Sex Differences in Autonomic Blood Pressure Regulation: Sex Chromosome Complement and Hormonal Involvement
Abstract
1. Introduction
2. Sexual Dimorphism in Autonomic and Baroreflex Blood Pressure Regulation
3. But Why Do Males and Females Show Differences in Autonomic Blood Pressure Regulation?
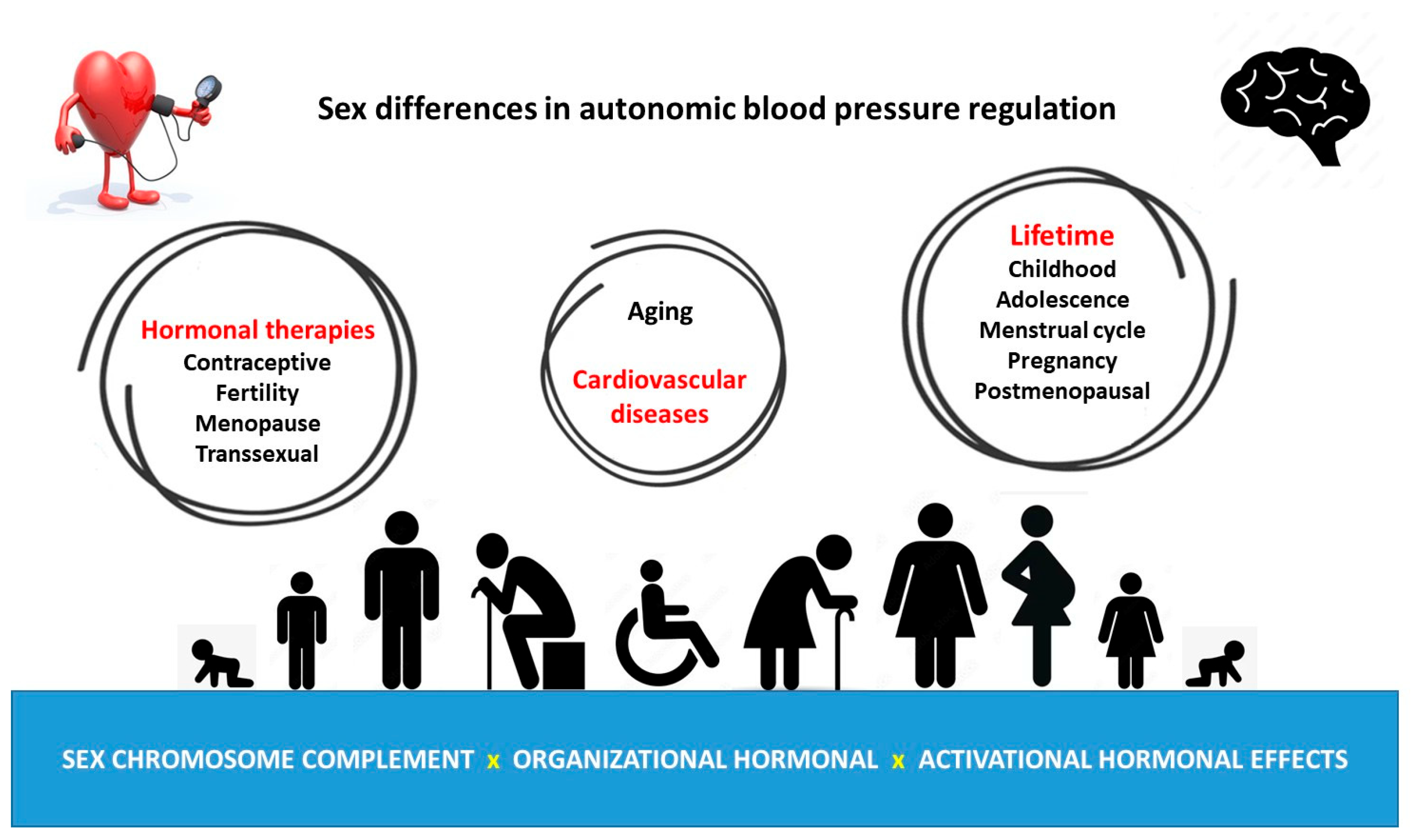
4. Female Autonomic Blood Pressure Regulation during Lifetime
4.1. Activational Effect of Estrogen on Autonomic Blood Pressure Regulation
4.2. Activational Effect of Progesterone on Autonomic Blood Pressure Regulation
4.3. Pregnancy, Progesterone Metabolites, and Baroreflex Function
4.4. Polycystic Ovarian Syndrome and Autonomic Cardiovascular Dysfunction
5. Male Autonomic Blood Pressure Regulation during Lifetime
Activational Effect of Testosterone on Autonomic Blood Pressure Regulation
6. Effect of Hormonal Therapies on Autonomic Blood Pressure Regulation
6.1. Contraceptive Treatment Effects and Autonomic Blood Pressure Regulation
6.2. Fertility Treatments and Autonomic Blood Pressure Regulation
6.3. Hormone Replacement Treatment in Menopause on Autonomic Blood Pressure Regulation
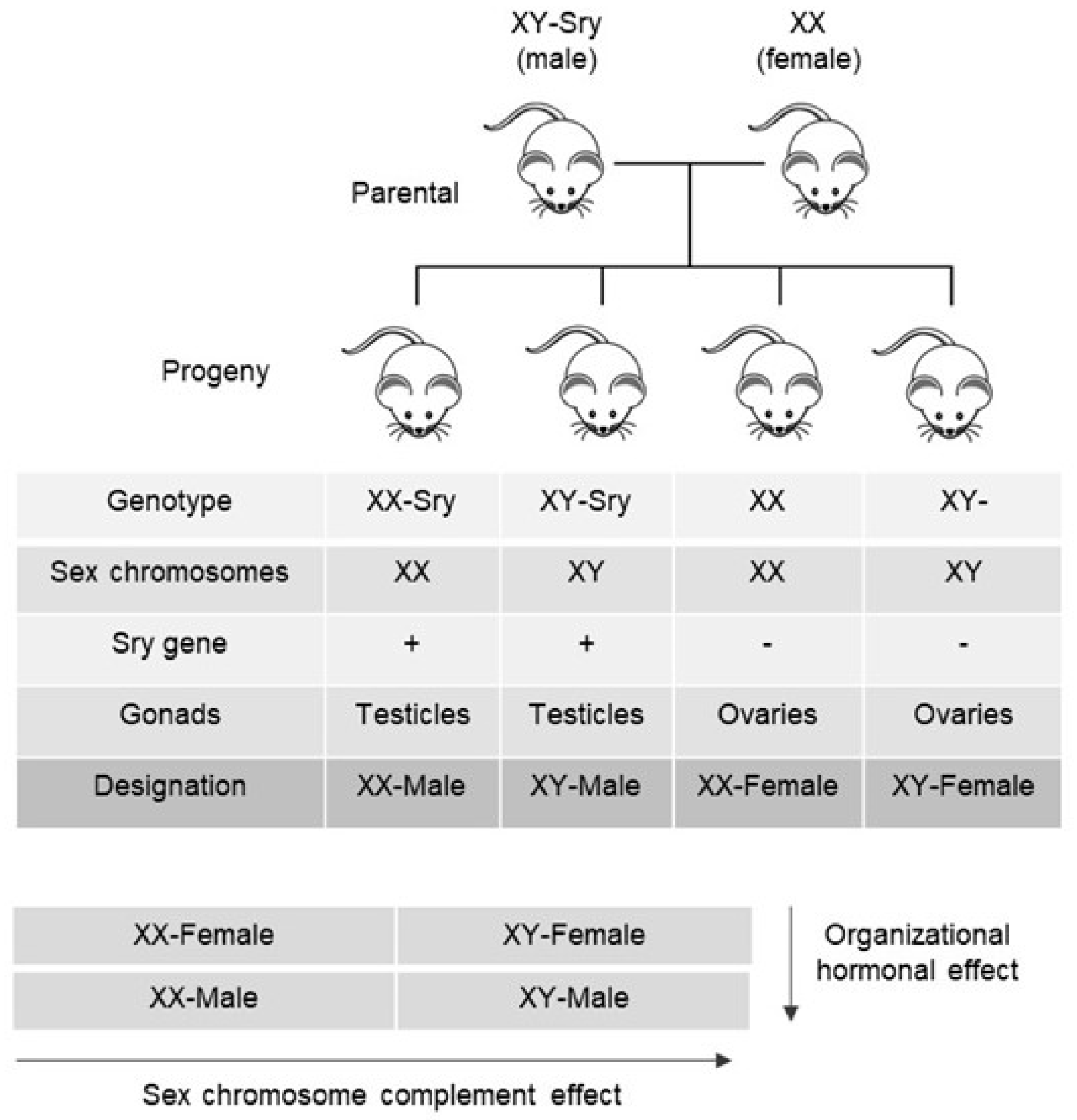
7. Organizational Sex Hormonal and SCC Effects on Sexually Dimorphic Blood Pressure Regulation
SCC and Organizational Sex Hormonal Effects on Sexually Dimorphic Bradycardic Baroreflex Response
8. Transsexual Sympathovagal Imbalances during Hormonal Treatments—Interaction of SCC, Organizational and Activational Hormonal Effects
9. SCC and Cardiovascular Diseases
10. Conclusions
Limitations and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ang II | Angiotensin II |
| AP | Area postrema |
| Agtr1a | AT1 type a receptor gene |
| Agtr2 | AT2 receptor type 2 gene |
| AT1 | Angiotensinergic type 1 receptor |
| AT2 | Angiotensinergic type 2 receptor |
| E2 | Estradiol |
| ERα | Estrogen receptor alpha |
| ERαKO | Estrogen receptor alpha knockout mice |
| FtoM | Female-to-male transsexual |
| HRV | Heart rate variability |
| LF/HF | Low-to-high-frequency power ratio |
| Mas | Angiotensinergic Mas receptor |
| MtoF | Male-to-female transsexual |
| NTS | Nucleus of the solitary tract |
| PE | Phenylephrine |
| RAS | Renin–angiotensin system |
| RVLM | Rostro ventrolateral medulla |
| SCC | Sex chromosome complement |
| WKY | Wistar Kyoto |
References
- Stramba-Badiale, M.; Fox, K.M.; Priori, S.G.; Collins, P.; Daly, C.; Graham, I.; Jonsson, B.; Schenck-Gustafsson, K.; Tendera, M. Cardiovascular diseases in women: A statement from the policy conference of the European Society of Cardiology. Eur. Heart J. 2006, 27, 994–1005. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex. Differ. 2012, 3, 7. [Google Scholar] [CrossRef]
- Mercuro, G.; Deidda, M.; Piras, A.; Dessalvi, C.C.; Maffei, S.; Rosano, G.M. Gender determinants of cardiovascular risk factors and diseases. J. Cardiovasc. Med. 2010, 11, 207–220. [Google Scholar] [CrossRef]
- Arain, F.A.; Kuniyoshi, F.H.; Abdalrhim, A.D.; Miller, V.M. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ. J. 2009, 73, 1774–1782. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Dampney, R.A.L. Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms. Front. Neurosci. 2017, 11, 461. [Google Scholar] [CrossRef]
- Koenig, J.; Rash, J.A.; Campbell, T.S.; Thayer, J.F.; Kaess, M. A Meta-Analysis on Sex Differences in Resting-State Vagal Activity in Children and Adolescents. Front. Physiol. 2017, 8, 582. [Google Scholar] [CrossRef]
- Evans, J.M.; Ziegler, M.G.; Patwardhan, A.R.; Ott, J.B.; Kim, C.S.; Leonelli, F.M.; Knapp, C.F. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. 2001, 91, 2611–2618. [Google Scholar] [CrossRef]
- Ramesh, S.; Wilton, S.B.; Holroyd-Leduc, J.M.; Turin, T.C.; Sola, D.Y.; Ahmed, S.B. Testosterone is associated with the cardiovascular autonomic response to a stressor in healthy men. Clin. Exp. Hypertens. 2015, 37, 184–191. [Google Scholar] [CrossRef]
- Resmini, E.; Casu, M.; Patrone, V.; Rebora, A.; Murialdo, G.; Minuto, F.; Ferone, D. Sympathovagal imbalance in transsexual subjects. J. Endocrinol. Investig. 2008, 31, 1014–1019. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Kuo, T.B.J.; Li, J.-Y.; Kuo, K.-L.; Chern, C.-M.; Yang, C.C.H.; Huang, H.-Y. Effects of age and sex on vasomotor activity and baroreflex sensitivity during the sleep-wake cycle. Sci. Rep. 2022, 12, 22424. [Google Scholar] [CrossRef]
- Kuo, T.B.; Lin, T.; Yang, C.C.; Li, C.L.; Chen, C.F.; Chou, P. Effect of aging on gender differences in neural control of heart rate. Am. J. Physiol. 1999, 277, H2233–H2239. [Google Scholar] [CrossRef]
- Sachse, C.; Trozic, I.; Brix, B.; Roessler, A.; Goswami, N. Sex differences in cardiovascular responses to orthostatic challenge in healthy older persons: A pilot study. Physiol. Int. 2019, 106, 236–249. [Google Scholar] [CrossRef]
- Lavi, S.; Nevo, O.; Thaler, I.; Rosenfeld, R.; Dayan, L.; Hirshoren, N.; Gepstein, L.; Jacob, G. Effect of aging on the cardiovascular regulatory systems in healthy women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R788–R793. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hasser, E.M.; Bishop, V.S. Central effect of angiotensin II on baroreflex regulation in conscious rabbits. Am. J. Physiol. 1989, 256 Pt 2, R694–R700. [Google Scholar] [CrossRef]
- Pamidimukkala, J.; Taylor, J.A.; Welshons, W.V.; Lubahn, D.B.; Hay, M. Estrogen modulation of baroreflex function in conscious mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R983–R989. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.R.; Merrill, R.H.; Wooles, W.R. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J. Appl. Physiol. 1994, 77, 606–613. [Google Scholar] [CrossRef]
- Convertino, V.A. Gender differences in autonomic functions associated with blood pressure regulation. Am. J. Physiol. 1998, 275, R1909–R1920. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Pikkuja¨msa¨, S.M.; Airaksinen, K.J.; Ika¨heimo, M.J.; Rantala, A.O.; Kauma, H.; Lilja, M.; Kesa¨niemi, Y.A. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 1996, 94, 122–125. [Google Scholar] [CrossRef]
- Tank, J.; Diedrich, A.; Szczech, E.; Luft, F.C.; Jordan, J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 2005, 45, 1159–1164. [Google Scholar] [CrossRef]
- Caeiro, X.E.; Mir, F.R.; Vivas, L.M.; Carrer, H.F.; Cambiasso, M.J. Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertension 2011, 58, 505–511. [Google Scholar] [CrossRef]
- Goodfellow, P.N.; Lovell-Badge, R. SRY and sex determination in mammals. Annu. Rev. Genet. 1993, 27, 71–92. [Google Scholar] [CrossRef]
- Mäkelä, J.A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef]
- Arnold, A.P.; Gorski, R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984, 7, 413–442. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Quraishy, S.; Abdel-Baki, A.-A.; Ghanjati, F.; Arauzo-Bravo, M.J.; Delic, D.; Wunderlich, F. Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. J. Steroid Biochem. Mol. Biol. 2015, 145, 121–130. [Google Scholar] [CrossRef]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Augui, S.; Nora, E.P.; Heard, E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat. Rev. Genet. 2011, 12, 429–442. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, J.T.; Rissman, E.F.; Bekiranov, S. Sexual differentiation in the developing mouse brain: Contributions of sex chromosome genes. Genes. Brain Behav. 2013, 12, 166–180. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef]
- Berletch, J.B.; Yang, F.; Xu, J.; Carrel, L.; Disteche, C.M. Genes that escape from X inactivation. Hum. Genet. 2011, 130, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Carrel, L.; Willard, H.F. Heterogeneous gene expression from the inactive X chromosome: An X-linked gene that escapes X inactivation in some human cell lines but is inactivated in others. Proc. Natl. Acad. Sci. USA 1999, 96, 7364–7369. [Google Scholar] [CrossRef]
- Zhang, Y.; Castillo-Morales, A.; Jiang, M.; Zhu, Y.; Hu, L.; Urrutia, A.O.; Kong, X.; Hurst, L.D. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol. Biol. Evol. 2013, 30, 2588–2601, Erratum in Mol. Biol. Evol. 2016, 33, 302. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Babak, T.; Shendure, J.; Disteche, C.M. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010, 20, 614–622. [Google Scholar] [CrossRef]
- Arnold, A.P.; Xu, J.; Grisham, W.; Chen, X.; Kim, Y.H.; Itoh, Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology 2004, 145, 1057–1062. [Google Scholar] [CrossRef]
- Cabrera Zapata, L.E.; Garcia-Segura, L.M.; Cambiasso, M.J.; Arevalo, M.A. Genetics and Epigenetics of the X and Y Chromosomes in the Sexual Differentiation of the Brain. Int. J. Mol. Sci. 2022, 23, 12288. [Google Scholar] [CrossRef]
- Dart, A.M.; Du, X.J.; Kingwell, B.A. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res. 2002, 53, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Yildirir, A.; Kabakci, G.; Akgul, E.; Tokgozoglu, L.; Oto, A. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Ann. Noninvasive Electrocardiol. 2002, 7, 60–63. [Google Scholar] [CrossRef]
- Tada, Y.; Yoshizaki, T.; Tomata, Y.; Yokoyama, Y.; Sunami, A.; Hida, A.; Kawano, Y. The Impact of Menstrual Cycle Phases on Cardiac Autonomic Nervous System Activity: An Observational Study Considering Lifestyle (Diet, Physical Activity, and Sleep) among Female College Students. J. Nutr. Sci. Vitaminol. 2017, 63, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.F.; Eagan, L.E.; Ranadive, S.M. Heart rate variability between hormone phases of the menstrual and oral contraceptive pill cycles of young women. Clin. Auton. Res. 2023, 33, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Hirshoren, N.; Tzoran, I.; Makrienko, I.; Edoute, Y.; Plawner, M.M.; Itskovitz-Eldor, J.; Jacob, G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J. Clin. Endocrinol. Metab. 2002, 87, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.K.; Azar, A.S.; Mulvaney, J.M.; Hinojosa-Laborde, C.; Haywood, J.R.; Brooks, V.L. Baroreflex sensitivity varies during the rat estrous cycle: Role of gonadal steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1419–R1426. [Google Scholar] [CrossRef]
- Du, X.J.; Dart, A.M.; Riemersma, R.A. Sex differences in the parasympathetic nerve control of rat heart. Clin. Exp. Pharmacol. Physiol. 1994, 21, 485–493. [Google Scholar] [CrossRef]
- Olsen, K.L.; Edwards, E.; Schechter, N.; Whalen, R.E. Muscarinic receptors in preoptic area and hypothalamus: Effects of cyclicity, sex and estrogen treatment. Brain Res. 1988, 448, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, T.C.; Degroff, V.; Luine, V.N.; McEwen, B.S. Estradiol 17 beta increases the number of muscarinic receptors in hypothalamic nuclei. Brain Res. 1980, 198, 239–243. [Google Scholar] [CrossRef]
- De Melo, V.U.; Saldanha, R.R.M.; Dos Santos, C.R.; Cruz, J.D.C.; Lira, V.A.; Santana-Filho, V.J.; Michelini, L.C. Ovarian Hormone Deprivation Reduces Oxytocin Expression in Paraventricular Nucleus Preautonomic Neurons and Correlates with Baroreflex Impairment in Rats. Front. Physiol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Tezini, G.C.; Becari, C.; Zanotto, C.Z.; Salgado, M.C.; Passaglia Rde, C.; Souza, H.C. Ageing is the main determinant of haemodynamics and autonomic cardiac changes observed in post-menopausal female rats. Auton. Neurosci. 2013, 174, 36–41. [Google Scholar] [CrossRef]
- Mohamed, M.K.; El-Mas, M.M.; Abdel-Rahman, A.A. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am. J. Physiol. 1999, 276, R1030–R1037. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.C.; Connell, B.J.; Saleh, T.M. Medullary and intrathecal injections of 17beta-estradiol in male rats. Brain Res. 2000, 867, 200–209. [Google Scholar] [CrossRef]
- El-Mas, M.M.; Abdel-Rahman, A.A. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can. J. Physiol. Pharmacol. 1998, 76, 381–386. [Google Scholar] [CrossRef]
- Saleh, T.M.; Connell, B.J. 17beta-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J. Auton. Nerv. Syst. 2000, 80, 148–161. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Lane, M.V.; Numan, S.; Dellovade, T.L. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004, 473, 270–291. [Google Scholar] [CrossRef]
- Schlenker, E.H.; Hansen, S.N. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006, 1123, 89–100. [Google Scholar] [CrossRef]
- Simerly, R.B.; Chang, C.; Muramatsu, M.; Swanson, L.W. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Spary, E.J.; Maqbool, A.; Batten, T.F. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J. Chem. Neuroanat. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.C.; Connell, B.J.; Saleh, T.M. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000, 879, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.M.; Connell, B.J.; McQuaid, T.; Cribb, A.E. Estrogen-induced neurochemical and electrophysiological changes in the parabrachial nucleus of the male rat. Brain Res. 2003, 990, 58–65. [Google Scholar] [CrossRef]
- Littlejohn, E.L.; Fedorchak, S.; Boychuk, C.R. Sex-steroid-dependent plasticity of brain-stem autonomic circuits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R60–R68. [Google Scholar] [CrossRef]
- Pamidimukkala, J.; Xue, B.; Newton, L.G.; Lubahn, D.B.; Hay, M. Estrogen receptor-alpha mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1063–H1070. [Google Scholar] [CrossRef]
- Head, G.A.; Obeyesekere, V.R.; Jones, M.E.; Simpson, E.R.; Krozowski, Z.S. Aromatase-deficient (ArKO) mice have reduced blood pressure and baroreflex sensitivity. Endocrinology 2004, 145, 4286–4291. [Google Scholar] [CrossRef][Green Version]
- Farquhar, W.B.; Taylor, J.A.; Darling, S.E.; Chase, K.P.; Freeman, R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation 2000, 102, 3086–3091. [Google Scholar] [CrossRef]
- Wenner, M.M.; Haddadin, A.S.; Taylor, H.S.; Stachenfeld, N.S. Mechanisms contributing to low orthostatic tolerance in women: The influence of oestradiol. J. Physiol. 2013, 591, 2345–2355. [Google Scholar] [CrossRef]
- Minson, C.T.; Halliwill, J.R.; Young, T.M.; Joyner, M.J. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 2000, 101, 862–868. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Chatterjee, S.; Patra, S.; Mishra, J. A study of sympathetic autonomic function in different phases of menstrual cycle among young adult females. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 579. [Google Scholar]
- Kammar, C.K.F.; Medabala, T.; Patil, P.; Sayana, S.B. A Comparative Study of Cardiovascular Autonomic Function Tests during Different Phases of Menstrual Cycle. Int. J. Health Sci. Res. 2013, 6, 34–40. [Google Scholar]
- Fu, Q.; Ogoh, S. Sex differences in baroreflex function in health and disease. J. Physiol. Sci. 2019, 69, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Miner, J.A.; Kaplan, P.F.; Halliwill, J.R.; Strycker, L.A.; Minson, C.T. Short-term administration of progesterone and estradiol independently alter carotid-vasomotor, but not carotid-cardiac, baroreflex function in young women. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1041–H1049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brooks, V.L.; Dampney, R.A.; Heesch, C.M. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R439–R451. [Google Scholar] [CrossRef]
- Heesch, C.M.; Rogers, R.C. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin. Exp. Pharmacol. Physiol. 1995, 22, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Heesch, C.M.; Foley, C.M. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann. N. Y. Acad. Sci. 2001, 940, 348–360. [Google Scholar] [CrossRef]
- Brooks, V.L.; Cassaglia, P.A.; Zhao, D.; Goldman, R.K. Baroreflex function in females: Changes with the reproductive cycle and pregnancy. Gend. Med. 2012, 9, 61–67. [Google Scholar] [CrossRef]
- Heesch, C.M. Neurosteroid modulation of arterial baroreflex function in the rostral ventrolateral medulla. Auton. Neurosci. 2011, 161, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Laiprasert, J.D.; Rogers, R.C.; Heesch, C.M. Neurosteroid modulation of arterial baroreflex-sensitive neurons in rat rostral ventrolateral medulla. Am. J. Physiol. 1998, 274, R903–R911. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Heesch, C.M. Effects of pregnancy and progesterone metabolites on arterial baroreflex in conscious rats. Am. J. Physiol. 1997, 272 Pt 2, R924–R934. [Google Scholar] [CrossRef]
- Malachias, M.V.B. Polycystic Ovary Syndrome and Cardiovascular Diseases: Still an Open Door. Arq. Bras. Cardiol. 2019, 112, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Luo, X.; Wang, W.; Sun, R.; Qi, M.; Yu, J. Cardiovascular Risk According to Body Mass Index in Women of Reproductive Age with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 822079, Erratum in Front. Cardiovasc. Med. 2023, 10, 1186990. [Google Scholar] [CrossRef]
- Ozkececi, G.; Unlu, B.S.; Dursun, H.; Akci, O.; Koken, G.; Onrat, E.; Avsar, A. Heart rate variability and heart rate turbulence in patients with polycystic ovary syndrome. Anatol. J. Cardiol. 2016, 16, 323–327. [Google Scholar] [CrossRef]
- Ollila, M.-M.; Kiviniemi, A.; Stener-Victorin, E.; Tulppo, M.; Puukka, K.; Tapanainen, J.; Franks, S.; Morin-Papunen, L.; Piltonen, T. Effect of polycystic ovary syndrome on cardiac autonomic function at a late fertile age: A prospective Northern Finland Birth Cohort 1966 study. BMJ Open 2019, 9, e033780. [Google Scholar] [CrossRef]
- Monahan, K.D. Effect of aging on baroreflex function in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R3–R12. [Google Scholar] [CrossRef]
- Babcock, M.C.; DuBose, L.E.; Hildreth, K.L.; Stauffer, B.L.; Cornwell, W.K.; Kohrt, W.M.; Moreau, K.L. Age-associated reductions in cardiovagal baroreflex sensitivity are exaggerated in middle-aged and older men with low testosterone. J. Appl. Physiol. 2022, 133, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Surampudi, P.N.; Wang, C.; Swerdloff, R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int. J. Endocrinol. 2012, 2012, 625434. [Google Scholar] [CrossRef]
- Rodrigues Dos Santos, M.; Bhasin, S. Benefits and Risks of Testosterone Treatment in Men with Age-Related Decline in Testosterone. Annu. Rev. Med. 2021, 72, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Di Lodovico, E.; Facondo, P.; Delbarba, A.; Pezzaioli, L.C.; Maffezzoni, F.; Cappelli, C.; Ferlin, A. Testosterone, Hypogonadism, and Heart Failure. Circ. Heart Fail. 2022, 15, e008755. [Google Scholar] [CrossRef]
- Marques Neto, S.R.; da HSilva, A.; dos Santos, M.C.; Ferraz, E.F.; Nascimento, J.H. The blockade of angiotensin AT1 and aldosterone receptors protects rats from synthetic androgen-induced cardiac autonomic dysfunction. Acta Physiol. 2013, 208, 166–171. [Google Scholar] [CrossRef] [PubMed]
- El-Mas, M.M.; Afify, E.A.; Mohy El-Din, M.M.; Omar, A.G.; Sharabi, F.M. Testosterone facilitates the baroreceptor control of reflex bradycardia: Role of cardiac sympathetic and parasympathetic components. J. Cardiovasc. Pharmacol. 2001, 38, 754–763. [Google Scholar] [CrossRef]
- Ward, G.R.; Abdel-Rahman, A.A. Orchiectomy or androgen receptor blockade attenuates baroreflex-mediated bradycardia in conscious rats. BMC Pharmacol. 2006, 6, 2. [Google Scholar] [CrossRef]
- Ward, G.R.; Abdel-Rahman, A.A. Effect of testosterone replacement or duration of castration on baroreflex bradycardia in conscious rats. BMC Pharmacol. 2005, 5, 9. [Google Scholar] [CrossRef]
- Minson, C.T.; Halliwill, J.R.; Young, T.M.; Joyner, M.J. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 2000, 102, 1473–1476. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Ramos, P.S.; Vianna, L.C.; Ricardo, D.R. Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiology 2015, 52, 1451–1455. [Google Scholar] [CrossRef]
- Schueller, P.O.; Feuring, M.; Sharkova, Y.; Grimm, W.; Christ, M. Effects of synthetic progestagens on autonomic tone, neurohormones and C-reactive protein levels in young healthy females in reproductive age. Int. J. Cardiol. 2006, 111, 42–48. [Google Scholar] [CrossRef]
- Weissman, A.; Lowenstein, L.; Tal, J.; Ohel, G.; Calderon, I.; Lightman, A. Modulation of heart rate variability by estrogen in young women undergoing induction of ovulation. Eur. J. Appl. Physiol. 2009, 105, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yildirir, A.; Kabakci, G.; Yarali, H.; Aybar, F.; Akgul, E.; Bukulmez, O.; Tokgozoglu, L.; Gurgan, T.; Oto, A. Effects of hormone replacement therapy on heart rate variability in postmenopausal women. Ann. Noninvasive Electrocardiol. 2001, 6, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Rubinow, D.R.; Watkins, L.; Hinderliter, A.L.; Caughey, M.C.; Girdler, S.S. The Effect of Perimenopausal Transdermal Estradiol and Micronized Progesterone on Markers of Risk for Arterial Disease. J. Clin. Endocrinol. Metab. 2020, 105, e2050–e2060. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.G.; Mlček, M.; Kittnar, O. Estrogen can modulate menopausal women’s heart rate variability. Physiol. Res. 2013, 62 (Suppl. 1), S165–S171. [Google Scholar] [CrossRef]
- Vongpatanasin, W.; Tuncel, M.; Mansour, Y.; Arbique, D.; Victor, R.G. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation 2001, 103, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Nijland, M.J. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1941–R1952. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G. Hormonal Imprinting: The First Cellular-level Evidence of Epigenetic Inheritance and its Present State. Curr. Genomics 2019, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Jurgens, J.A.; Evans, S.A.; Ennis, R.C.; Villar, V.A.; Jose, P.A. Mechanisms of fetal programming in hypertension. Int. J. Pediatr. 2012, 2012, 584831. [Google Scholar] [CrossRef] [PubMed]
- Ghorashi, V.; Sheikhvatan, M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol. Pol. 2008, 59, 390–392. [Google Scholar]
- Serin, I.S.; Kula, M.; Başbuğ, M.; Unlühizarci, K.; Güçer, S.; Tayyar, M. Androgen levels of preeclamptic patients in the third trimester of pregnancy and six weeks after delivery. Acta Obstet. Gynecol. Scand. 2001, 80, 1009–1013. [Google Scholar] [CrossRef]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Pérez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Olivier, N.B.; Mohankumar, P.S.; Lee, J.S.; Padmanabhan, V.; Fink, G.D. Hypertension caused by prenatal testosterone excess in female sheep. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1837–E1841. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Chinnathambi, V.; Hankins, G.D.; Yallampalli, C.; Sathishkumar, K. Prenatal testosterone exposure induces hypertension in adult females via androgen receptor-dependent protein kinase Cδ-mediated mechanism. Hypertension 2015, 65, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, V.; Balakrishnan, M.; Yallampalli, C.; Sathishkumar, K. Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biol. Reprod. 2012, 86, 137. [Google Scholar] [CrossRef]
- Le-Ha, C.; Beilin, L.J.; Burrows, S.; Keelan, J.A.; Hickey, M.; Mori, T.A. Prenatal Testosterone Associates with Blood Pressure in Young Adults: A Prospective Cohort Study. Hypertension 2021, 77, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Association of the human Y chromosome with high blood pressure in the general population. Hypertension 2000, 36, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Ely, D.L.; Turner, M.E. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension 1990, 16, 277–281. [Google Scholar] [CrossRef]
- Wiley, D.H.; Dunphy, G.; Daneshvar, H.; Salisbury, R.; Neeki, M.; Ely, D.L. Neonatal sympathectomy reduces adult blood pressure and cardiovascular pathology in Y chromosome consomic rats. Blood Press. 1999, 8, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Czech, D.P.; Lee, J.; Sim, H.; Parish, C.L.; Vilain, E.; Harley, V.R. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J. Neurochem. 2012, 122, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Milsted, A.; Serova, L.; Sabban, E.L.; Dunphy, G.; Turner, M.E.; Ely, D.L. Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci. Lett. 2004, 369, 203–207. [Google Scholar] [CrossRef]
- Shi, W.; Sheng, X.; Dorr, K.M.; Hutton, J.E.; Emerson, J.I.; Davies, H.A.; Andrade, T.D.; Wasson, L.K.; Greco, T.M.; Hashimoto, Y.; et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev. Cell 2021, 56, 3019–3034.e7. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef]
- Cox, B.F.; Bishop, V.S. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am. J. Physiol. 1991, 261 Pt 2, H1284–H1291. [Google Scholar] [CrossRef]
- Gandhi, S.K.; Gainer, J.; King, D.; Brown, N.J. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension 1998, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Blessing, W.W.; Hedger, S.C.; Joh, T.H.; Willoughby, J.O. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res. 1987, 419, 336–340. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Miselis, R.R. The central neural connections of the area postrema of the rat. J. Comp. Neurol. 1985, 234, 344–364. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.D.; Bruner, C.A.; Mangiapane, M.L. Area postrema is critical for angiotensin-induced hypertension in rats. Hypertension 1987, 9, 355–361. [Google Scholar] [CrossRef]
- Matsukawa, S.; Reid, I.A. Role of the area postrema in the modulation of the baroreflex control of heart rate by angiotensin II. Circ. Res. 1990, 67, 1462–1473. [Google Scholar] [CrossRef]
- Xue, B.; Gole, H.; Pamidimukkala, J.; Hay, M. Role of the area postrema in angiotensin II modulation of baroreflex control of heart rate in conscious mice. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1003–H1007. [Google Scholar] [CrossRef] [PubMed]
- Chaves, G.Z.; Caligiorne, S.M.; Santos, R.A.; Khosla, M.C.; Campagnole-Santos, M.J. Modulation of the baroreflex control of heart rate by angiotensin-(1-7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J. Hypertens. 2000, 18, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Y.; Zhang, F.; Han, Y.; Wang, H.-J.; Zhang, Y.; Guo, R.; Zhu, G.-Q. AT1 receptor in rostral ventrolateral medulla mediating blunted baroreceptor reflex in spontaneously hypertensive rats. Acta Pharmacol. Sin. 2004, 25, 1433–1438. [Google Scholar] [PubMed]
- Sakima, A.; Averill, D.B.; Gallagher, P.E.; Kasper, S.O.; Tommasi, E.N.; Ferrario, C.M.; Diz, D.I. Impaired heart rate baroreflex in older rats: Role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension 2005, 46, 333–340. [Google Scholar] [CrossRef]
- Koike, G.; Horiuchi, M.; Yamada, T.; Szpirer, C.; Jacob, H.J.; Dzau, V.J. Human type 2 angiotensin II receptor gene: Cloned, mapped to the X chromosome, and its mRNA is expressed in the human lung. Biochem. Biophys. Res. Commun. 1994, 203, 1842–1850. [Google Scholar] [CrossRef]
- Dadam, F.M.; Cisternas, C.D.; Macchione, A.F.; Godino, A.; Antunes-Rodrigues, J.; Cambiasso, M.J.; Vivas, L.M.; Caeiro, X.E. Sex chromosome complement involvement in angiotensin receptor sexual dimorphism. Mol. Cell Endocrinol. 2017, 447, 98–105. [Google Scholar] [CrossRef]
- Hall, J.E.; Brands, M.W.; Henegar, J.R. Angiotensin II and long-term arterial pressure regulation: The overriding dominance of the kidney. J. Am. Soc. Nephrol. 1999, 10 (Suppl. S12), S258–S265. [Google Scholar]
- Ji, H.; Zheng, W.; Wu, X.; Liu, J.; Ecelbarger, C.M.; Watkins, R.; Arnold, A.P.; Sandberg, K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 2010, 55, 1275–1282. [Google Scholar] [CrossRef]
- Gooren, L.J.; Wierckx, K.; Giltay, E.J. Cardiovascular disease in transsexual persons treated with cross-sex hormones: Reversal of the traditional sex difference in cardiovascular disease pattern. Eur. J. Endocrinol. 2014, 170, 809–819. [Google Scholar] [CrossRef]
- Wiik, A.; Andersson, D.P.; Brismar, T.B.; Chanpen, S.; Dhejne, C.; Ekström, T.J.; Flanagan, J.N.; Holmberg, M.; Kere, J.; Lilja, M.; et al. Metabolic and functional changes in transgender individuals following cross-sex hormone treatment: Design and methods of the GEnder Dysphoria Treatment in Sweden (GETS) study. Contemp. Clin. Trials Commun. 2018, 10, 148–153. [Google Scholar] [CrossRef]
- De Andrade, P.E.; Amaral, J.A.T.D.; Paiva, L.d.S.; Adami, F.; Raimudo, J.Z.; Valenti, V.E.; de Abreu, L.C.; Raimundo, R.D. Reduction of heart rate variability in hypertensive elderly. Blood Press. 2017, 26, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Lombardi, F.; Pagani, M.; Cerutti, S. Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J. Cardiovasc. Electrophysiol. 1994, 5, 274–286. [Google Scholar] [CrossRef]
- Nathwani, N.C.; Unwin, R.; Brook, C.G.; Hindmarsh, P.C. Blood pressure and Turner syndrome. Clin. Endocrinol. 2000, 52, 363–370. [Google Scholar] [CrossRef]
- Zuckerman-Levin, N.; Zinder, O.; Greenberg, A.; Levin, M.; Jacob, G.; Hochberg, Z. Physiological and catecholamine response to sympathetic stimulation in turner syndrome. Clin. Endocrinol. 2006, 64, 410–415. [Google Scholar] [CrossRef]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Bertozzi, M.; Penta, L.; Esposito, S. Different Clinical Presentations and Management in Complete Androgen Insensitivity Syndrome (CAIS). Int. J. Environ. Res. Public. Health 2019, 16, 1268. [Google Scholar] [CrossRef]
- Tyutyusheva, N.; Mancini, I.; Baroncelli, G.I.; D’elios, S.; Peroni, D.; Meriggiola, M.C.; Bertelloni, S. Complete Androgen Insensitivity Syndrome: From Bench to Bed. Int. J. Mol. Sci. 2021, 22, 1264. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebaek, N.; Gravholt, C.H. Increased mortality in Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 3830–3834. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Schoemaker, M.J.; Wright, A.F.; Jacobs, P.A.; United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: A cohort study. J. Clin. Endocrinol. Metab. 2005, 90, 6516–6522. [Google Scholar] [CrossRef]
- Pasquali, D.; Arcopinto, M.; Renzullo, A.; Rotondi, M.; Accardo, G.; Salzano, A.; Esposito, D.; Saldamarco, L.; Isidori, A.M.; Marra, A.M.; et al. Cardiovascular abnormalities in Klinefelter syndrome. Int. J. Cardiol. 2013, 168, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, N.; Fennoy, I.; Carel, J.C.; Roger, M. Inhibin B and anti-Müllerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Samango-Sprouse, C.A.; Sadeghin, T.; Mitchell, F.L.; Dixon, T.; Stapleton, E.; Kingery, M.; Gropman, A.L. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47,XXY syndrome at 36 and 72 months of age. Am. J. Med. Genet. A 2013, 161, 501–508. [Google Scholar] [CrossRef]
- Samango-Sprouse, C.; Stapleton, E.J.; Lawson, P.; Mitchell, F.; Sadeghin, T.; Powell, S.; Gropman, A.L. Positive effects of early androgen therapy on the behavioral phenotype of boys with 47,XXY. Am. J. Med. Genet. C Semin. Med. Genet. 2015, 169, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.; Clemente Fuentes, R.W. Jacobs Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ratcliffe, S.G.; Read, G.; Pan, H.; Fear, C.; Lindenbaum, R.; Crossley, J. Prenatal testosterone levels in XXY and XYY males. Horm. Res. 1994, 42, 106–109. [Google Scholar] [CrossRef]
- Tartaglia, N.; Howell, S.; Davis, S.; Kowal, K.; Tanda, T.; Brown, M.; Boada, C.; Alston, A.; Crawford, L.; Thompson, T.; et al. Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 428–443. [Google Scholar] [CrossRef]
- Davis, S.M.; Bloy, L.; Roberts, T.P.; Kowal, K.; Alston, A.; Tahsin, A.; Truxon, A.; Ross, J.L. Testicular function in boys with 47,XYY and relationship to phenotype. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 371–385. [Google Scholar] [CrossRef]
- Aksglaede, L.; Skakkebaek, N.E.; Juul, A. Abnormal sex chromosome constitution and longitudinal growth: Serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. J. Clin. Endocrinol. Metab. 2008, 93, 169–176. [Google Scholar] [CrossRef]
- Boisen, E.; Owen, D.R.; Rasmussen, L.; Sergeant, J. Cardiac functioning and blood pressure of 47,XYY and 47,XXY men in a double-blind, double-matched population survey. Am. J. Hum. Genet. 1981, 33, 77–84. [Google Scholar]
- Shen, Z.; Zou, C.C.; Shang, S.Q.; Jiang, K.W. Down-Klinefelter syndrome (48,XXY,+21) in a child with congenital heart disease: Case report and literature review. Intern. Med. 2012, 51, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caeiro, X.E.; Silva, G.V.; Godino, A. Sex Differences in Autonomic Blood Pressure Regulation: Sex Chromosome Complement and Hormonal Involvement. Sexes 2023, 4, 536-554. https://doi.org/10.3390/sexes4040035
Caeiro XE, Silva GV, Godino A. Sex Differences in Autonomic Blood Pressure Regulation: Sex Chromosome Complement and Hormonal Involvement. Sexes. 2023; 4(4):536-554. https://doi.org/10.3390/sexes4040035
Chicago/Turabian StyleCaeiro, Ximena E., Gabriela V. Silva, and Andrea Godino. 2023. "Sex Differences in Autonomic Blood Pressure Regulation: Sex Chromosome Complement and Hormonal Involvement" Sexes 4, no. 4: 536-554. https://doi.org/10.3390/sexes4040035
APA StyleCaeiro, X. E., Silva, G. V., & Godino, A. (2023). Sex Differences in Autonomic Blood Pressure Regulation: Sex Chromosome Complement and Hormonal Involvement. Sexes, 4(4), 536-554. https://doi.org/10.3390/sexes4040035





