Is Consumption of Ginger in Daily Life Associated with Sexual Response?
Abstract
:1. Introduction
1.1. Disgust, Sexual Arousal, and Sexual Dysfunction
1.2. Consumption of Ginger and Improvement in Sexual Behavior
1.3. Potential Pathways of Z Consumption on Sexual Desire and Sexual Behaviors
1.4. Aims and Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Background Information
2.2.2. Sex Drive Questionnaire
2.2.3. Frequency of Sexual Behaviors
2.2.4. Consumption of Ginger Inventory
2.3. Procedures
2.4. Data Analyses
3. Results
3.1. Correlations between Consumption of Z, Sexual Desire, and Sexual Behavior-Related Variables
3.2. Associations between Z Consumption and Sexual Behavior: Mediating Effects of Sexual Arousal, Disgust, and Sexual Desire
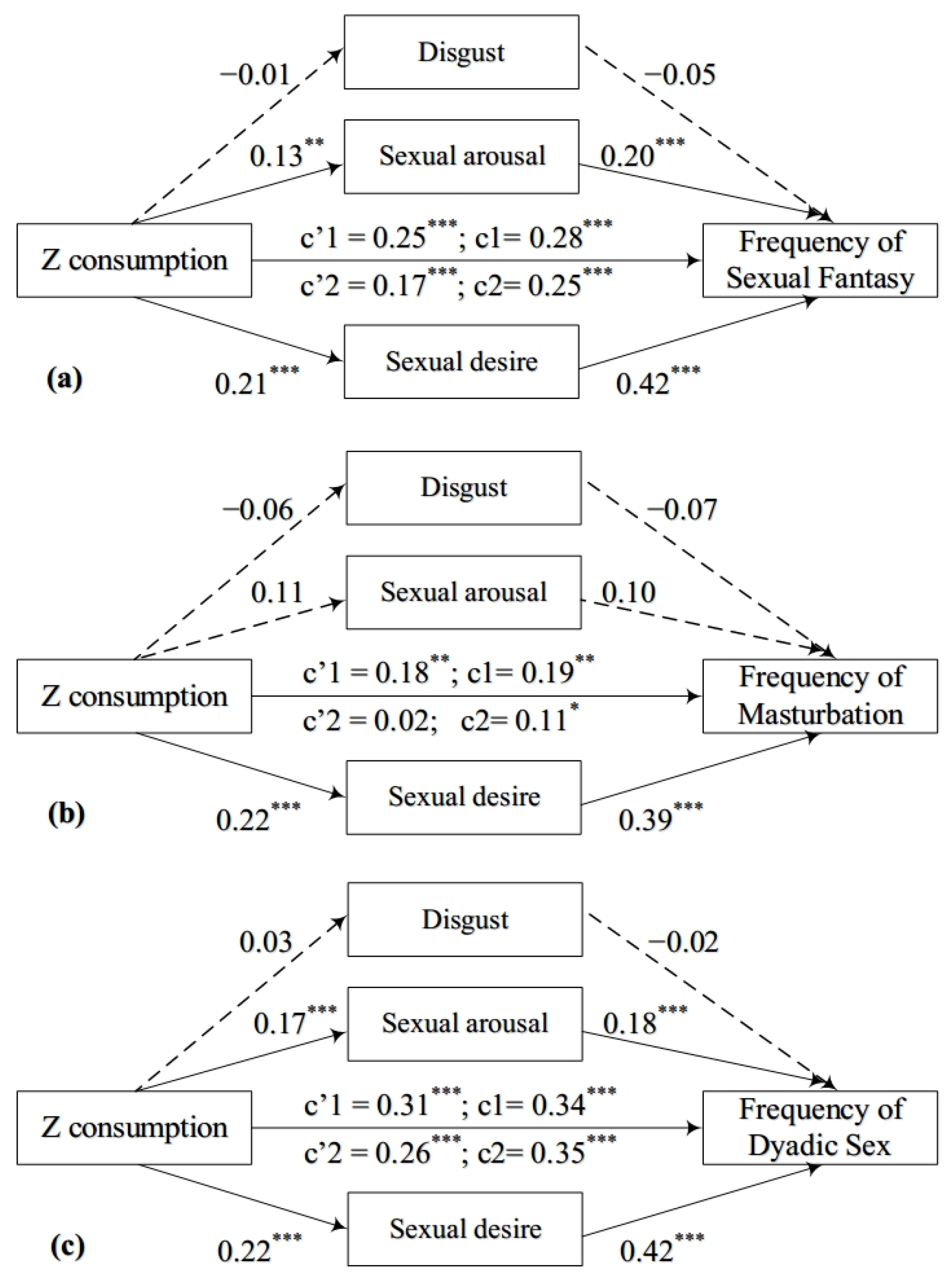
3.2.1. Mediating Analyses for the Relationship between Zwk and the Frequency of Sexual Fantasy
3.2.2. Mediating Analyses for the Relationship between Zwk and the Frequency of Masturbation
3.2.3. Mediating Analyses for the Relationship between Zwk and the Frequency of Dyadic Sex
3.3. Exploratory Mediation Analyses
4. Discussion
4.1. The Association between Z Consumption, Sexual Desire, and Sexual Behaviors-Related Variables
4.2. Potential Pathways behind Association between Z Consumption and Sexual Behaviors
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basson, R.; Gilks, T. Women’s Sexual Dysfunction Associated with Psychiatric Disorders and Their Treatment. Womens Health 2018, 14, 1745506518762664. [Google Scholar] [CrossRef]
- Bonierbale, M.; Lançon, C.; Tignol, J. The ELIXIR Study: Evaluation of Sexual Dysfunction in 4557 Depressed Patients in France. Curr. Med. Res. Opin. 2003, 19, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Brody, S. The Relative Health Benefits of Different Sexual Activities. J. Sex. Med. 2010, 7, 1336–1361. [Google Scholar] [CrossRef] [PubMed]
- Impett, E.A.; Strachman, A.; Finkel, E.J.; Gable, S.L. Maintaining Sexual Desire in Intimate Relationships: The Importance of Approach Goals. J. Pers. Soc. Psychol. 2008, 94, 808–823. [Google Scholar] [CrossRef]
- Laumann, E.O.; Paik, A.; Rosen, R.C. Sexual Dysfunction in the United StatesPrevalence and Predictors. JAMA 1999, 281, 537–544. [Google Scholar] [CrossRef]
- Lewis, R.W.; Fugl-Meyer, K.S.; Corona, G.; Hayes, R.D.; Laumann, E.O.; Moreira, E.D.; Rellini, A.H.; Segraves, T. Definitions/Epidemiology/Risk Factors for Sexual Dysfunction. J. Sex. Med. 2010, 7, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Santtila, P.; Wager, I.; Witting, K.; Harlaar, N.; Jern, P.; Johansson, A.; Varjonen, M.; Sandnabba, N.K. Discrepancies between Sexual Desire and Sexual Activity: Gender Differences and Associations with Relationship Satisfaction. J. Sex Marital Ther. 2008, 34, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, J.A.; Novick, A.M. Hypoactive Sexual Desire Disorder in Women: Physiology, Assessment, Diagnosis, and Treatment. J. Midwifery Womens Health 2021, 66, 740–748. [Google Scholar] [CrossRef]
- Curtis, V.; de Barra, M. The Structure and Function of Pathogen Disgust. Philos. Trans. R. Soc. B-Biol. Sci. 2018, 373, 20170208. [Google Scholar] [CrossRef]
- De Jong, P.J.; van Lankveld, J.; Elgersma, H.J.; Borg, C. Disgust and Sexual Problems-Theoretical Conceptualization and Case Illustrations. Int. J. Cogn. Ther. 2010, 3, 23–39. [Google Scholar] [CrossRef]
- Crosby, C.L.; Buss, D.M.; Meston, C.M. Sexual Disgust: Evolutionary Perspectives and Relationship to Female Sexual Function. Curr. Sex. Health Rep. 2019, 11, 300–306. [Google Scholar] [CrossRef]
- DePesa, N.S.; Cassisi, J.E. Affective and Autonomic Responses to Erotic Images: Evidence of Disgust-Based Mechanisms in Female Sexual Interest/Arousal Disorder. J. Sex Res. 2017, 54, 877–886. [Google Scholar] [CrossRef]
- Borg, C.; de Jong, P.J.; Schultz, W.W. Vaginismus and Dyspareunia: Automatic vs. Deliberate Disgust Responsivity. J. Sex. Med. 2010, 7, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.J.; van Overveld, M.; Schultz, W.W.; Peters, M.L.; Buwalda, F.M. Disgust and Contamination Sensitivity in Vaginismus and Dyspareunia. Arch. Sex. Behav. 2009, 38, 244–252. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.J.; van Overveld, M.; Borg, C. Giving in to Arousal or Staying Stuck in Disgust? Disgust-Based Mechanisms in Sex and Sexual Dysfunction. J. Sex Res. 2013, 50, 247–262. [Google Scholar] [CrossRef]
- Andrews, A.R.; Crone, T.; Cholka, C.B.; Cooper, T.V.; Bridges, A.J. Correlational and Experimental Analyses of the Relation between Disgust and Sexual Arousal. Motiv. Emot. 2015, 39, 766–779. [Google Scholar] [CrossRef]
- Borg, C.; Oosterwijk, T.A.; Lisy, D.; Boesveldt, S.; de Jong, P.J. The Influence of Olfactory Disgust on (Genital) Sexual Arousal in Men. PLoS ONE 2019, 14, e0214330. [Google Scholar] [CrossRef]
- Fleischman, D.S.; Hamilton, L.D.; Fessler, D.M.T.; Meston, C.M. Disgust versus Lust: Exploring the Interactions of Disgust and Fear with Sexual Arousal in Women. PLoS ONE 2015, 10, e0118151. [Google Scholar] [CrossRef]
- Hinzmann, J.; Borg, C.; Verwoerd, J.R.L.; de Jong, P.J. The Reciprocal Relationship Between Sexual Arousal and Disgust as Evidenced in Automatic Approach-Avoidance Behavior. J. Sex Res. 2019, 57, 384–396. [Google Scholar] [CrossRef]
- Albertson, K. Acupuncture and Chinese Herbal Medicine for Women’s Health: Bridging the Gap between Western and Eastern Medicine, 10th ed.; Createspace Independent Publishing Platform: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Ang-Lee, M.K.; Moss, J.; Yuan, C.S. Herbal Medicines and Perioperative Care. JAMA 2001, 286, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shabanian, S.; Ebrahimbabaei, M.; Safavi, P.; Lotfizadeh, M. Comparing the Effect of Rose Drop, Ginger, and Cinnamon on Sexual Function in Depressed Women with Sexual Dysfunction. Pharmacogn. Res. 2018, 10, 314–318. [Google Scholar] [CrossRef]
- Stein, R.A.; Schmid, K.; Bolivar, J.; Swick, A.G.; Joyal, S.V.; Hirsh, S.P. Kaempferia Parviflora Ethanol Extract Improves Self-Assessed Sexual Health in Men: A Pilot Study. J. Integr. Med.-JIM 2018, 16, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Adedara, I.A.; Thome, G.R.; Morsch, V.M.; Rovani, M.T.; Mujica, L.K.S.; Duarte, T.; Duarte, M.; Oboh, G.; Schetinger, M.R.C. Dietary Supplementation of Ginger and Turmeric Improves Reproductive Function in Hypertensive Male Rats. Toxicol. Rep. 2015, 2, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, B.J.; McInnis, D.; Morelli, R.; Castro, R.M.; Garziera, L.; Paranhos, L.G.; Costa, K.; Gava, C.; Costa, M.L.Z.; Walder, J.M.M. Optimum Dose of Ginger Root Oil to Treat Sterile Mediterranean Fruit Fly Males (Diptera: Tephritidae). J. Appl. Entomol. 2013, 137, 83–90. [Google Scholar] [CrossRef]
- Wen, G.; Zhang, Y.; Nyman, T.J.; Jern, P.; Santtila, P. Effects of Ginger on Disgust, Sexual Arousal, and Sexual Engagement: A Placebo-Controlled Experiment. J. Sex Res. 2023. ahead of print. [Google Scholar] [CrossRef]
- Tracy, J.L.; Steckler, C.M.; Heltzel, G. The Physiological Basis of Psychological Disgust and Moral Judgments. J. Pers. Soc. Psychol. 2019, 116, 15–32. [Google Scholar] [CrossRef]
- Shenhav, A.; Mendes, W.B. Aiming for the Stomach and Hitting the Heart: Dissociable Triggers and Sources for Disgust Reactions. Emotion 2014, 14, 301–309. [Google Scholar] [CrossRef]
- Eberhart, L.H.J.; Mayer, R.; Betz, O.; Tsolakidis, S.; Hilpert, W.; Morin, A.M.; Geldner, G.; Wulf, H.; Seeling, W. Ginger Does Not Prevent Postoperative Nausea and Vomiting after Laparoscopic Surgery. Anesth. Analg. 2003, 96, 995–998. [Google Scholar] [CrossRef]
- Montazeri, A.; Hamidzadeh, A.; Raei, M.; Mohammadiun, M.; Montazeri, A.S.; Mirshahi, R.; Rohani, H. Evaluation of Oral Ginger Efficacy against Postoperative Nausea and Vomiting: A Randomized, Double—Blinded Clinical Trial. Iran. Red Crescent Med. J. 2013, 15, e12268. [Google Scholar] [CrossRef]
- Nale, R.; Bhave, S.; Divekar, D.S. A Comparative Study of Ginger and Other Routinely Used Antiemetics for Prevention of Post Operative Nausea and Vomiting. J. Anaesthesiol. Clin. Pharmacol. 2007, 23, 405–410. [Google Scholar]
- Ostovich, J.M.; Sabini, J. How Are Sociosexuality, Sex Drive, and Lifetime Number of Sexual Partners Related? Pers. Soc. Psychol. Bull. 2004, 30, 1255–1266. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Melisaratos, N. The DSFI: A Multidimensional Measure of Sexual Functioning. J. Sex Marital Ther. 1979, 5, 244–281. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K. A Review of Food Frequency Questionnaires Developed and Validated in Japan. J. Epidemiol. 2009, 19, 1–11. [Google Scholar] [CrossRef]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared with Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am. J. Epidemiol. 2018, 187, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A Meta-Analysis of the Reproducibility of Food Frequency Questionnaires in Nutritional Epidemiological Studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. Validity of the Food Frequency Questionnaire for Adults in Nutritional Epidemiological Studies: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2021, 63, 1670–1688. [Google Scholar] [CrossRef]
- Willett, W.; Lenart, E. Reproducibility and Validity of Food-Frequency Questionnaires. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2012; pp. 96–141. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Guilford Press: New York, NY, USA, 2018. [Google Scholar]
- Marx, W.; Ried, K.; McCarthy, A.L.; Vitetta, L.; Sali, A.; McKavanagh, D.; Isenring, L. Ginger-Mechanism of Action in Chemotherapy-Induced Nausea and Vomiting: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 141–146. [Google Scholar] [CrossRef]
- Toth, B.; Lantos, T.; Hegyi, P.; Viola, R.; Vasas, A.; Benko, R.; Gyongyi, Z.; Vincze, A.; Csecsei, P.; Miko, A.; et al. Ginger (Zingiber Officinale): An Alternative for the Prevention of Postoperative Nausea and Vomiting. A Meta-Analysis. Phytomedicine 2018, 50, 8–18. [Google Scholar] [CrossRef]
- Cappelletti, M.; Wallen, K. Increasing Women’s Sexual Desire: The Comparative Effectiveness of Estrogens and Androgens. Horm. Behav. 2016, 78, 178–193. [Google Scholar] [CrossRef]
- Davis, S.R.; Worsley, R.; Miller, K.K.; Parish, S.J.; Santoro, N. Androgens and Female Sexual Function and Dysfunction—Findings from the Fourth International Consultation of Sexual Medicine. J. Sex. Med. 2016, 13, 168–178. [Google Scholar] [CrossRef]
- Yoest, K.E.; Quigley, J.A.; Becker, J.B. Rapid Effects of Ovarian Hormones in Dorsal Striatum and Nucleus Accumbens. Horm. Behav. 2018, 104, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Panay, N.; Al-Azzawi, F.; Bouchard, C.; Davis, S.R.; Eden, J.; Lodhi, I.; Rees, M.; Rodenberg, C.A.; Rymer, J.; Schwenkhagen, A.; et al. Testosterone Treatment of HSDD in Naturally Menopausal Women: The ADORE Study. Climacteric 2010, 13, 121–131. [Google Scholar] [CrossRef]
- Shifren, J.L.; Davis, S.R.; Moreau, M.; Waldbaum, A.; Bouchard, C.; DeRogatis, L.; Derzko, C.; Bearnson, P.; Kakos, N.; O’Neill, S.; et al. Testosterone Patch for the Treatment of Hypoactive Sexual Desire Disorder in Naturally Menopausal Women: Results from the INTIMATE NM1 Study. Menopause 2006, 13, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.A.; Forbes, E.A.; Strauss, B.J.G.; McLachlan, R.I. Testosterone Therapy Increases Sexual Desire in Ageing Men with Low-Normal Testosterone Levels and Symptoms of Androgen Deficiency. Int. J. Impot. Res. 2008, 20, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.; Cole, N.; Bhartia, M.; Kennedy, D.; Raju, J.; Wilkinson, P.; Saghir, A.; Blast Study Group. The Response to Testosterone Undecanoate in Men with Type 2 Diabetes Is Dependent on Achieving Threshold Serum Levels (the BLAST Study). Int. J. Clin. Pract. 2014, 68, 203–215. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the Diagnosis, Treatment and Monitoring of Hypogonadism in Men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Mattern, A.; Hanisch, J.; Gooren, L.; Jones, H.; Maggi, M. IPASS: A Study on the Tolerability and Effectiveness of Injectable Testosterone Undecanoate for the Treatment of Male Hypogonadism in a Worldwide Sample of 1438 Men. J. Sex. Med. 2013, 10, 579–588. [Google Scholar] [CrossRef]
- Banihani, S.A. Ginger and Testosterone. Biomolecules 2018, 8, 119. [Google Scholar] [CrossRef]
- Pfaus, J.G. Pathways of Sexual Desire. J. Sex. Med. 2009, 6, 1506–1533. [Google Scholar] [CrossRef]
- Kedia, G.T.; Ückert, S.; Tsikas, D.; Becker, A.J.; Kuczyk, M.A.; Bannowsky, A. The Use of Vasoactive Drugs in the Treatment of Male Erectile Dysfunction: Current Concepts. J. Clin. Med. 2020, 9, 2987. [Google Scholar] [CrossRef]
- Brotto, L.A. Evidence-Based Treatments for Low Sexual Desire in Women. Front. Neuroendocrinol. 2017, 45, 11–17. [Google Scholar] [CrossRef] [PubMed]
| N | % | ||
|---|---|---|---|
| Age (M ± SD) | 28.72 (5.22) | 5.22 | |
| The number of sex partners (M ± SD) | 1.88 (1.73) | 1.73 | |
| Sex | Men | 249 | 49.9 |
| Women | 250 | 50.1 | |
| Relationship status | Single (including divorce) | 78 | 15.6 |
| In committed relationship | 421 | 84.4 | |
| Education | Primary school | 1 | 0.2 |
| Junior school | 2 | 0.4 | |
| Senior school | 16 | 3.2 | |
| College | 418 | 83.8 | |
| Postgraduate and above | 62 | 12.4 | |
| Sexual orientation | Heterosexual | 487 | 97.6 |
| Homosexual | 6 | 1.2 | |
| Bisexual | 6 | 1.2 | |
| Relationship length | Single | 75 | 15 |
| <1 month | 6 | 1.2 | |
| 1–3 months | 8 | 1.6 | |
| 4–6 months | 14 | 2.8 | |
| 7–12 months | 25 | 5 | |
| 1–2 years | 69 | 13.8 | |
| 3–5 years | 151 | 30.3 | |
| 6–10 years | 112 | 22.4 | |
| >10 years | 39 | 7.8 | |
| Monthly income (¥) | No | 14 | 2.8 |
| <5 k | 114 | 22.8 | |
| 5~8 k | 160 | 32.1 | |
| 9~15 k | 168 | 33.7 | |
| 16~30 k | 40 | 8 | |
| 31~50 k | 2 | 0.4 | |
| >50 k | 1 | 0.2 | |
| General physical health (“In general, would you say your physical health is”) | Excellent | 157 | 31.5 |
| Very good | 224 | 44.9 | |
| Good | 82 | 16.4 | |
| Fair | 32 | 6.4 | |
| Poor | 4 | 0.8 | |
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | - | - | 1 | ||||||
| 2. Age | 28.72 | 5.22 | 0.076 | 1 | |||||
| 3. Relationship length | 6.03 | 2.48 | 0.057 | 0.676 *** | 1 | ||||
| 4. The number of sexual partners | 1.88 | 1.73 | 0.191 *** | 0.234 *** | 0.222 *** | 1 | |||
| 5. Health condition a | 2.00 | 0.90 | −0.04 | −0.100 * | −0.151 *** | −0.072 | |||
| 6. Zwk | 2.36 | 1.85 | 0.01 | 0.215 *** | 0.224 *** | 0.169 ** | −0.086 | 1 | |
| 7. Sexual desire | 3.75 | 0.94 | 0.306 *** | 0.190 *** | 0.300 ** | 0.284 ** | −0.239 *** | 0.215 *** | 1 |
| Frequency of Sexual Fantasy | 2.14 | 2.26 | 0.214 *** | −0.017 | −0.001 | 0.260 ** | −0.065 | 0.254 *** | 0.453 *** |
| Frequency of Masturbation | 0.70 | 1.37 | 0.086 | −0.108 * | −0.103 * | 0.062 | 0.04 | § 0.107 * | 0.397 *** |
| Frequency of Dyadic Sex | 1.09 | 1.27 | 0.148 *** | 0.148 *** | 0.275 *** | 0.281 ** | −0.157 *** | 0.350 *** | 0.473 *** |
| Disgust during Sexual Fantasy | 1.89 | 1.47 | 0.005 | −0.099 * | −0.076 | −0.099 * | 0.135 ** | −0.006 | −0.156 ** |
| Disgust during Masturbation | 2.16 | 1.61 | 0.085 | −0.016 | 0.001 | −0.129 | 0.033 | −0.06 | −0.079 |
| Disgust during Dyadic Sex | 1.42 | 1.22 | −0.029 | −0.082 | −0.043 | −0.047 | 0.093 | 0.03 | −0.043 |
| Sexual arousal during Sexual Fantasy | 5.39 | 1.61 | 0.041 | 0.199 *** | 0.220 *** | 0.085 | −0.144 ** | 0.129 ** | 0.405 *** |
| Sexual arousal during Masturbation | 5.87 | 1.64 | −0.101 | −0.012 | 0.043 | −0.148 * | 0.029 | § 0.113 | 0.177 * |
| Sexual arousal during Dyadic Sex | 6.26 | 1.31 | 0.082 | 0.208 *** | 0.262 *** | 0.151 ** | −0.241 *** | 0.170 *** | 0.428 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, G.; Zhang, Y.; Nyman, T.J.; Jern, P.; Santtila, P. Is Consumption of Ginger in Daily Life Associated with Sexual Response? Sexes 2023, 4, 555-568. https://doi.org/10.3390/sexes4040036
Wen G, Zhang Y, Nyman TJ, Jern P, Santtila P. Is Consumption of Ginger in Daily Life Associated with Sexual Response? Sexes. 2023; 4(4):555-568. https://doi.org/10.3390/sexes4040036
Chicago/Turabian StyleWen, Guangju, Yikang Zhang, Thomas J. Nyman, Patrick Jern, and Pekka Santtila. 2023. "Is Consumption of Ginger in Daily Life Associated with Sexual Response?" Sexes 4, no. 4: 555-568. https://doi.org/10.3390/sexes4040036
APA StyleWen, G., Zhang, Y., Nyman, T. J., Jern, P., & Santtila, P. (2023). Is Consumption of Ginger in Daily Life Associated with Sexual Response? Sexes, 4(4), 555-568. https://doi.org/10.3390/sexes4040036







