renew. Sustain. Energy Rev. 2017, 73, 53–74. [Google Scholar] [CrossRef]
- Klein, S.A.; Reindl, D.T.; Brownell, K. Refrigeration system performance using liquid-suction heat exchangers. Int. J. Refrig. 2000, 23, 588–596. [Google Scholar] [CrossRef]
- Domanski, P.A.; Didion, D.A.; Doyle, J.P. Evaluation of suction-line/liquid-line heat exchange in the refrigeration cycle. Int. J. Refrig. 1994, 17, 487–493. [Google Scholar] [CrossRef]
- Aprea, C.; Ascani, M.; Rossi, F. A criterion for predicting the possible advantage of adopting a suction/liquid heat exchanger in refrigerating system. Appl. Therm. Eng. 1999, 19, 329–336. [Google Scholar] [CrossRef]
- Torrella, E.; Sánchez, D.; Llopis, R.; Cabello, R. Energetic evaluation of an internal heat exchanger in a CO2 transcritical refrigeration plant using experimental data. Int. J. Refrig. 2011, 34, 40–49. [Google Scholar] [CrossRef]
- Rigola, J.; Ablanque, N.; Pérez-Segarra, C.D.; Oliva, A. Numerical simulation and experimental validation of internal heat exchanger influence on CO2 trans-critical cycle performance. Int. J. Refrig. 2010, 33, 664–674. [Google Scholar] [CrossRef]
- Sánchez, D.; Patiño, J.; Llopis, R.; Cabello, R.; Torrella, E.; Fuentes, F.V. New positions for an internal heat exchanger in a CO2 supercritical refrigeration plant. Experimental analysis and energetic evaluation. Appl. Therm. Eng. 2014, 63, 129–139. [Google Scholar] [CrossRef]
- Llopis, R.; Nebot-Andrés, L.; Sánchez, D.; Catalán-Gil, J.; Cabello, R. Subcooling methods for CO2 refrigeration cycles: A review. Int. J. Refrig. 2018, 93, 85–107. [Google Scholar] [CrossRef]
- Llopis, R.; Cabello, R.; Sánchez, D.; Torrella, E. Energy improvements of CO2 transcritical refrigeration cycles using dedicated mechanical subcooling. Int. J. Refrig. 2015, 55, 129–141. [Google Scholar] [CrossRef]
- Schoenfield, J.; Muehlbauer, J.; Hwang, Y.; Radermacher, R. Integration of a thermoelectric subcooler into a carbon dioxide transcritical vapor compression cycle refrigeration system. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 14–17 July 2008; p. 903. Available online: http://docs.lib.purdue.edu/iracc/903 (accessed on 3 July 2025).
- Arora, A.; Singh, N.K.; Monga, S.; Kumar, O. Energy and exergy analysis of a combined transcritical CO2 compression refrigeration and single effect H2O-LiBr vapour absorption system. Int. J. Exergy 2011, 9, 453–471. [Google Scholar] [CrossRef]
- Mazzola, D.; Sheehan, J.; Bortoluzzi, D.; Smitt, G.; Orlandi, M. Supermarket application. Effects of sub-cooling on real R744 based trans-critical plants in warm and hot climate. In Proceedings of the 12th IIR Gustav Lorentzen Conference on Natural Refrigerants (GL2016), Edinburgh, UK, 21–24 August 2016; pp. 551–558. [Google Scholar] [CrossRef]
- Chen, G.; Volovyk, O.; Zhu, D.; Ierin, V.; Shestopalov, K. Theoretical analysis and optimization of a hybrid CO2 transcritical mechanical compression—Ejector cooling cycle. Int. J. Refrig. 2017, 74, 86–94. [Google Scholar] [CrossRef]
- Kantchev, J.; Lesage, G. Mechanical Subcooling of Transcritical R-744 Refrigeration Systems with Heat Pump Heat Reclaim and Floating Head Pressure. European Patent Application EP 2 632 561 A3, 20 November 2013. Available online: https://patentimages.storage.googleapis.com/cf/fe/95/6b7e456e3a9165/EP2631561A3.pdf (accessed on 7 July 2025).
- Cecchinato, L.; Chiarello, M.; Corradi, M.; Fornasieri, E.; Minetto, S.; Stringari, P.; Zilio, C. Thermodynamic analysis of different two-stage transcritical carbon dioxide cycles. Int. J. Refrig. 2009, 32, 1058–1067. [Google Scholar] [CrossRef]
- Corberán, J.M.; Martínez-Galván, A.; Martínez-Ballester, S.; Gonzálvez-Maciá, J.; Alias, C. Impact of an internal heat exchanger on a transcritical CO2 heat pump under optimal pressure conditions: Optimal-pressure performance of CO2 heat pump with IHX. Int. J. Refrig. 2008, 31, 1059–1067. [Google Scholar] [CrossRef]
- Song, Y.; Cui, C.; Yin, X.; Cao, F. Advanced development and application of transcritical CO2 refrigeration and heat pump technology—A review. Energy Rep. 2022, 8, 7840–7869. [Google Scholar] [CrossRef]
- Nebot-Andrés, L.; Cabello, R.; Sánchez, D.; Catalán-Gil, J.; Llopis, R.; Torrella, E. Experimental assessment of different extraction points for the integrated mechanical subcooling system of a CO2 transcritical plant. Appl. Therm. Eng. 2021, 197, 117370. [Google Scholar] [CrossRef]
- Bellos, E.; Zafar Said, Z.; Lykas, P.; Tzivanidis, C. A review of polygeneration systems with CO2 working fluid. Therm. Sci. Eng. Progress. 2022, 31, 101325. [Google Scholar] [CrossRef]
- Li, W.; Korolija, I.; Tang, R.; Mumovic, D. Performance analysis of heat recovery in CO2 refrigeration systems for heating electrification in supermarkets. Appl. Energy 2025, 383, 125461. [Google Scholar] [CrossRef]
- Aghagoli, A.; Sorin, M.; Khennich, M. Exergy efficiency and COP improvement of a CO2 transcritical heat pump system by replacing an expansion valve with a Tesla turbine. Energies 2022, 15, 4973. [Google Scholar] [CrossRef]
- Gambini, M.; Manno, M.; Vellini, M. Energy and exergy analysis of transcritical CO2 cycles for heat pump applications. Sustainability 2024, 16, 7511. [Google Scholar] [CrossRef]
- Shi, W.; Chang, H.; Zhou, J.; Mu, B.; Quan, S.; Pan, L. Research progress on CO2 transcritical cycle technology for building heating and cooling applications. Buildings 2025, 15, 2952. [Google Scholar] [CrossRef]
- Karampour, M.; Sawalha, S. Integration of heating and air conditioning into a CO2 transcritical booster system with parallel compression. Part II: Performance analysis based on field measurements. In Proceedings of the 12th IIR Gustav Lorentzen Conference on Natural Refrigerants (GL2016), Edinburgh, UK, 21–24 August 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Pan, Z.; Zhao, J.; Hu, B.; Cao, F. Hot-gas bypass defrosting method and analysis of defrosting time for transcritical CO2 heat pump. J. Shanghai Jiaotong Univ. 2019, 53, 1367–1374. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, L.; Chen, Y.; Tong, L. Effect of an internal heat exchanger on performance of the transcritical carbon dioxide refrigeration cycle with an expander. Entropy 2014, 16, 5919–5934. [Google Scholar] [CrossRef]
- Shi, B.; Chen, M.; Chi, W.; Yang, Q.; Liu, G.; Zhao, Y.; Li, L. Effects of internal heat exchanger on two-stage compression transcritical CO2 refrigeration cycle combined with expander and intercooling. Energies 2023, 16, 115. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, D.; Jin, Z.; Wang, J.; Zhang, G.; Li, H. A comprehensive investigation on the effect of internal heat exchanger based on a novel evaluation method in the transcritical CO2 heat pump system. Renew. Energy 2021, 178, 574–586. [Google Scholar] [CrossRef]
- Nebot-Andrés, L.; Calleja-Anta, D.; Sánchez, D.; Cabello, R.; Llopis, R. Thermodynamic analysis of a CO2 refrigeration cycle with integrated mechanical subcooling. Energies 2020, 13, 4. [Google Scholar] [CrossRef]
- Satrústegui, Á.; Aranguren, P.; Araiz, M.; Sánchez, D.; Cabello, R.; Astrain, D. Experimental evaluation of a transcritical CO2 refrigeration facility working with an internal heat exchanger and a thermoelectric subcooler: Performance assessment and comparative. Int. J. Refrig. 2022, 141, 66–75. [Google Scholar] [CrossRef]
- Wang, J.; Belusko, M.; Evans, M.; Liu, M.; Zhao, C.; Bruno, F. A comprehensive review and analysis on CO2 heat pump water heaters. Energy Convers. Manag. X 2022, 15, 100277. [Google Scholar] [CrossRef]
- Besagni, G.; Mereu, R.; Inzoli, F. Ejector refrigeration: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 53, 373–407. [Google Scholar] [CrossRef]
- Chen, X.; Omer, S.; Worall, M.; Riffat, S. Recent developments in ejector refrigeration technologies. Renew. Sustain. Energy Rev. 2013, 19, 629–651. [Google Scholar] [CrossRef]
- Sumeru, K.; Nasution, H.; Ani, F. A review on two-phase ejector as an expansion device in vapor compression refrigeration cycle. Renew. Sustain. Energy Rev. 2012, 16, 4927–4937. [Google Scholar] [CrossRef]
- Sarkar, J. Ejector enhanced vapor compression refrigeration and heat pump systems—A review. Renew. Sustain. Energy Rev. 2012, 16, 6647–6659. [Google Scholar] [CrossRef]
- Li, D.; Groll, E.A. Transcritical CO2 refrigeration cycle with ejector-expansion device. Int. J. Refrig. 2005, 28, 766–773. [Google Scholar] [CrossRef]
- Deng, J.-Q.; Jiang, P.-X.; Lu, T.; Lu, W. Particular characteristics of transcritical CO2 refrigeration cycle with an ejector. Appl. Therm. Eng. 2007, 27, 381–388. [Google Scholar] [CrossRef]
- Haida, M.; Banasiak, K.; Smolka, J.; Hafner, A.; Eikevik, T.M. Experimental analysis of the R744 vapour compression rack equipped with the multi-ejector expansion work recovery module. Int. J. Refrig. 2016, 64, 93–107. [Google Scholar] [CrossRef]
- Schönenberger, J. Experience with R744 refrigerating systems and implemented multi ejectors and liquid overfeed. In Proceedings of the 12th IIR Gustav Lorentzen Natural Working Fluids Conference, Edinburgh, UK, 21–24 August 2016. ID: 1107. [Google Scholar]
- Hafner, A.; Shönenberger, J.; Banasiak, K.; Girotto, S. R744 ejector supported parallel vapour compression system. In Proceedings of the 3rd IIR International Conference on Sustainability and Cold Chain, London, UK, 23–25 June 2014. ID: 129. [Google Scholar]
- Hafner, A.; Försterling, S.; Banasiak, K. Multi-ejector concept for R-744 supermarket refrigeration. Int. J. Refrig. 2014, 43, 1–13. [Google Scholar] [CrossRef]
- Gullo, P.; Hafner, A.; Cortella, G. Multi-ejector R744 booster refrigerating plant and air conditioning system integration—A theoretical evaluation of energy benefits for supermarket applications. Int. J. Refrig. 2017, 75, 164–176. [Google Scholar] [CrossRef]
- Lv, J.; Lv, D.; Yang, Q.; Zhao, Y.; Liu, G.; Li, L. Comparative performance analysis of a self-heat recuperation driven ejector subcooling transcritical CO2 refrigeration systems using different throttling devices. Int. J. Refrig. 2025, 134, 106–119. [Google Scholar] [CrossRef]
- Chen, X.; Worall, M.; Omer, S.; Su, Y.; Riffat, S. Theoretical studies of a hybrid ejector CO2 compression cooling system for vehicles and preliminary experimental investigations of an ejector cycle. Appl. Energy 2013, 102, 931–942. [Google Scholar] [CrossRef]
- Ahamed, J.U.; Saidur, R.; Masjuki, H.H. A review on exergy analysis of vapor compression refrigeration system. Renew. Sustain. Energy Rev. 2011, 15, 1593–1600. [Google Scholar] [CrossRef]
- Murthy, A.A.; Subiantoro, A.; Norris, S.; Fukuta, M. A review on expanders and their performance in vapour compression refrigeration systems. Int. J. Refrig. 2019, 106, 427–446. [Google Scholar] [CrossRef]
- Elbel, S.; Lawrence, N. Review of recent developments in advanced ejector technology. Int. J. Refrig. 2016, 62, 1–18. [Google Scholar] [CrossRef]
- Lorentzen, G.; Pettersen, J. A new, efficient and environmentally benign system for car air-conditioning. Int. J. Refrig. 1993, 16, 4–12. [Google Scholar] [CrossRef]
- Kim, M.H.; Pettersen, J.; Bullard, C.W. Fundamental process and system design issues in CO2 vapor compression systems. Progress. Energy Combust. Sci. 2004, 30, 119–174. [Google Scholar] [CrossRef]
- Dai, B.; Liu, S.; Zhu, K.; Sun, Z.; Ma, Y. Thermodynamic performance evaluation of transcritical carbon dioxide refrigeration cycle integrated with thermoelectric subcooler and expander. Energy 2017, 122, 787–800. [Google Scholar] [CrossRef]
- Söylemez, E.; Hafner, A.; Schlemminger, C.; Kriezi, E.E.; Khorshidi, V. Performance analysis of an integrated CO2 refrigeration system with multi-ejectors installed in a supermarket. Energies 2022, 15, 3142. [Google Scholar] [CrossRef]
- Elbarghthi, A.F.A.; Dvořák, V. Evaluation of various ejector profiles on CO2 transcritical refrigeration system performance. Entropy 2022, 24, 1173. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.; Hao, L.; Lian, H.; Deng, J.; Lu, W. Modelling, optimization, and experimental studies of refrigeration CO2 ejectors: A review. Mathematics 2022, 10, 4325. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, L.; Wang, X. Thermodynamic analysis of double-stage compression transcritical CO2 refrigeration cycles with an expander. Entropy 2015, 17, 2544–2555. [Google Scholar] [CrossRef]
- Mei, S.; Liu, Z.; Liu, X. Research progress and applications of transcritical carbon dioxide heat pumps: A review. Clean Energy Sci. Technol. 2023, 1, 118. [Google Scholar] [CrossRef]
- Taslimitaleghani, S.; Sorin, M.; Poncet, S. Energy and exergy efficiencies of different configurations of the ejector-based CO2 refrigeration systems. Int. J. Energy Prod. Manag. 2018, 3, 22–33. [Google Scholar] [CrossRef]




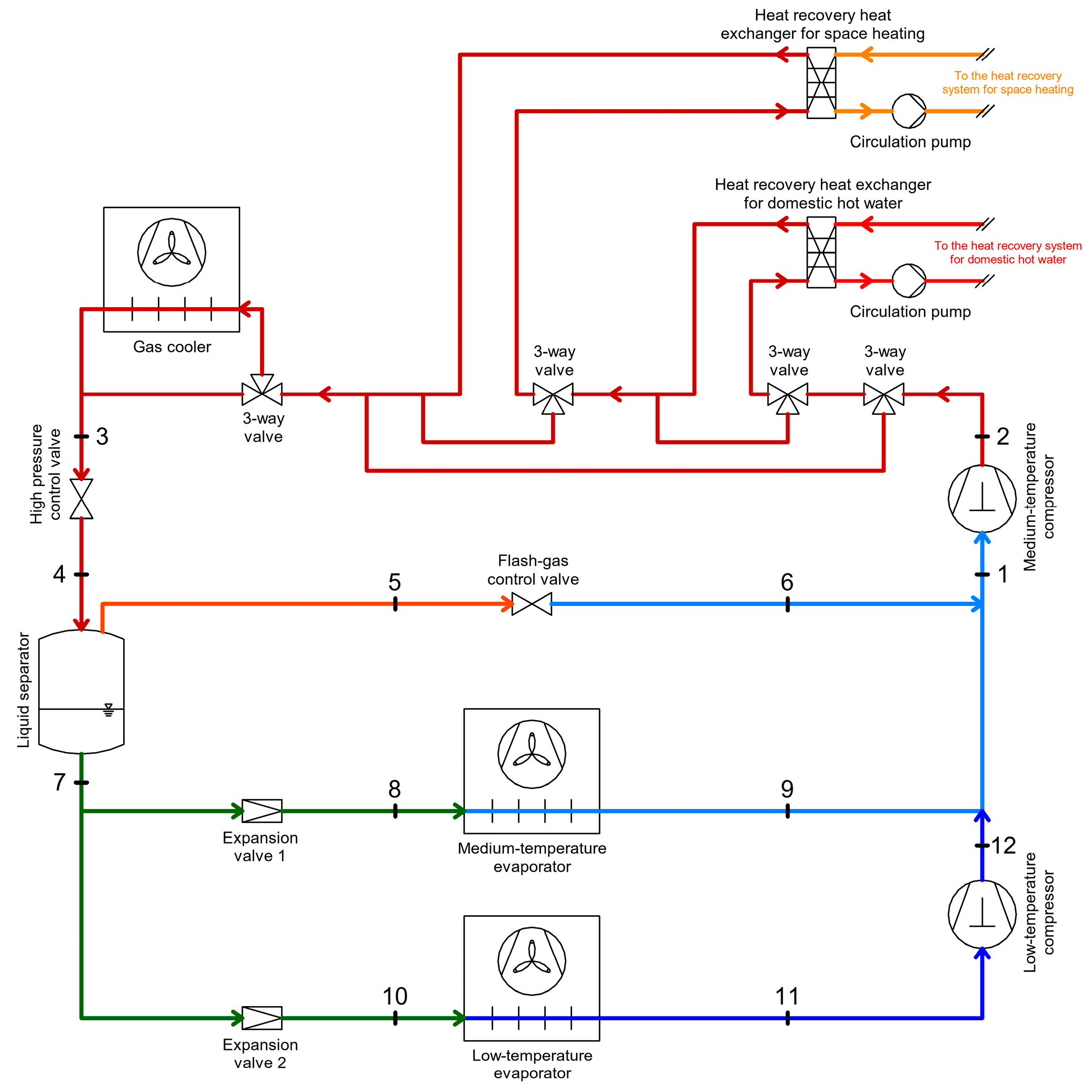

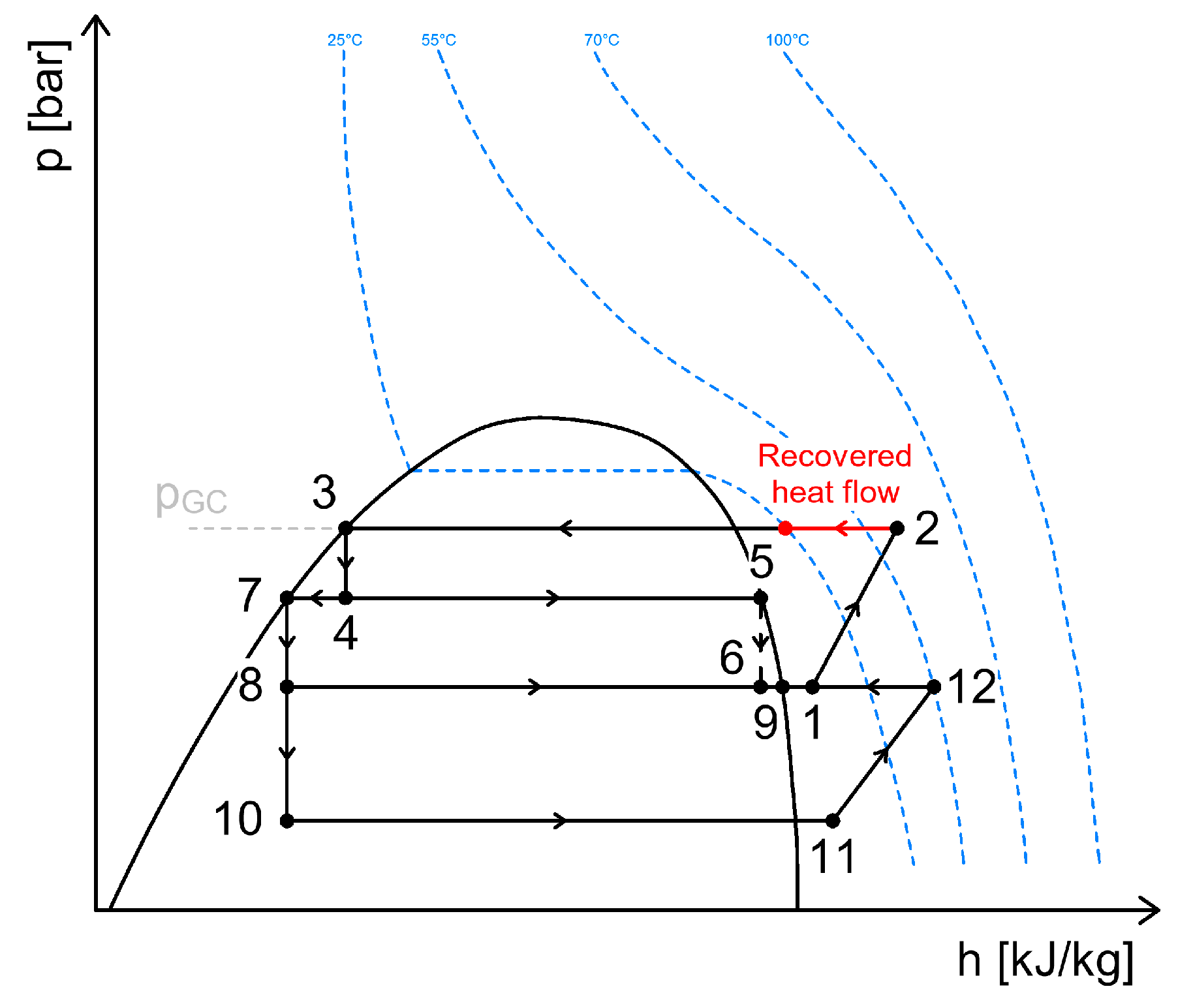
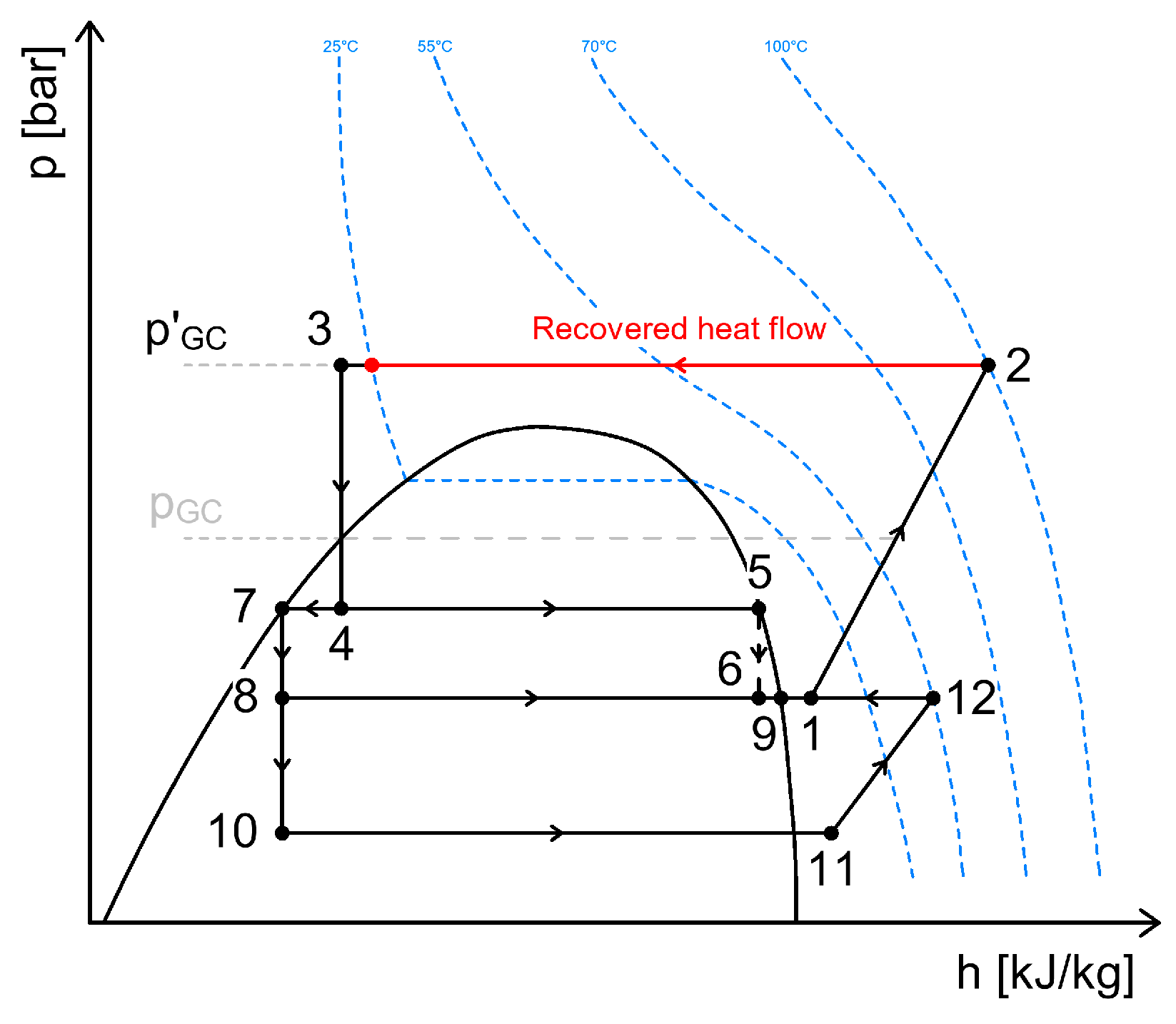



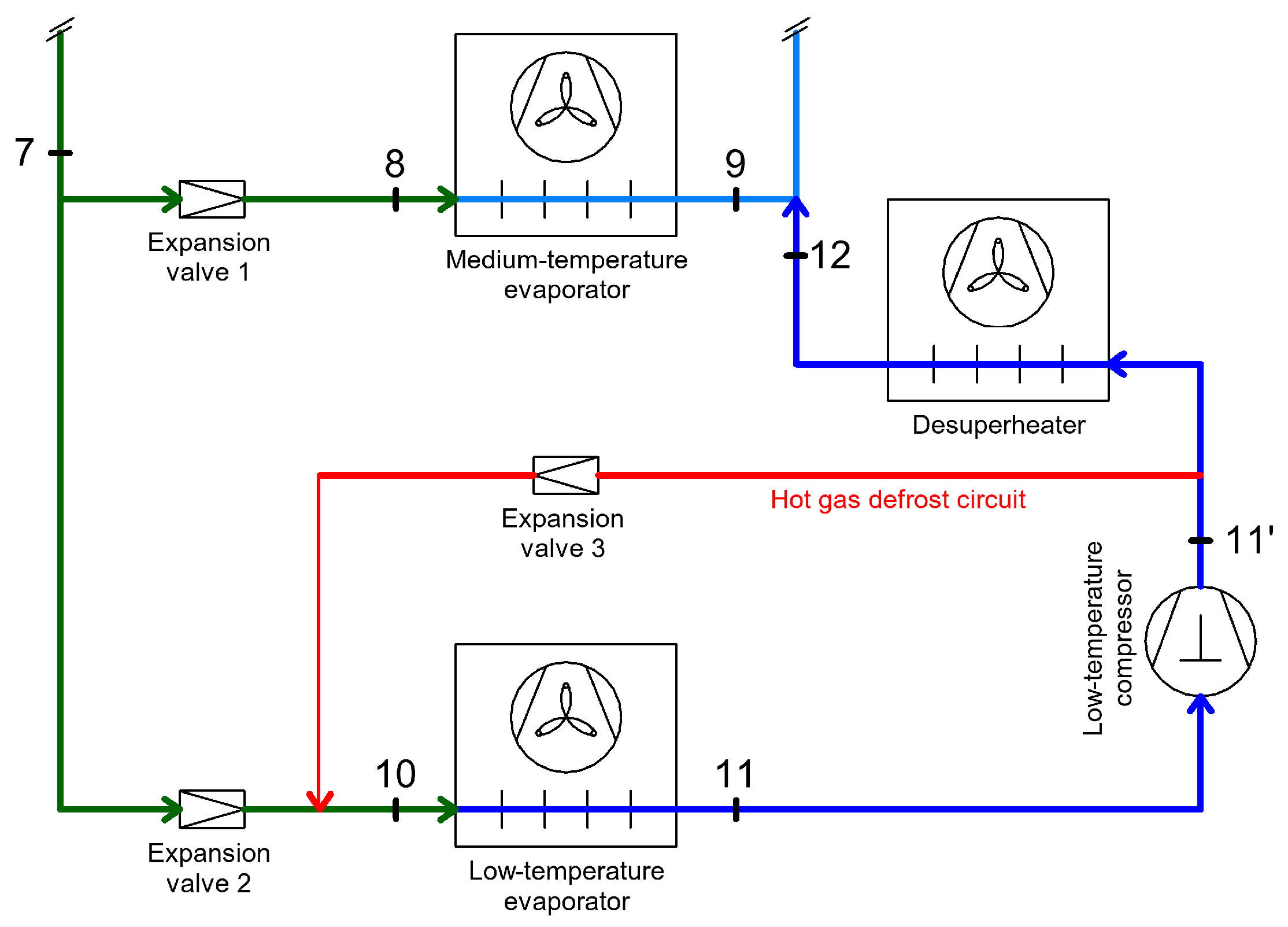









| Heat Recovery for DHW, SH, AC | Heat Recovery for Defrosting of Evaporators | Heat Recovery Through Internal Heat Exchangers | Energy Recovery | |||||
|---|---|---|---|---|---|---|---|---|
| HR from the High-Pressure Compressor Discharge | HR Between the Gas Cooler and the Intermediate Separator Receiver | HR Through Defrosting Evaporators with Hot Gases | HR Through Defrosting of Evaporators with Hot Glycol | HR Through Internal Heat Exchangers Acting as Superheaters | HR via Internal Heat Exchangers with Subcooling Function | Energy Recovery Using Ejectors | Energy Recovery Using Expansion Turbines | |
| Cascade Refrigeration System Using CO2 and Ammonia | ✓ | ✓ | ✓ | ✓ | ||||
| Single-Stage CO2 Refrigeration System | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Single-Stage CO2 Refrigeration System with Parallel Compression | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Single-Stage CO2 Refrigeration System with Ejector | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| CO2 Booster Refrigeration System | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CO2 Booster Refrigeration System with Parallel Compression | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CO2 Booster Refrigeration System with Parallel Compression and Ejector | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Energy Recovery Possibilities | COP Improvement | Exergy Efficiency Improvement | System Integration/Complexity | Advantages | Limitations | Cost (Estimated) | |
|---|---|---|---|---|---|---|---|
| Energy Recovery | Energy Recovery Using Ejectors | +5–18% [92,93,94] | +10–30% [93,94] | High |
| +5–15% investment cost, payback period 2–5 years | |
| Energy Recovery Using Expansion Turbines | +5–30% [95,96] | +10–30% [97] | Medium |
|
| +10–30% investment cost | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, C.C.; Apostol, V.; Pop, H.; Prisecaru, T.; Ionita, C.; Chiriac, A. A Review of Heat and Energy Recovery Possibilities Within CO2 Refrigeration Systems. Inventions 2025, 10, 105. https://doi.org/10.3390/inventions10060105
Pavel CC, Apostol V, Pop H, Prisecaru T, Ionita C, Chiriac A. A Review of Heat and Energy Recovery Possibilities Within CO2 Refrigeration Systems. Inventions. 2025; 10(6):105. https://doi.org/10.3390/inventions10060105
Chicago/Turabian StylePavel, Cornel Constantin, Valentin Apostol, Horatiu Pop, Tudor Prisecaru, Claudia Ionita, and Adrian Chiriac. 2025. "A Review of Heat and Energy Recovery Possibilities Within CO2 Refrigeration Systems" Inventions 10, no. 6: 105. https://doi.org/10.3390/inventions10060105
APA StylePavel, C. C., Apostol, V., Pop, H., Prisecaru, T., Ionita, C., & Chiriac, A. (2025). A Review of Heat and Energy Recovery Possibilities Within CO2 Refrigeration Systems. Inventions, 10(6), 105. https://doi.org/10.3390/inventions10060105






