Sex Differences in Mouse Models of Autism Spectrum Disorders: Their Potential to Uncover the Impact of Brain Sexual Differentiation on Gender Bias
Abstract
1. Introduction
2. Mouse Models of ASD
2.1. Mouse Models of Psychiatric Disorders
2.2. Tools to Evaluate Face Validity of Mouse Models of ASD and Limitations Related to Sex
2.2.1. Tests to Evaluate Sociability and Communication Deficits in Mice
2.2.2. Tests to Evaluate Repetitive and Stereotyped Behaviors in Mice
2.2.3. Tests for the Evaluation of Associated Symptoms
2.2.4. Non-Behavioral Associated Symptoms
2.3. Sex Differences in Mouse Models of ASD
| Pharmacological Animal Models | Sociability-Associated Behaviors | Repetitive Behaviors | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Tests | Results | Test | Results | ||||
| VPA Valproic acid maternal injection | rSI 3ch-SI 3ch-SN JSP SM | ↓ Social interaction ↓ Juvenile social play ↓ Social novelty preference | ♂ | SG MB | ↑ Self-grooming ↑ Marble burying | ♂ | [42,55,138,182,183,184,185,186] |
| = Social preference | ♀ | ND | ♀ | ||||
| MIA Maternal immune activation: polyinosinic-polycytidylic acid (PolyI:C) via maternal injection at GD12.5 or 3 injections (3×) at GD10.5–12.5–14.5 | 3ch-SI 3ch-SN rSI SM USVs | ↓ Social interaction ↓/= Social novelty preference (GD12.5/3 × GD10.5–12.5–14.5) ↓ USVs (young and adult) | ♂ | MB SG sT-maze | ↑ Self-grooming ↑ Marble burying ↓ Spontaneous alternation | ♂ | [98,99,100,102,105,106,107,108,112,140] |
| ↓/= Social interaction (GD12.5/3 × GD10.5–12.5–14.5) = Social novelty preference (3 × GD10.5–12.5–14.5) ↑ USVs (3 × GD10.5–12.5–14.5) | ♀ | ↑/= Self-grooming (GD12.5/3 × GD10.5–12.5–14.5) ↑ Marble burying ↓ Spontaneous alteration | ♀ | ||||
| Bacterial lipopolysaccharides (LPS) via maternal injection at GD17 | rSI JSP | ↓ Social interaction ↓ Juvenile social play | ♂ | HBT | = Hole-poke frequency ↑ Self-grooming | ♂ | [109,110,141] |
| ↓ Social interaction = Juvenile social play | ♀ | ND | ♀ | ||||
| Human influenza virus via maternal injection at GD9.5 | rSI | ↓ Social interaction | ♂♀ | ND | [98] | ||
| Neonatal LPS LPS injection at different post-natal ages | rSI SM USVs | ↓/= Social interaction ↓ USVs (pups and young) | ♂ | SG | ↑ Self-grooming (in open field) | ♂ | [140,142,143,144,145] |
| ↓/= Social interaction = USVs (pups) ↓ Social novelty preference | ♀ | = Self-grooming (in open field) | ♀ | ||||
| PPA Propionic acid (PPA) via icv, sc, or ip injection or pre-natal maternal injection | rSI JSP 3ch-SI | ↓/= Social interaction = Juvenile social play | ♂ | rT-maze | ↓ Reversal learning | ♂ | [146,147,148,149,150] |
| = Social interaction = Juvenile social play | ♀ | ND | ♀ | ||||
| C6 mice In utero exposure to a maternal antibody reactive to contactin-associated protein-like 2 (Caspr2). | 3ch-SI | =/↓ Social interaction | ♂ | SG MB Clock | = Self-grooming =/↑ Marble burying ↓ Reversal learning | ♂ | [77,104] |
| = Social interaction | ♀ | = Self-grooming = Marble burying | ♀ | ||||
| GENETIC ANIMAL MODELS | Test | Results | Test | Results | Ref. | ||
| Nlgn4 Null mutation of the murine ortholog of the human Nlgn4 (neuroligin-4) gene | 3ch-SI 3ch-SN rSI SM USVs Nesting | ↓ Social interaction ↓/= Social novelty preference ↓ USVs ↓ Nesting behavior | ♂ | SG MB | = Self-grooming = Marble burying ↑ Circling episodes (spontaneous) | ♂ | [120,121] |
| ↓ Social interaction ↓ Social novelty preference ↓ USVs = Nesting behavior (tendency) | ♀ | ↑ Self-grooming = Marble burying ↑ Circling episodes (spontaneous) | ♀ | ||||
| Nlgn3 Homozygous mutation of humanized R451C mutation of the Nlgn3 (neuroligin-3) gene | JSP 3ch-SI 3ch-SN rSI SM | ↓ Juvenile social play ↓/= Social interaction = Social novelty preference | ♂ | SG | = Self-grooming | ♂ | [122,123] |
| Null mutation in the murine ortholog of the human Nlgn3 gene | 3ch-SI 3ch-SN rSI SM | = Social interaction ↓ Social novelty preference ↓ USVs | ♂ | ND | [124,125] | ||
| Neurexin 1α Null mutation in the murine neurexin 1α gene | 3ch-SI 3ch-SN SM Nesting | = Social interaction = Social novelty preference ↓ Nesting behavior | ♂♀ | SG | ↑ Self-grooming | ♂♀ | [127] |
| Nlgn1 Null mutation in the murine ortholog of the human Nlgn1 (neuroligin-1) gene | 3ch-SI 3ch-SN rSI SM Nesting Olfactory | ↓/= Social interaction = Social novelty preference = Social olfaction ↓ Nesting behavior | ♂♀ | SG MB | ↑ Self-grooming = Marble burying | ♂♀ | [126] |
| Pten Conditional null mutation of the mouse ortholog of the human pten gene, which is inactivated in neurons of the cortex and hippocampus | 3ch-SI 3ch-SN rSI SM Nesting USVs | ↓ Social interaction ↓ Social novelty preference ↓ Social memory ↓ Nesting behavior = USVs | ♂♀ | MB HBT | ↓ Marble burying ↓ Hole-poke frequency | ♂♀ | [156,157] |
| Haploinsufficent mutant line in which exon 5, which encodes the core catalytic phosphatase domain, is deleted | 3ch-SI 3ch-SN SM | = Social interaction ↓ Social novelty preference | ♂ | MB | ↑ Marble burying | ♂ | [161,162] |
| ↓/= Social interaction = Social novelty preference | ♀ | = Marble burying | ♀ | ||||
| En2 Null mutation in the murine ortholog of the human En2 (engrailed 2) gene | JSP rSI | ↓ Juvenile social play =/↓ Social interaction | ♂ | SG | ↑ Self-grooming | ♂ | [166,187] |
| ↓ Juvenile social play =/↓ Social interaction | ♀ | ↑ Self-grooming | ♀ | ||||
| 15q11–13 Paternal duplication of the genomic region on mouse chromosome 7, which corresponds to the human genomic region 15q11-13, which is observed to be maternally duplicated in some cases of ASD | 3ch-SI 3ch-SN USVs SM | ↓ Social interaction ↓ Social novelty preference ↓ USVs | ♂ | MB | ↓ Marble burying | ♂ | [163,164] |
| 17p11.2 Duplication in the genomic region of murine chromosome 11, which is homologous to the human genomic region 17p11.2 | 3ch-SI 3ch-SN SM Nesting | ↓ Social interaction = Social novelty preference ↓ Nesting behavior | ♂ | ND | [165] | ||
| Slc6a4 Null mutation in the murine ortholog of the human serotonin transporter (Slc6a4) gene | 3ch-SI SM | ↓ Social interaction = Social novelty preference | ♂ | ND | [166] | ||
| ↓ Social interaction = Social novelty preference | ♀ | ||||||
| OT Null mutation in the murine oxytocin gene | 3ch-SI SM Olfactory | ↓/= Social interaction = Social novelty preference ↓ Social memory | ♂ | SG | ND | [151,152] | |
| = Social olfaction | ♂♀ | = Self-grooming | ♂♀ | ||||
| V1aR and V1bR Null mutations in the murine vasopressin receptors (Avpr1a or Avpr1b genes) | rSI SM USVs | ↓ Social interaction ↓ Social memory ↓ USVs | ♂ | ND | [153,154,155] | ||
| ↓ USVs | ♀ | ||||||
| Mecp2 Conditional mutation in methyl-CpG-binding protein 2 gene | rSI SM Nesting Olfactory | ↓ Social interaction ↓/= Social memory ↓ Nesting behavior = Social olfaction | ♂ | SG | = Self-grooming ↑ Forepaw stereotypical movements | ♂ | [167,168] |
| Fmr1 Null mutant mouse with a targeted mutation in the Fmr1 gene (phenotype is dependent on the genetic background) | 3ch-SI SM | ↓ Social interaction (FVB/129) = Social novelty preference | ♂ | SG MB | ↑ Self-grooming ↑/↓ Marble burying | ♂ | [158,159,160] |
| Shank1 Null mutation in the murine shank1 gene | 3ch-SI rSI Olfactory | ↓/= Social interaction = Social olfaction | ♂♀ | SG | = Self-grooming | ♂♀ | [128] |
| Shank2 Null mutation in the murine shank2 gene | 3ch-SI 3ch-SN SM USVs Olfactory Nesting | =/↓ Social interaction ↓ Social novelty preference ↓ USVs (adult) = Social olfaction ↓ Nesting behavior | ♂ | SG | = Self-grooming ↑ Repetitive jumping | ♂ | [129,130] |
| = Social interaction ↓ Social novelty preference = Social olfaction | ♀ | ↑/= Self-grooming ↑ Repetitive jumping | ♀ | ||||
| Shank3 Mutations in the ankyrin domain | 3ch-SI rSI SM USVs Olfactory | =/↓ Social interaction = Social novelty preference ↑/↓ USVs = Social olfaction | ♂ | SG HBT | ↑ Self-grooming ↑ Hole-poke frequency | ♂ | [131,132,133,134] |
| ↓ Social interaction = Social novelty preference ↓ USVs | ♀ | ↑ Self-grooming ↑ Hole-poke frequency | ♀ | ||||
| Mutations in the PDZ domain | 3ch-SI rSI SM | ↓ Social interaction (juvenile) ↓ Social novelty preference | ♂ | SG | ↑ Self-grooming (juvenile and adult) | ♂ | [132,136] |
| =/↓ Social interaction (juvenile) | ♀ | ↑ Self-grooming (juvenile) | ♀ | ||||
| Mutations in the Homer binding domain | 3ch-SI rSI SM USV Olfactory Nesting | ↓/= Social interaction ↓/= Social novelty preference = USVs (Adult) = Social olfaction ↓ Nesting | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [135] |
| = Social interaction ↓ Social novelty preference ↓ Nesting | ♀ | ↑ Self-grooming ↓ Marble burying | ♀ | ||||
| Chd8 Chromodomain helicase DNA-binding protein 8 haploinsuficiency | rSI 3ch-SN 3ch-SI | ↓/=/↑ Social interaction (rSI/3ch-SI/3ch-SI) ↓ Social novelty preference | ♂ | ND | [188,189,190] | ||
| = Social interaction ↑ Social novelty preference | ♀ | ||||||
| Arid1b AT-rich interaction domain 1B | SBHC 3ch-SI rSI USV | ↓ Social interaction ↓ Social interaction (juvenile) Altered USVs (pups) | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [191,192,193] |
| ND | ♀ | ↑ Self-grooming | ♀ | ||||
| Myt1l Myelin transcription factor 1-like gene | 3ch-SN USVs | ↓/= Social novelty preference ↓ Social interaction ↓ USVs (pups) | ♂♀ | SG MB | ↓ Self-grooming ↓ Marble burying | ♂♀ | [194,195,196] |
| Scn2a Nav1.2 gen, which is a member of the voltage-gated sodium channels family | 3ch-SI 3ch-SN USV | ↑Social interaction ↓ Social novelty preference ↓ USVs (pups and adults) | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [197,198,199,200,201] |
| = Social interaction ↓ Social novelty preference | ♀ | ↑ Self-grooming ↓ Marble burying | ♀ | ||||
| Adnp Activity-dependent neuroprotective protein | USVs 3ch-SI | ↓ USVs (pups) = Social interaction | ♂ | ND | [202,203,204] | ||
| ↓ USVs (pups) ↓ Social interaction | ♀ | ||||||
| INBRED STRAINS | Tests | Results | Test | Results | Ref. | ||
| BTBR + tf/J | 3ch-SI 3ch-SN rSI SM JSP Olfactory USVs | ↓ Social interaction = Social novelty preference ↓ Juvenile social play = Social olfaction ↓ USVs (adults) | ♂ | SG MB | ↑ Self-grooming ↑ Marble burying | ♂ | [59,169,170,176,177,178,179,180,181] |
| ↓ Social interaction | ♂♀ | ↑ Self-grooming | ♂♀ | ||||
| ↓ Juvenile social play ↓ Social interaction | ♀ | ↑ Self-grooming ↑ Marble burying | ♀ | ||||
| BALB/c | 3ch-SI SM JSP USVs | ↓ Social interaction = Social novelty preference ↓ USVs (juvenile) | ♂ | ND | [39,60,166,171,172] | ||
| ↓ Social interaction ↓ Juvenile social play | ♀ | ||||||
| C58/J | 3ch-SI SM Olfactory | ↓/= Social interaction = Social novelty preference = Social olfaction | ♂ | SG Revearsal-HBT | ↑ Self-grooming behavior ↑ Repetitive motor stereotypes ↑ Repetitive jumping = Reversal learning | ♂ | [172,173,174,175] |
| = Social interaction ↓ Social novelty preference = Social olfaction | ♀ | ↑ Self-grooming behavior ↑ Repetitive jumping = Reversal learning | ♀ | ||||
3. Brain Sexual Differentiation and Sex Differences: Relevance to Mouse Models of ASD
3.1. Perinatal Sexual Differentiation of the Brain
3.2. Pre-Pubertal Sexual Differentiation of the Brain
4. Brain Inflammation as a Mechanism of Convergence of Diverse ASD Etiological Factors and Brain Sexual Differentiation
5. Limitations and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, TX, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The Genetics of Autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic Heritability and Shared Environmental Factors among Twin Pairs with Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Larsson, H.; Hultman, C.M.; Reichenberg, A. The Familial Risk of Autism. JAMA J. Am. Med. Assoc. 2014, 311, 1770–1777. [Google Scholar] [CrossRef]
- Elsabbagh, M. Linking Risk Factors and Outcomes in Autism Spectrum Disorder: Is There Evidence for Resilience? BMJ 2020, 368, l6880. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Hull, L.; Petrides, K.V.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.C.; Mandy, W. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord. 2017, 47, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.V.; Mandy, W. The Female Autism Phenotype and Camouflaging: A Narrative Review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef]
- Skuse, D.H. Imprinting, the X-Chromosome, and the Male Brain: Explaining Sex Differences in the Liability to Autism. Pediatr. Res. 2000, 47, 9. [Google Scholar] [CrossRef]
- Robinson, E.B.; Lichtenstein, P.; Anckarsäter, H.; Happé, F.; Ronald, A. Examining and Interpreting the Female Protective Effect against Autistic Behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 5258–5262. [Google Scholar] [CrossRef] [PubMed]
- Wigdor, E.M.; Weiner, D.J.; Grove, J.; Fu, J.M.; Thompson, W.K.; Carey, C.E.; Baya, N.; van der Merwe, C.; Walters, R.K.; Satterstrom, F.K.; et al. The Female Protective Effect against Autism Spectrum Disorder. Cell Genomics 2022, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Jacquemont, S.; Coe, B.P.; Hersch, M.; Duyzend, M.H.; Krumm, N.; Bergmann, S.; Beckmann, J.S.; Rosenfeld, J.A.; Eichler, E.E. A Higher Mutational Burden in Females Supports a “Female Protective Model” in Neurodevelopmental Disorders. Am. J. Hum. Genet. 2014, 94, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.M.; Warrier, V.; Allison, C.; Baron-Cohen, S. Testing the Empathizing–Systemizing Theory of Sex Differences and the Extreme Male Brain Theory of Autism in Half a Million People. Proc. Natl. Acad. Sci. USA 2018, 115, 12152–12157. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Chapman, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Foetal Testosterone and the Child Systemizing Quotient. Eur. J. Endocrinol. Suppl. 2006, 155, 123–130. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; et al. Fetal Testosterone and Autistic Traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef]
- Kung, K.T.F.; Spencer, D.; Pasterski, V.; Neufeld, S.; Glover, V.; O’Connor, T.G.; Hindmarsh, P.C.; Hughes, I.A.; Acerini, C.L.; Hines, M. No Relationship between Prenatal Androgen Exposure and Autistic Traits: Convergent Evidence from Studies of Children with Congenital Adrenal Hyperplasia and of Amniotic Testosterone Concentrations in Typically Developing Children. J. Child Psychol. Psychiatry 2016, 57, 1455–1462. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Belzung, C.; Griebel, G. Measuring Normal and Pathological Anxiety-like Behaviour in Mice: A Review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Kaiser, T.; Feng, G. Modeling Psychiatric Disorders for Developing Effective Treatments. Nat. Med. 2015, 21, 979–988. [Google Scholar] [CrossRef]
- Willner, P. The Validity of Animal Models of Depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Lemoine, M. Criteria of Validity for Animal Models of Psychiatric Disorders: Focus on Anxiety Disorders and Depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Lipska, B.K.; Weinberger, D.R. To Model a Psychiatric Disorder in Animals: Schizophrenia as a Reality Test. Neuropsychopharmacology 2000, 23, 223–239. [Google Scholar] [CrossRef]
- Uliana, D.L.; Zhu, X.; Gomes, F.V.; Grace, A.A. Using Animal Models for the Studies of Schizophrenia and Depression: The Value of Translational Models for Treatment and Prevention. Front. Behav. Neurosci. 2022, 16, 935320. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM-IV-TR; American Psychiatric Association: Arlington, TX, USA, 2000; ISBN 0-89042-024-6. [Google Scholar]
- Crawley, J.N. Translational Animal Models of Autism and Neurodevelopmental Disorders. Dialogues Clin. Neurosci. 2012, 14, 293–305. [Google Scholar] [CrossRef]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural Phenotyping Assays for Mouse Models of Autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K. Inclusion of Females Does Not Increase Variability in Rodent Research Studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; D’Amato, F.R. Deficit in Attachment Behavior in Mice Lacking the μ-Opioid Receptor Gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Maxson, S.C.; Fish, E.W.; Faccidomo, S. Aggressive Behavioral Phenotypes in Mice. Behav. Brain Res. 2001, 125, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Burns-Cusato, M.; Scordalakes, E.M.; Rissman, E.F. Of Mice and Missing Data: What We Know (and Need to Learn) about Male Sexual Behavior. Physiol. Behav. 2004, 83, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Rissman, E.F. Sex Differences in Juvenile Mouse Social Behavior Are Influenced by Sex Chromosomes and Social Context. Genes Brain Behav. 2011, 10, 465–472. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.W.; Lauder, J.M.; et al. Mouse Behavioral Tasks Relevant to Autism: Phenotypes of 10 Inbred Strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef]
- Nadler, J.J.; Moy, S.S.; Dold, G.; Trang, D.; Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.P.; Piven, J.; Magnuson, T.R.; et al. Automated Apparatus for Quantitation of Social Approach Behaviors in Mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- Brodkin, E.S.; Hagemann, A.; Nemetski, S.M.; Silver, L.M. Social Approach-Avoidance Behavior of Inbred Mouse Strains towards DBA/2 Mice. Brain Res. 2004, 1002, 151–157. [Google Scholar] [CrossRef]
- Depino, A.M.; Lucchina, L.; Pitossi, F. Early and Adult Hippocampal TGF-Β1 Overexpression Have Opposite Effects on Behavior. Brain. Behav. Immun. 2011, 25. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-like Symptoms in Fmr1 Knock-out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef]
- Kazlauskas, N.; Seiffe, A.; Campolongo, M.; Zappala, C.; Depino, A.M. Sex-Specific Effects of Prenatal Valproic Acid Exposure on Sociability and Neuroinflammation: Relevance for Susceptibility and Resilience in Autism. Psychoneuroendocrinology 2019, 110, 104441. [Google Scholar] [CrossRef]
- Seiffe, A.; Federico Ramirez, M.; Darío Barrios, C.; Milagros Albarrán, M.; Mara Depino, A.; Ramirez, M.F.; Barrios, C.D.; Albarrán, M.M.; Depino, A.M. Early Estradiol Exposure Masculinizes Disease-relevant Behaviors in Female Mice. Eur. J. Neurosci. 2021, 53, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Kopachev, N.; Netser, S.; Wagner, S. Sex-Dependent Features of Social Behavior Differ between Distinct Laboratory Mouse Strains and Their Mixed Offspring. iScience 2022, 25, 103735. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Kennedy, A.; Burgos-Artizzu, X.P.; Zelikowsky, M.; Navonne, S.G.; Perona, P.; Anderson, D.J. Automated Measurement of Mouse Social Behaviors Using Depth Sensing, Video Tracking, and Machine Learning. Proc. Natl. Acad. Sci. USA 2015, 112, E5351–E5360. [Google Scholar] [CrossRef] [PubMed]
- Argue, K.J.; McCarthy, M.M. Utilization of Same- vs. Mixed-Sex Dyads Impacts the Observation of Sex Differences in Juvenile Social Play Behavior. Curr. Neurobiol. 2000, 6, 17–23. [Google Scholar] [CrossRef]
- Argue, K.J.; McCarthy, M.M. Characterization of Juvenile Play in Rats: Importance of Sex of Self and Sex of Partner. Biol. Sex Differ. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Lidster, K.; Owen, K.; Browne, W.J.; Prescott, M.J. Cage Aggression in Group-Housed Laboratory Male Mice: An International Data Crowdsourcing Project. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Auger, A.P.; Olesen, K.M. Brain Sex Differences and the Organisation of Juvenile Social Play Behaviour. J. Neuroendocrinol. 2009, 21, 519–525. [Google Scholar] [CrossRef]
- Terranova, M.L.; Laviola, G.; Alleva, E. Ontogeny of Amicable Social Behavior in the Mouse: Gender Differences and Ongoing Isolation Outcomes. Dev. Psychobiol. 1993, 26, 467–481. [Google Scholar] [CrossRef]
- Hörnberg, H.; Pérez-Garci, E.; Schreiner, D.; Hatstatt-Burklé, L.; Magara, F.; Baudouin, S.; Matter, A.; Nacro, K.; Pecho-Vrieseling, E.; Scheiffele, P. Rescue of Oxytocin Response and Social Behaviour in a Mouse Model of Autism. Nature 2020, 584, 252–256. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Lahvis, G.P. Social Reward among Juvenile Mice. Genes Brain Behav. 2007, 6, 661–671. [Google Scholar] [CrossRef]
- Cann, C.; Venniro, M.; Hope, B.T.; Ramsey, L.A. Parametric Investigation of Social Place Preference in Adolescent Mice. Behav. Neurosci. 2020, 134, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Crawley, J.N. Simple Behavioral Assessment of Mouse Olfaction. Curr. Protoc. Neurosci. 2009, 48. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Höcht, C.; Depino, A.M. Sociability Deficits after Prenatal Exposure to Valproic Acid Are Rescued by Early Social Enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Bale, T.L. Stress and Sex Influences on Food-Seeking Behaviors. Obesity 2008, 16, 1539–1544. [Google Scholar] [CrossRef]
- Baum, M.J.; Keverne, E.B. Sex Difference in Attraction Thresholds for Volatile Odors from Male and Estrous Female Mouse Urine. Horm. Behav. 2002, 41, 213–219. [Google Scholar] [CrossRef]
- Kass, M.D.; Czarnecki, L.A.; Moberly, A.H.; McGann, J.P. Differences in Peripheral Sensory Input to the Olfactory Bulb between Male and Female Mice. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS ONE 2008, 3, 48–52. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Jochman, K.A.; Kim, J.U.; Koy, J.K.; Wilson, E.D.; Chen, Q.; Wilson, C.R.; Lahvis, G.P. Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice. PLoS ONE 2007, 2, e351. [Google Scholar] [CrossRef]
- Branchi, I.; Santucci, D.; Alleva, E. Ultrasonic Vocalisation Emitted by Infant Rodents: A Tool for Assessment of Neurobehavioural Development. Behav. Brain Res. 2001, 125, 49–56. [Google Scholar] [CrossRef]
- Egnor, S.E.R.; Seagraves, K.M. The Contribution of Ultrasonic Vocalizations to Mouse Courtship. Curr. Opin. Neurobiol. 2016, 38, 1–5. [Google Scholar] [CrossRef]
- Moles, A.; Costantini, F.; Garbugino, L.; Zanettini, C.; D’Amato, F.R. Ultrasonic Vocalizations Emitted during Dyadic Interactions in Female Mice: A Possible Index of Sociability? Behav. Brain Res. 2007, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic Vocalizations: A Tool for Behavioural Phenotyping of Mouse Models of Neurodevelopmental Disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Pittaras, E.; Nosjean, A.; Chabout, J.; Cressant, A.; Granon, S. Social Behaviors and Acoustic Vocalizations in Different Strains of Mice. Behav. Brain Res. 2017, 320, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.; Lee, C.C. Neural Mechanisms Underlying Repetitive Behaviors in Rodent Models of Autism Spectrum Disorders. Front. Cell. Neurosci. 2021, 14, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.; Depino, A.M. Altered Peripheral and Central Inflammatory Responses in a Mouse Model of Autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of Rodent Self-Grooming and Its Value for Translational Neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; Andrade da Costa, B.L.S.; De Stasi, A.M.; Bacci, A.; Imamura Kawasawa, Y.; Vaidya, V.A.; Gaspar, P. Early-Life Stress Impairs Postnatal Oligodendrogenesis and Adult Emotional Behaviour through Activity-Dependent Mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef]
- Pitzer, C.; Kurpiers, B.; Eltokhi, A. Sex Differences in Depression-Like Behaviors in Adult Mice Depend on Endophenotype and Strain. Front. Behav. Neurosci. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble Burying Reflects a Repetitive and Perseverative Behavior More than Novelty-Induced Anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- de Brouwer, G.; Fick, A.; Harvey, B.H.; Wolmarans, D.W. A Critical Inquiry into Marble-Burying as a Preclinical Screening Paradigm of Relevance for Anxiety and Obsessive–Compulsive Disorder: Mapping the Way Forward. Cogn. Affect. Behav. Neurosci. 2019, 19, 1–39. [Google Scholar] [CrossRef]
- Schneider, T.; Popik, P. Attenuation of Estrous Cycle-Dependent Marble Burying in Female Rats by Acute Treatment with Progesterone and Antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Dember, W.N.; Fowler, H. Spontaneous Alternation Behavior. Psychol. Bull. 1958, 55, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R. The Neurobiological Basis of Spontaneous Alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Isseroff, A. Limited Recovery of Spontaneous Alternation after Extensive Hippocampal Damage: Evidence for a Memory Impairment. Exp. Neurol. 1979, 64, 284–294. [Google Scholar] [CrossRef]
- Brimberg, L.; Mader, S.; Jeganathan, V.; Berlin, R.; Coleman, T.R.; Gregersen, P.K.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Caspr2-Reactive Antibody Cloned from a Mother of an ASD Child Mediates an ASD-like Phenotype in Mice. Mol. Psychiatry 2016, 21, 1663–1671. [Google Scholar] [CrossRef]
- Remmelink, E.; Smit, A.B.; Verhage, M.; Loos, M. Measuring Discrimination- and Reversal Learning in Mouse Models within 4 Days and without Prior Food Deprivation. Learn. Mem. 2016, 23, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Aarde, S.M.; Genner, R.M.; Hrncir, H.; Arnold, A.P.; Jentsch, J.D. Sex Chromosome Complement Affects Multiple Aspects of Reversal-Learning Task Performance in Mice. Genes Brain Behav. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Poe, M.D.; Nonneman, R.J.; Young, N.B.; Koller, B.H.; Crawley, J.N.; Duncan, G.E.; Bodfish, J.W. Development of a Mouse Test for Repetitive, Restricted Behaviors: Relevance to Autism. Behav. Brain Res. 2008, 188, 178–194. [Google Scholar] [CrossRef]
- Bettis, T.; Jacobs, L.F. Sex Differences in Object Recognition Are Modulated by Object Similarity. Behav. Brain Res. 2012, 233, 288–292. [Google Scholar] [CrossRef]
- Kazlauskas, N.; Campolongo, M.; Lucchina, L.; Zappala, C.; Depino, A.M. Postnatal Behavioral and Inflammatory Alterations in Female Pups Prenatally Exposed to Valproic Acid. Psychoneuroendocrinology 2016, 72, 11–21. [Google Scholar] [CrossRef]
- Plappert, C.F.; Rodenbücher, A.M.; Pilz, P.K.D. Effects of Sex and Estrous Cycle on Modulation of the Acoustic Startle Response in Mice. Physiol. Behav. 2005, 84, 585–594. [Google Scholar] [CrossRef]
- Mitrovic, I.; Margeta-Mitrovic, M.; Bader, S.; Stoffel, M.; Jan, L.Y.; Basbaum, A.I. Contribution of GIRK2-Mediated Postsynaptic Signaling to Opiate and A2-Adrenergic Analgesia and Analgesic Sex Differences. Proc. Natl. Acad. Sci. USA 2003, 100, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.R.; Rivarola, M.A. Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us? Sexes 2022, 3, 141–163. [Google Scholar] [CrossRef]
- Crawley, J.N.; Paylor, R. A Proposed Test Battery and Constellations of Specific Behavioral Paradigms to Investigate the Behavioral Phenotypes of Transgenic and Knockout Mice. Horm. Behav. 1997, 31, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, N.; Ganster, T.; O’Leary, A.; Popp, S.; Freudenberg, F.; Reif, A.; Soler Artigas, M.; Ribasés, M.; Ramos-Quiroga, J.A.; Lesch, K.P.; et al. Dissociation of Impulsivity and Aggression in Mice Deficient for the ADHD Risk Gene Adgrl3: Evidence for Dopamine Transporter Dysregulation. Neuropharmacology 2019, 156, 107557. [Google Scholar] [CrossRef]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef]
- Jhun, M.; Panwar, A.; Cordner, R.; Irvin, D.K.; Veiga, L.; Yeager, N.; Pechnick, R.N.; Schubloom, H.; Black, K.L.; Wheeler, C.J. CD103 Deficiency Promotes Autism (ASD) and Attention-Deficit Hyperactivity Disorder (ADHD) Behavioral Spectra and Reduces Age-Related Cognitive Decline. Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Casanova, M.F. The Neuropathology of Autism. Brain Pathol. 2007, 17, 422–433. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Le, H. Proinflammatory and Regulatory Cytokine Production Associated with Innate and Adaptive Immune Responses in Children with Autism Spectrum Disorders and Developmental Regression. J. Neuroimmunol. 2001, 120, 170–179. [Google Scholar] [CrossRef]
- Gupta, S.; Aggarwal, S.; Rashanravan, B.; Lee, T. Th1- and Th2-like Cytokines in CD4+ and CD8+ T Cells in Autism. J. Neuroimmunol. 1998, 85, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Warren, R.; Averett, R.; Ghaziuddin, M. Circulating Autoantibodies to Neuronal and Glial Filament Proteins in Autism. Pediatr. Neurol. 1997, 17, 88–90. [Google Scholar] [CrossRef]
- Vojdani, A.; Campbell, A.; Anyanwu, E.; Kashanian, A.; Bock, K.; Vojdani, E. Erratum: Antibodies to Neuron-Specific Antigens in Children with Autism: Possible Cross-Reaction with Encephalitogenic Proteins from Milk, Chlamydia Pneumoniae and Streptococcus Group A (Journal of Neuroimmunology (2002) 129 (168) S0165572802001807). J. Neuroimmunol. 2002, 130, 248. [Google Scholar] [CrossRef]
- Depino, A.M. Peripheral and Central Inflammation in Autism Spectrum Disorders. Mol. Cell. Neurosci. 2013, 53. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal Influenza Infection Causes Marked Behavioral and Pharmacological Changes in the Offspring. J. Neurosci. 2003, 23, 297–302. [Google Scholar] [CrossRef]
- Weiser, M.J.; Mucha, B.; Denheyer, H.; Atkinson, D.; Schanz, N.; Vassiliou, E.; Benno, R.H. Dietary Docosahexaenoic Acid Alleviates Autistic-like Behaviors Resulting from Maternal Immune Activation in Mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 27–37. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic Diet Improves Behaviors in a Maternal Immune Activation Model of Autism Spectrum Disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Fortunato, J.J.; da Rosa, N.; Martins Laurentino, A.O.; Goulart, M.; Michalak, C.; Borges, L.P.; da Cruz Cittadin Soares, E.; Reis, P.A.; de Castro Faria Neto, H.C.; Petronilho, F. Effects of ω-3 Fatty Acids on Stereotypical Behavior and Social Interactions in Wistar Rats Prenatally Exposed to Lipopolysaccarides. Nutrition 2017, 35, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves-Kirsten, G.P.; Chaible, L.M.; Silva, A.C.; Martins, D.O.; Britto, L.R.G.; Dagli, M.L.Z.; Torrão, A.S.; Palermo-Neto, J.; Bernardi, M.M. Hypoactivity of the Central Dopaminergic System and Autistic-like Behavior Induced by a Single Early Prenatal Exposure to Lipopolysaccharide. J. Neurosci. Res. 2012, 90, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Gata-Garcia, A.; Porat, A.; Brimberg, L.; Volpe, B.T.; Huerta, P.T.; Diamond, B. Contributions of Sex Chromosomes and Gonadal Hormones to the Male Bias in a Maternal Antibody-Induced Model of Autism Spectrum Disorder. Front. Neurol. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal Immune Activation Yields Offspring Displaying Mouse Versions of the Three Core Symptoms of Autism. Brain. Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Villani, A.; Ajmone-Cat, M.A.; Minghetti, L.; Ricceri, L.; Pazienza, V.; De Simone, R.; Calamandrei, G. Maternal Immune Activation Induces Autism-like Changes in Behavior, Neuroinflammatory Profile and Gut Microbiota in Mouse Offspring of Both Sexes. Transl. Psychiatry 2022, 12, 384. [Google Scholar] [CrossRef]
- Wu, W.-L.; Hsiao, E.Y.; Yan, Z.; Mazmanian, S.K.; Patterson, P.H. The Placental Interleukin-6 Signaling Controls Fetal Brain Development and Behavior. Brain. Behav. Immun. 2017, 62, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Golan, H.M.; Lev, V.; Hallak, M.; Sorokin, Y.; Huleihel, M. Specific Neurodevelopmental Damage in Mice Offspring Following Maternal Inflammation during Pregnancy. Neuropharmacology 2005, 48, 903–917. [Google Scholar] [CrossRef]
- Hava, G.; Vered, L.; Yael, M.; Mordechai, H.; Mahoud, H. Alterations in Behavior in Adult Offspring Mice Following Maternal Inflammation during Pregnancy. Dev. Psychobiol. 2006, 48, 162–168. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal Lipopolysaccharide Reduces Social Behavior in Male Offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef]
- Wu, W.-L.; Adams, C.E.; Stevens, K.E.; Chow, K.-H.; Freedman, R.; Patterson, P.H. The Interaction between Maternal Immune Activation and Alpha 7 Nicotinic Acetylcholine Receptor in Regulating Behaviors in the Offspring. Brain. Behav. Immun. 2015, 46, 192–202. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering Sex Differences of Rodent Microglia. J. Neuroinflammation 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Levitt, P. Autism Spectrum Disorders: Developmental Disconnection Syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Luhmann, H.J. Early Patterns of Electrical Activity in the Developing Cerebral Cortex of Humans and Rodents. Trends Neurosci. 2006, 29, 414–418. [Google Scholar] [CrossRef]
- Hutsler, J.J.; Zhang, H. Increased Dendritic Spine Densities on Cortical Projection Neurons in Autism Spectrum Disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Bonsi, P.; De Jaco, A.; Fasano, L.; Gubellini, P. Postsynaptic Autism Spectrum Disorder Genes and Synaptic Dysfunction. Neurobiol. Dis. 2022, 162, 105564. [Google Scholar] [CrossRef]
- Coiro, P.; Padmashri, R.; Suresh, A.; Spartz, E.; Pendyala, G.; Chou, S.; Jung, Y.; Meays, B.; Roy, S.; Gautam, N.; et al. Impaired Synaptic Development in a Maternal Immune Activation Mouse Model of Neurodevelopmental Disorders. Brain. Behav. Immun. 2015, 50, 249–258. [Google Scholar] [CrossRef]
- Sgritta, M.; Vignoli, B.; Pimpinella, D.; Griguoli, M.; Santi, S.; Bialowas, A.; Wiera, G.; Zacchi, P.; Malerba, F.; Marchetti, C.; et al. Impaired Synaptic Plasticity in an Animal Model of Autism Exhibiting Early Hippocampal GABAergic-BDNF/TrkB Signaling Alterations. iScience 2023, 26, 105728. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced Social Interaction and Ultrasonic Communication in a Mouse Model of Monogenic Heritable Autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef]
- El-Kordi, A.; Winkler, D.; Hammerschmidt, K.; Kästner, A.; Krueger, D.; Ronnenberg, A.; Ritter, C.; Jatho, J.; Radyushkin, K.; Bourgeron, T.; et al. Development of an Autism Severity Score for Mice Using Nlgn4 Null Mutants as a Construct-Valid Model of Heritable Monogenic Autism. Behav. Brain Res. 2013, 251, 41–49. [Google Scholar] [CrossRef]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal Aberrant Behavioral Phenotypes of Neuroligin-3 R451C Knockin Mice. Autism Res. 2008, 1, 147–158. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A Neuroligin-3 Mutation Implicated in Autism Increases Inhibitory Synaptic Transmission in Mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Radyushkin, K.; Hammerschmidt, K.; Boretius, S.; Varoqueaux, F.; El-Kordi, A.; Ronnenberg, A.; Winter, D.; Frahm, J.; Fischer, J.; Brose, N.; et al. Neuroligin-3-Deficient Mice: Model of a Monogenic Heritable Form of Autism with an Olfactory Deficit. Genes Brain Behav. 2009, 8, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.E.; Fuccillo, M.V.; Maxeiner, S.; Hayton, S.J.; Gokce, O.; Lim, B.K.; Fowler, S.C.; Malenka, R.C.; Südhof, T.C. Autism-Associated Neuroligin-3 Mutations Commonly Impair Striatal Circuits to Boost Repetitive Behaviors. Cell 2014, 158, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Blaiss, C.A.; Etherton, M.R.; Espinosa, F.; Tabuchi, K.; Walz, C.; Bolliger, M.F.; Südhof, T.C.; Powell, C.M. Neuroligin-1 Deletion Results in Impaired Spatial Memory and Increased Repetitive Behavior. J. Neurosci. 2010, 30, 2115–2129. [Google Scholar] [CrossRef]
- Etherton, M.R.; Blaiss, C.A.; Powell, C.M.; Südhof, T.C. Mouse Neurexin-1α Deletion Causes Correlated Electrophysiological and Behavioral Changes Consistent with Cognitive Impairments. Proc. Natl. Acad. Sci. USA 2009, 106, 17998–18003. [Google Scholar] [CrossRef]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and Motor Functions in Shank1 Mutant Mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.-L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like Behaviours and Hyperactivity in Mice Lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Won, H.; Lee, H.-R.; Gee, H.Y.; Mah, W.; Kim, J.-I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like Social Behaviour in Shank2-Mutant Mice Improved by Restoring NMDA Receptor Function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the Autism-Associated Shank3 Gene Leads to Deficits in Synaptic Function, Social Interaction, and Social Communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 Mutant Mice Display Autistic-like Behaviours and Striatal Dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic Dysfunction and Abnormal Behaviors in Mice Lacking Major Isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Chen, Q.; van der Goes, M.-S.; Hawrot, J.; Yao, A.Y.; Gao, X.; Lu, C.; Zang, Y.; Zhang, Q.; et al. Striatopallidal Dysfunction Underlies Repetitive Behavior in Shank3-Deficient Model of Autism. J. Clin. Investig. 2017, 127, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Kouser, M.; Speed, H.E.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of Predominant Shank3 Isoforms Results in Hippocampus-Dependent Impairments in Behavior and Synaptic Transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef]
- Balaan, C.; Corley, M.J.; Eulalio, T.; Leite-ahyo, K.; Pang, A.P.S.; Fang, R.; Khadka, V.S.; Maunakea, A.K.; Ward, M.A. Juvenile Shank3b Deficient Mice Present with Behavioral Phenotype Relevant to Autism Spectrum Disorder. Behav. Brain Res. 2019, 356, 137–147. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The Valproic Acid-Induced Rodent Model of Autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewłocki, R. Gender-Specific Behavioral and Immunological Alterations in an Animal Model of Autism Induced by Prenatal Exposure to Valproic Acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal Immune Activation: Reporting Guidelines to Improve the Rigor, Reproducibility, and Transparency of the Model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and Early Postnatal Immune Activation Produce Sex-Specific Effects on Autism-like Behaviors and Neuroimmune Function in Mice. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Hsueh, P.-T.; Lin, H.-H.; Wang, H.-H.; Liu, C.-L.; Ni, W.-F.; Liu, J.-K.; Chang, H.-H.; Sun, D.-S.; Chen, Y.-S.; Chen, Y.-L. Immune Imbalance of Global Gene Expression, and Cytokine, Chemokine and Selectin Levels in the Brains of Offspring with Social Deficits via Maternal Immune Activation. Genes, Brain Behav. 2018, 17, e12479. [Google Scholar] [CrossRef]
- MacRae, M.; Macrina, T.; Khoury, A.; Migliore, M.M.; Kentner, A.C. Tracing the Trajectory of Behavioral Impairments and Oxidative Stress in an Animal Model of Neonatal Inflammation. Neuroscience 2015, 298, 455–466. [Google Scholar] [CrossRef]
- Kentner, A.C.; Scalia, S.; Shin, J.; Migliore, M.M.; Rondón-Ortiz, A.N. Targeted Sensory Enrichment Interventions Protect against Behavioral and Neuroendocrine Consequences of Early Life Stress. Psychoneuroendocrinology 2018, 98, 74–85. [Google Scholar] [CrossRef]
- Smith, C.J.; Kingsbury, M.A.; Dziabis, J.E.; Hanamsagar, R.; Malacon, K.E.; Tran, J.N.; Norris, H.A.; Gulino, M.; Bordt, E.A.; Bilbo, S.D. Neonatal Immune Challenge Induces Female-Specific Changes in Social Behavior and Somatostatin Cell Number. Brain. Behav. Immun. 2020, 90, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Custódio, C.S.; Mello, B.S.F.; Filho, A.J.M.C.; de Carvalho Lima, C.N.; Cordeiro, R.C.; Miyajima, F.; Réus, G.Z.; Vasconcelos, S.M.M.; Barichello, T.; Quevedo, J.; et al. Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-Lasting Sex- and Age-Related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol. Neurobiol. 2018, 55, 3775–3788. [Google Scholar] [CrossRef] [PubMed]
- Macfabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.; Cain, D.P. Effects of the Enteric Bacterial Metabolic Product Propionic Acid on Object-Directed Behavior, Social Behavior, Cognition, and Neuroinflammation in Adolescent Rats: Relevance to Autism Spectrum Disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- Shultz, S.R.; Aziz, N.A.B.; Yang, L.; Sun, M.; Macfabe, D.F.; Brien, T.J.O. Intracerebroventricular Injection of Propionic Acid, an Enteric Metabolite Implicated in Autism, Induces Social Abnormalities That Do Not Differ between Seizure-Prone (FAST) and Seizure-Resistant (SLOW) Rats. Behav. Brain Res. 2015, 278, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.; Kim, J.; Lee, S.; Hong, Y. Pathophysiological and Neurobehavioral Characteristics of a Propionic Acid-Mediated Autism-like Rat Model. PLoS ONE 2018, 13, e0192925. [Google Scholar] [CrossRef]
- Shams, S.; Foley, K.A.; Kavaliers, M.; Macfabe, D.F.; Ossenkopp, K.P. Systemic Treatment with the Enteric Bacterial Metabolic Product Propionic Acid Results in Reduction of Social Behavior in Juvenile Rats: Contribution to a Rodent Model of Autism Spectrum Disorder. Dev. Psychobiol. 2019, 61, 688–699. [Google Scholar] [CrossRef]
- Foley, K.A.; MacFabe, D.F.; Vaz, A.; Ossenkopp, K.; Kavaliers, M. Sexually Dimorphic Effects of Prenatal Exposure to Propionic Acid and Lipopolysaccharide on Social Behavior in Neonatal, Adolescent, and Adult Rats: Implications for Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2014, 39, 68–78. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social Amnesia in Mice Lacking the Oxytocin Gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Crawley, J.N.; Chen, T.; Puri, A.; Washburn, R.; Sullivan, T.L.; Hill, J.M.; Young, N.B.; Nadler, J.J.; Moy, S.S.; Young, L.J.; et al. Social Approach Behaviors in Oxytocin Knockout Mice: Comparison of Two Independent Lines Tested in Different Laboratory Environments. Neuropeptides 2007, 41, 145–163. [Google Scholar] [CrossRef]
- Scattoni, M.; Mcfarlane, H.; Zhodzishsky, V.; Caldwell, H.; Young, W.; Ricceri, L.; Crawley, J. Reduced Ultrasonic Vocalizations in Vasopressin 1b Knockout Mice. Behav. Brain Res. 2008, 187, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.F.; Hu, S.-B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.F.; Hu, S.-B.; Ren, X.; Terwilliger, E.F.; Young, L.J. The V1a Vasopressin Receptor Is Necessary and Sufficient for Normal Social Recognition: A Gene Replacement Study. Neuron 2005, 47, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.-H.; Luikart, B.W.; Powell, C.M.; Zhou, J.; Matheny, S.A.; Zhang, W.; Li, Y.; Baker, S.J.; Parada, L.F. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron 2006, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.N.; Smith, G.D.; Arbuckle, E.P.; White, J.; Holley, A.J.; Floruta, C.M.; Ahmed, N.; Gomez, M.C.; Okonkwo, O. Deletion of PTEN Produces Autism-like Behavioral Deficits and Alterations in Synaptic Proteins. Front. Mol. Neurosci. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Spencer, C.M.; Graham, D.F.; Yuva-Paylor, L.A.; Nelson, D.L.; Paylor, R. Social Behavior in Fmr1 Knockout Mice Carrying a Human FMR1 Transgene. Behav. Neurosci. 2008, 122, 710–715. [Google Scholar] [CrossRef]
- Spencer, C.M.; Alekseyenko, O.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Altered Anxiety-Related and Social Behaviors in the Fmr1 Knockout Mouse Model of Fragile X Syndrome. Genes, Brain Behav. 2005, 4, 420–430. [Google Scholar] [CrossRef]
- McNaughton, C.H.; Moon, J.; Strawderman, M.S.; Maclean, K.N.; Evans, J.; Strupp, B.J. Evidence for Social Anxiety and Impaired Social Cognition in a Mouse Model of Fragile X Syndrome. Behav. Neurosci. 2008, 122, 293–300. [Google Scholar] [CrossRef]
- Page, D.T.; Kuti, O.J.; Prestia, C.; Sur, M. Haploinsufficiency for Pten and Serotonin Transporter Cooperatively Influences Brain Size and Social Behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 1989–1994. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Page, D.T. Pten Haploinsufficient Mice Show Broad Brain Overgrowth but Selective Impairments in Autism-Relevant Behavioral Tests. Hum. Mol. Genet. 2014, 23, 3490–3505. [Google Scholar] [CrossRef]
- Nakatani, J.; Tamada, K.; Hatanaka, F.; Ise, S.; Ohta, H.; Inoue, K.; Tomonaga, S.; Watanabe, Y.; Chung, Y.J.; Banerjee, R.; et al. Abnormal Behavior in a Chromosome- Engineered Mouse Model for Human 15q11-13 Duplication Seen in Autism. Cell 2009, 137, 1235–1246. [Google Scholar] [CrossRef]
- Tamada, K.; Tomonaga, S.; Hatanaka, F.; Nakai, N.; Takao, K.; Miyakawa, T.; Nakatani, J.; Takumi, T. Decreased Exploratory Activity in a Mouse Model of 15q Duplication Syndrome; Implications for Disturbance of Serotonin Signaling. PLoS ONE 2010, 5, e15126. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Carmona-Mora, P.; Chrast, J.; Krall, P.M.; Canales, C.P.; Lupski, J.R.; Reymond, A.; Walz, K. Abnormal Social Behaviors and Altered Gene Expression Rates in a Mouse Model for Potocki-Lupski Syndrome. Hum. Mol. Genet. 2008, 17, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Grossman, A.W.; Murphy, D.L.; D’Ercole, A.J.; Crawley, J.N.; Magnuson, T.R.; Lauder, J.M. Social Approach in Genetically Engineered Mouse Lines Relevant to Autism. Genes, Brain Behav. 2009, 8, 129–142. [Google Scholar] [CrossRef]
- Gemelli, T.; Berton, O.; Nelson, E.D.; Perrotti, L.I.; Jaenisch, R.; Monteggia, L.M. Postnatal Loss of Methyl-CpG Binding Protein 2 in the Forebrain Is Sufficient to Mediate Behavioral Aspects of Rett Syndrome in Mice. Biol. Psychiatry 2006, 59, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Bouwknecht, J.A.; Teague, R.; Paylor, R.; Zoghbi, H.Y. Abnormalities of Social Interactions and Home-Cage Behavior in a Mouse Model of Rett Syndrome. Hum. Mol. Genet. 2005, 14, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Roullet, F.I.; Crawley, J.N. Reduced Scent Marking and Ultrasonic Vocalizations in the BTBR T+tf/J Mouse Model of Autism. Genes, Brain Behav. 2011, 10, 35–43. [Google Scholar] [CrossRef]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-Related Alterations of Gut Microbiota Composition in the BTBR Mouse Model of Autism Spectrum Disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef]
- Chen, Q.; Panksepp, J.B.; Lahvis, G.P. Empathy Is Moderated by Genetic Background in Mice. PLoS ONE 2009, 4, e4387. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Segall, S.K.; Andrade, G.M.; Crawley, J.N.; Magnuson, T.R. Social Approach and Repetitive Behavior in Eleven Inbred Mouse Strains. Behav. Brain Res. 2008, 191, 118–129. [Google Scholar] [CrossRef]
- Ryan, B.C.; Young, N.B.; Crawley, J.N.; Bodfish, J.W.; Moy, S.S. Social Deficits, Stereotypy and Early Emergence of Repetitive Behavior in the C58/J Inbred Mouse Strain. Behav. Brain Res. 2010, 208, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, A.M.; Edington, G.; Mihalik, A.C.; Buchwald, Z.; Koppuzha, D.; Korah, M.; Lewis, M.H. Further Characterization of Repetitive Behavior in C58 Mice: Developmental Trajectory and Effects of Environmental Enrichment. Behav. Brain Res. 2012, 235, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.M.; Curry-Pochy, L.S.; Shafer, R.; Rudy, J.; Lewis, M.H. Reversal Learning in C58 Mice: Modeling Higher Order Repetitive Behavior. Behav. Brain Res. 2017, 332, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism Is Blocked by the MGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Perry, K.; Weber, M.D.; Katz, A.M.; Crawley, J.N. Social Peers Rescue Autism-Relevant Sociability Deficits in Adolescent Mice. Autism Res. 2011, 4, 17–27. [Google Scholar] [CrossRef]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like Behavioral Phenotypes in BTBR T+tf/J Mice. Genes, Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef]
- Silverman, J.L.; Smith, D.G.; Rizzo, S.J.S.; Karras, M.N.; Turner, S.M.; Tolu, S.S.; Bryce, D.K.; Smith, D.L.; Fonseca, K.; Ring, R.H.; et al. Negative Allosteric Modulation of the MGluR5 Receptor Reduces Repetitive Behaviors and Rescues Social Deficits in Mouse Models of Autism. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Bolivar, V.; Walters, S.; Phoenix, J. Assessing Autism-like Behavior in Mice: Variations in Social Interactions among Inbred Strains. Behav. Brain Res. 2007, 176, 21–26. [Google Scholar] [CrossRef]
- Yang, M.; Clarke, A.M.; Crawley, J.N. Postnatal Lesion Evidence against a Primary Role for the Corpus Callosum in Mouse Sociability. Eur. J. Neurosci. 2009, 29, 1663–1677. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Schneider, T.; Turczak, J.; Przewłocki, R. Environmental Enrichment Reverses Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Issues for a Therapeutic Approach in Autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Seiffe, A.; Ramírez, M.F.; Sempé, L.; Depino, A.M. Juvenile Handling Rescues Autism-Related Effects of Prenatal Exposure to Valproic Acid. Sci. Rep. 2022, 12, 7174. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The Critical Period of Valproate Exposure to Induce Autistic Symptoms in Sprague–Dawley Rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hara, Y.; Ago, Y.; Takano, E.; Hasebe, S.; Nakazawa, T.; Hashimoto, H.; Matsuda, T.; Takuma, K. Environmental Enrichment Attenuates Behavioral Abnormalities in Valproic Acid-Exposed Autism Model Mice. Behav. Brain Res. 2017, 333, 67–73. [Google Scholar] [CrossRef]
- Cheh, M.A.; Millonig, J.H.; Roselli, L.M.; Ming, X.; Jacobsen, E.; Kamdar, S.; Wagner, G.C. En2 Knockout Mice Display Neurobehavioral and Neurochemical Alterations Relevant to Autism Spectrum Disorder. Brain Res. 2006, 1116, 166–176. [Google Scholar] [CrossRef]
- Katayama, Y.; Nishiyama, M.; Shoji, H.; Ohkawa, Y.; Kawamura, A.; Sato, T.; Suyama, M.; Takumi, T.; Miyakawa, T.; Nakayama, K.I. CHD8 Haploinsufficiency Results in Autistic-like Phenotypes in Mice. Nature 2016, 537, 675–679. [Google Scholar] [CrossRef]
- Cherepanov, S.M.; Gerasimenko, M.; Yuhi, T.; Furuhara, K.; Tsuji, C.; Yokoyama, S.; Nakayama, K.I.; Nishiyama, M.; Higashida, H. Oxytocin Ameliorates Impaired Social Behavior in a Chd8 Haploinsufficiency Mouse Model of Autism. BMC Neurosci. 2021, 22, 32. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Simon, J.M.; Hu, W.; Moy, S.S.; Harper, K.M.; Liu, C.-W.; Lu, K.; Zylka, M.J. Developmental Pyrethroid Exposure and Age Influence Phenotypes in a Chd8 Haploinsufficient Autism Mouse Model. Sci. Rep. 2022, 12, 5555. [Google Scholar] [CrossRef]
- Celen, C.; Chuang, J.-C.; Luo, X.; Nijem, N.; Walker, A.K.; Chen, F.; Zhang, S.; Chung, A.S.; Nguyen, L.H.; Nassour, I.; et al. Arid1b Haploinsufficient Mice Reveal Neuropsychiatric Phenotypes and Reversible Causes of Growth Impairment. eLife 2017, 6, e25730. [Google Scholar] [CrossRef]
- Jung, E.-M.; Moffat, J.J.; Liu, J.; Dravid, S.M.; Gurumurthy, C.B.; Kim, W.-Y. Arid1b Haploinsufficiency Disrupts Cortical Interneuron Development and Mouse Behavior. Nat. Neurosci. 2017, 20, 1694–1707. [Google Scholar] [CrossRef]
- Shibutani, M.; Horii, T.; Shoji, H.; Morita, S.; Kimura, M.; Terawaki, N.; Miyakawa, T.; Hatada, I. Arid1b Haploinsufficiency Causes Abnormal Brain Gene Expression and Autism-Related Behaviors in Mice. Int. J. Mol. Sci. 2017, 18, 1872. [Google Scholar] [CrossRef]
- Weigel, B.; Tegethoff, J.F.; Grieder, S.D.; Lim, B.; Nagarajan, B.; Liu, Y.-C.; Truberg, J.; Papageorgiou, D.; Adrian-Segarra, J.M.; Schmidt, L.K.; et al. MYT1L Haploinsufficiency in Human Neurons and Mice Causes Autism-Associated Phenotypes That Can Be Reversed by Genetic and Pharmacologic Intervention. Mol. Psychiatry 2023, 1–14. [Google Scholar] [CrossRef]
- Kim, S.; Oh, H.; Choi, S.H.; Yoo, Y.-E.; Noh, Y.W.; Cho, Y.; Im, G.H.; Lee, C.; Oh, Y.; Yang, E.; et al. Postnatal Age-Differential ASD-like Transcriptomic, Synaptic, and Behavioral Deficits in Myt1l-Mutant Mice. Cell Rep. 2022, 40, 111398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lambo, M.E.; Ge, X.; Dearborn, J.T.; Liu, Y.; McCullough, K.B.; Swift, R.G.; Tabachnick, D.R.; Tian, L.; Noguchi, K.; et al. A MYT1L Syndrome Mouse Model Recapitulates Patient Phenotypes and Reveals Altered Brain Development Due to Disrupted Neuronal Maturation. Neuron 2021, 109, 3775–3792.e14. [Google Scholar] [CrossRef]
- Wang, H.-G.; Bavley, C.C.; Li, A.; Jones, R.M.; Hackett, J.E.; Bayleyen, Y.; Lee, F.S.; Rajadhyaksha, A.M.; Pitt, G.S. Scn2a Severe Hypomorphic Mutation Decreases Excitatory Synaptic Input and Causes Autism-Associated Behaviors. JCI Insight 2021, 6, e150698. [Google Scholar] [CrossRef]
- Indumathy, J.; Pruitt, A.; Gautier, N.M.; Crane, K.; Glasscock, E. Kv1.1 Deficiency Alters Repetitive and Social Behaviors in Mice and Rescues Autistic-like Behaviors Due to Scn2a Haploinsufficiency. Brain Behav. 2021, 11, e02041. [Google Scholar] [CrossRef]
- Léna, I.; Mantegazza, M. NaV1.2 Haploinsufficiency in Scn2a Knock-out Mice Causes an Autistic-like Phenotype Attenuated with Age. Sci. Rep. 2019, 9, 12886. [Google Scholar] [CrossRef] [PubMed]
- Spratt, P.W.E.; Ben-Shalom, R.; Keeshen, C.M.; Burke, K.J.; Clarkson, R.L.; Sanders, S.J.; Bender, K.J. The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron 2019, 103, 673–685.e5. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, T.; Raveau, M.; Ogiwara, I.; Hattori, S.; Miyamoto, H.; Mazaki, E.; Itohara, S.; Miyakawa, T.; Montal, M.; Yamakawa, K. Scn2a Haploinsufficient Mice Display a Spectrum of Phenotypes Affecting Anxiety, Sociability, Memory Flexibility and Ampakine CX516 Rescues Their Hyperactivity. Mol. Autism 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Yizhar-Barnea, O.; Touloumi, O.; Lagoudaki, R.; Avraham, K.B.; Grigoriadis, N.; Gozes, I. Atypical Auditory Brainstem Response and Protein Expression Aberrations Related to ASD and Hearing Loss in the Adnp Haploinsufficient Mouse Brain. Neurochem. Res. 2019, 44, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenková, V.; McKinney, R.A.; Gozes, I. Activity-Dependent Neuroprotective Protein Deficiency Models Synaptic and Developmental Phenotypes of Autism-like Syndrome. J. Clin. Investig. 2018, 128, 4956–4969. [Google Scholar] [CrossRef]
- Malishkevich, A.; Amram, N.; Hacohen-Kleiman, G.; Magen, I.; Giladi, E.; Gozes, I. Activity-Dependent Neuroprotective Protein (ADNP) Exhibits Striking Sexual Dichotomy Impacting on Autistic and Alzheimer’s Pathologies. Transl. Psychiatry 2015, 5, e501. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing Action of Prenatally Administered Testosterone Propionate on the Tissues Mediating Mating Behavior in the Female Guinea Pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Molenda-Figueira, H.A.; Sisk, C.L. Back to the Future: The Organizational-Activational Hypothesis Adapted to Puberty and Adolescence. Horm. Behav. 2009, 55, 597–604. [Google Scholar] [CrossRef]
- Koopman, P.; Münsterberg, A.; Capel, B.; Vivian, N.; Lovell-Badge, R. Expression of a Candidate Sex-Determining Gene during Mouse Testis Differentiation. Nature 1990, 348, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M. Sex Differences in “Cognitive” Regions of the Rat Brain. Psychoneuroendocrinology 1991, 16, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Shryne, J.E.; Gorski, R.A. Structural Sexual Dimorphisms in the Anteroventral Periventricular Nucleus of the Rat Hypothalamus Are Sensitive to Gonadal Steroids Perinatally, but Develop Peripubertally. Neuroendocrinology 1996, 63, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What Does the “Four Core Genotypes” Mouse Model Tell Us about Sex Differences in the Brain and Other Tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef]
- De Vries, G.J.; Simerly, R.B. Anatomy, Development, and Function of Sexually Dimorphic Neural Circuits in the Mammalian Brain. Horm. Brain Behav. 2002, 4, 137–139, XXVII–XXIX. [Google Scholar] [CrossRef]
- De Vries, G.J.; Rissman, E.F.; Simerly, R.B.; Yang, L.Y.; Scordalakes, E.M.; Auger, C.J.; Swain, A.; Lovell-Badge, R.; Burgoyne, P.S.; Arnold, A.P. A Model System for Study of Sex Chromosome Effects on Sexually Dimorphic Neural and Behavioral Traits. J. Neurosci. 2002, 22, 9005–9014. [Google Scholar] [CrossRef]
- Wagner, C.K.; Xu, J.; Pfau, J.L.; Quadros, P.S.; De Vries, G.J.; Arnold, A.P. Neonatal Mice Possessing an Sry Transgene Show a Masculinized Pattern of Progesterone Receptor Expression in the Brain Independent of Sex Chromosome Status. Endocrinology 2004, 145, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Jurgens, H.A.; Auger, C.J.; De Vries, G.J.; Arnold, A.P.; Juraska, J.M. Sex Differences in Mouse Cortical Thickness Are Independent of the Complement of Sex Chromosomes. Neuroscience 2003, 116, 71–75. [Google Scholar] [CrossRef] [PubMed]
- McPhie-Lalmansingh, A.A.; Tejada, L.D.; Weaver, J.L.; Rissman, E.F. Sex Chromosome Complement Affects Social Interactions in Mice. Horm. Behav. 2008, 54, 565–570. [Google Scholar] [CrossRef]
- Gatewood, J.D.; Wills, A.; Shetty, S.; Xu, J.; Arnold, A.P.; Burgoyne, P.S.; Rissman, E.F. Sex Chromosome Complement and Gonadal Sex Influence Aggressive and Parental Behaviors in Mice. J. Neurosci. 2006, 26, 2335–2342. [Google Scholar] [CrossRef]
- Sato, T.; Matsumoto, T.; Kawano, H.; Watanabe, T.; Uematsu, Y.; Sekine, K.; Fukuda, T.; Aihara, K.I.; Krust, A.; Yamada, T.; et al. Brain Masculinization Requires Androgen Receptor Function. Proc. Natl. Acad. Sci. USA 2004, 101, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Raisman, G.; Field, P.M. Sexual Dimorphism in the Preoptic Area of the Rat. Science 1971, 173, 731–733. [Google Scholar] [CrossRef]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef] [PubMed]
- Grgurevic, N.; Büdefeld, T.; Rissman, E.F.; Tobet, S.A.; Majdic, G. Aggressive Behaviors in Adult SF-1 Knockout Mice That Are Not Exposed to Gonadal Steroids during Development. Behav. Neurosci. 2008, 122, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.V.; Manoli, D.S.; Fraser, E.J.; Coats, J.K.; Tollkuhn, J.; Honda, S.I.; Harada, N.; Shah, N.M. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell 2009, 139, 61–72. [Google Scholar] [CrossRef]
- Lee, N.S.; Beery, A.K. Neural Circuits Underlying Rodent Sociality: A Comparative Approach. In Brain Imaging in Behavioral Neuroscience; Springer: Berlin/Heidelberg, Germany, 2019; pp. 211–238. ISBN 9783642209246. [Google Scholar]
- Johnson, M.H.; Griffin, R.; Csibra, G.; Halit, H.; Farroni, T.; de Haan, M.; Tucker, L.A.; Baron-Cohen, S.; Richards, J. The Emergence of the Social Brain Network: Evidence from Typical and Atypical Development. Dev. Psychopathol. 2005, 17, 599–619. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of Minds: The Medial Frontal Cortex and Social Cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Shepard, K.N.; Michopoulos, V.; Toufexis, D.J.; Wilson, M.E. Genetic, Epigenetic and Environmental Impact on Sex Differences in Social Behavior. Physiol. Behav. 2009, 97, 157–170. [Google Scholar] [CrossRef]
- Newman, S.W. The Medial Extended Amygdala in Male Reproductive Behavior. A Node in the Mammalian Social Behavior Network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef]
- Schwarz, J.M. Sex and the Developing Brain; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128021149. [Google Scholar]
- McCarthy, M.M. Estradiol and the Developing Brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef]
- Mong, J.A.; Nuñez, J.L.; McCarthy, M.M. GABA Mediates Steroid-Induced Astrocyte Differentiation in the Neonatal Rat Hypothalamus. J. Neuroendocrinol. 2002, 14, 45–55. [Google Scholar] [CrossRef]
- Todd, B.J.; Schwarz, J.M.; Mong, J.A.; McCarthy, M.M. Glutamate AMPA/Kainate Receptors, Not GABAA Receptors, Mediate Estradiol-Induced Sex Differences in the Hypothalamus. Dev. Neurobiol. 2007, 67, 304–315. [Google Scholar] [CrossRef]
- Davis, E.C.; Popper, P.; Gorski, R.A. The Role of Apoptosis in Sexual Differentiation of the Rat Sexually Dimorphic Nucleus of the Preoptic Area. Brain Res. 1996, 734, 10–18. [Google Scholar] [CrossRef]
- Pickett, L.A.; VanRyzin, J.W.; Marquardt, A.E.; McCarthy, M.M. Microglia Phagocytosis Mediates the Volume and Function of the Rat Sexually Dimorphic Nucleus of the Preoptic Area. Proc. Natl. Acad. Sci. USA 2023, 120, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, T.M.; Campbell, R.E.; Porteous, R.; Bock, D.; Gröne, H.J.; Todman, M.G.; Korach, K.S.; Greiner, E.; Pérez, C.A.; Schütz, G.; et al. Definition of Estrogen Receptor Pathway Critical for Estrogen Positive Feedback to Gonadotropin-Releasing Hormone Neurons and Fertility. Neuron 2006, 52, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Houtsmuller, E.J.; Brand, T.; de Jonge, F.H.; Joosten, R.N.J.M.A.; van de Poll, N.E.; Slob, A.K. SDN-POA Volume, Sexual Behavior, and Partner Preference of Male Rats Affected by Perinatal Treatment with ATD. Physiol. Behav. 1994, 56, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Pickett, L.A.; Wright, C.L.; Davis, K.T.; Joshi, A.; McCarthy, M.M. Mast Cells in the Developing Brain Determine Adult Sexual Behavior. J. Neurosci. 2018, 38, 8044–8059. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia Are Essential to Masculinization of Brain and Behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Amateau, S.K.; McCarthy, M.M. Induction of PGE2 by Estradiol Mediates Developmental Masculinization of Sex Behavior. Nat. Neurosci. 2004, 7, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Wright, C.L.; Martin, R.C.; McCarthy, M.M. Prostaglandin E2 Regulates AMPA Receptor Phosphorylation and Promotes Membrane Insertion in Preoptic Area Neurons and Glia during Sexual Differentiation. PLoS ONE 2011, 6, e18500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Hu, M.H.; Hanley, B.P.; Lin, Y.S.; Poston, L.; Lightman, S.L.; O’Byrne, K.T. The Posterodorsal Medial Amygdala Regulates the Timing of Puberty Onset in Female Rats. Endocrinology 2015, 156, 3725–3736. [Google Scholar] [CrossRef]
- Morris, J.A.; Jordan, C.L.; King, Z.A.; Northcutt, K.V.; Breedlove, S.M. Sexual Dimorphism and Steroid Responsiveness of the Posterodorsal Medial Amygdala in Adult Mice. Brain Res. 2008, 1190, 115–121. [Google Scholar] [CrossRef]
- Cooke, B.M.; Stokas, M.R.; Woolley, C.S. Morphological Sex Differences and Laterality in the Prepubertal Medial Amygdala. J. Comp. Neurol. 2007, 501, 904–915. [Google Scholar] [CrossRef]
- Johnson, R.T.; Breedlove, S.M.; Jordan, C.L. Androgen Receptors Mediate Masculinization of Astrocytes in the Rat Posterodorsal Medial Amygdala during Puberty. J. Comp. Neurol. 2013, 521, 2298–2309. [Google Scholar] [CrossRef]
- VanRyzin, J.W.; Marquardt, A.E.; Argue, K.J.; Vecchiarelli, H.A.; Ashton, S.E.; Arambula, S.E.; Hill, M.N.; McCarthy, M.M. Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron 2019, 102, 435–449.e6. [Google Scholar] [CrossRef]
- Donaldson, Z.R.; Young, L.J. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef]
- Young, L.J.; Wang, Z. The Neurobiology of Pair Bonding. Nat. Neurosci. 2004, 7, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.L.; Bass, A.H. Social Behavior Functions and Related Anatomical Characteristics of Vasotocin/Vasopressin Systems in Vertebrates. Brain Res. Rev. 2001, 35, 246–265. [Google Scholar] [CrossRef]
- Lim, M.M.; Young, L.J. Neuropeptidergic Regulation of Affiliative Behavior and Social Bonding in Animals. Horm. Behav. 2006, 50, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Insel, T.R.; Harbaugh, C.R.; Carter, C.S. Oxytocin Administered Centrally Facilitates Formation of a Partner Preference in Female Prairie Voles (Microtus Ochrogaster). J. Neuroendocrinol. 1994, 6, 247–250. [Google Scholar] [CrossRef]
- Winslow, J.T.; Hastings, N.; Carter, C.S.; Harbaugh, C.R.; Insel, T.R. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature 1993, 365, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Kalló, I.; Steinhauser, A.; Merchenthaler, I.; Coen, C.W.; Petersen, S.L.; Liposits, Z. Estrogen Receptor-β in Oxytocin and Vasopressin Neurons of the Rat and Human Hypothalamus: Immunocytochemical and in Situ Hybridization Studies. J. Comp. Neurol. 2004, 473, 315–333. [Google Scholar] [CrossRef]
- Choleris, E.; Gustafsson, J.Å.; Korach, K.S.; Muglia, L.J.; Pfaff, D.W.; Ogawa, S. An Estrogen-Dependent Four-Gene Micronet Regulating Social Recognition: A Study with Oxytocin and Estrogen Receptor-α and -β Knockout Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 6192–6197. [Google Scholar] [CrossRef]
- Young, L.J. The Neurobiology of Social Recognition, Approach, and Avoidance. Biol. Psychiatry 2002, 51, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. J. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef]
- Cataldo, I.; Azhari, A.; Esposito, G. A Review of Oxytocin and Arginine-Vasopressin Receptors and Their Modulation of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Piochon, C.; Kloth, A.D.; Grasselli, G.; Titley, H.K.; Nakayama, H.; Hashimoto, K.; Wan, V.; Simmons, D.H.; Eissa, T.; Nakatani, J.; et al. Cerebellar Plasticity and Motor Learning Deficits in a Copy-Number Variation Mouse Model of Autism. Nat. Commun. 2014, 5, 5586. [Google Scholar] [CrossRef]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like Behaviour and Cerebellar Dysfunction in Purkinje Cell Tsc1 Mutant Mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef]
- Mercer, A.A.; Palarz, K.J.; Tabatadze, N.; Woolley, C.S.; Raman, I.M. Sex Differences in Cerebellar Synaptic Transmission and Sex-Specific Responses to Autism-Linked Gabrb3 Mutations in Mice. eLife 2016, 5. [Google Scholar] [CrossRef]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; Mccarthy, M.M. Prostaglandin E2 Is an Endogenous Modulator of Cerebellar Development and Complex Behavior during a Sensitive Postnatal Period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.F.; Wright, C.L.; McCarthy, M.M. A Critical Period in Purkinje Cell Development Is Mediated by Local Estradiol Synthesis, Disrupted by Inflammation, and Has Enduring Consequences Only for Males. J. Neurosci. 2016, 36, 10039–10049. [Google Scholar] [CrossRef]
- Lemaigre-dubreuil, Y.; Doulazmi, M.; Fre, F.; Hadj-sahraoui, N.; Delhaye-bouchaud, N.; Mariani, J. Span of the Heterozygous Staggerer Mouse (Rora/Rora Sg) Is Gender-Related. J. Comp. Neurol. 1999, 411, 267–273. [Google Scholar]
- Hadj-Sahraoui, N.; Frédéric, F.; Delhaye-Bouchaud, N.; Mariani, J. Gender Effect on Purkinje Cell Loss in the Cerebellum of the Heterozygous Reeler Mouse. J. Neurogenet. 1996, 11, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.M.; Witt, D.M.; Rissman, E.F. Sex Differences in the Cerebellum and Frontal Cortex: Roles of Estrogen Receptor Alpha and Sex Chromosome Genes. Neuroendocrinology 2011, 93, 230–240. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Mathews, I.Z. Adolescent Development, Hypothalamic-Pituitary-Adrenal Function, and Programming of Adult Learning and Memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zapatero-Caballero, H.; Sanchez-Franco, F.; Fernandez-Mendez, C.; García-San Frutos, M.; Botella-Cubells, L.M.; Fernandez-Vazquez, G. Gonadotropin-Releasing Hormone Receptor Gene Expression during Pubertal Development of Female Rats. Biol. Reprod. 2004, 70, 348–355. [Google Scholar] [CrossRef]
- Pignatelli, D.; Xiao, F.; Gouveia, A.M.; Ferreira, J.G.; Vinson, G.P. Adrenarche in the Rat. J. Endocrinol. 2006, 191, 301–308. [Google Scholar] [CrossRef]
- Zapatero-Caballero, H.; Sanchez-Franco, F.; Guerra-Perez, N.; Fernandez-Mendez, C.; Fernandez-Vazquez, G. Gonadotropin-Releasing Hormone Receptor Gene Expression during Pubertal Development of Male Rats. Biol. Reprod. 2003, 68, 1764–1770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, R.; Meng, Q.; Nautiyal, J.; Flurkey, K.; Tsaih, S.W.; Krier, R.; Parker, M.G.; Harrison, D.E.; Paigen, B. Genetic Coregulation of Age of Female Sexual Maturation and Lifespan through Circulating IGF1 among Inbred Mouse Strains. Proc. Natl. Acad. Sci. USA 2012, 109, 8224–8229. [Google Scholar] [CrossRef]
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin Accelerates the Onset of Puberty in Normal Female Mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Selmanoff, M.K.; Goldman, B.D.; Ginsburg, B.E. Developmental Changes in Serum Luteinizing Hormone, Follicle Stimulating Hormone and Androgen Levels in Males of Two Inbred Mouse Strains. Endocrinology 1977, 100, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Sisk, C.L. Pubertal Hormones, the Adolescent Brain, and the Maturation of Social Behaviors: Lessons from the Syrian Hamster. Mol. Cell. Endocrinol. 2006, 254–255, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M.; Sisk, C.L.; DonCarlos, L.L. Sexual Differentiation of the Adolescent Rodent Brain: Hormonal Influences and Developmental Mechanisms. Horm. Behav. 2013, 64, 203–210. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. The Organizing Actions of Adolescent Gonadal Steroid Hormones on Brain and Behavioral Development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef]
- Brock, O.; Baum, M.J.; Bakker, J. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. J. Neurosci. 2011, 31, 5574–5578. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Richardson, H.N.; Zehr, J.L.; Osetek, A.J.; Menard, T.A.; Sisk, C.L. Gonadal Hormones Masculinize and Defeminize Reproductive Behaviors during Puberty in the Male Syrian Hamster. Horm. Behav. 2004, 45, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Menard, T.A.; Smith, D.A.; Albers, H.E.; Sisk, C.L. Testicular Hormone Exposure during Adolescence Organizes Flank-Marking Behavior and Vasopressin Receptor Binding in the Lateral Septum. Horm. Behav. 2006, 50, 477–483. [Google Scholar] [CrossRef]
- Shrenker, P.; Maxson, S.C.; Ginsburg, B.E. The Role of Postnatal Testosterone in the Development of Sexually Dimorphic Behaviors in DBA/1Bg Mice. Physiol. Behav. 1985, 35, 757–762. [Google Scholar] [CrossRef]
- Bloch, G.J.; Mills, R.; Gale, S. Prepubertal Testosterone Treatment of Female Rats: Defeminization of Behavioral and Endocrine Function in Adulthood. Neurosci. Biobehav. Rev. 1995, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.J.; Mills, R. Prepubertal Testosterone Treatment of Neonatally Gonadectomized Male Rats: Defeminization and Masculinization of Behavioral and Endocrine Function in Adulthood. Neurosci. Biobehav. Rev. 1995, 19, 187–200. [Google Scholar] [CrossRef]
- Kercmar, J.; Snoj, T.; Tobet, S.A.; Majdic, G. Gonadectomy Prior to Puberty Decreases Normal Parental Behavior in Adult Mice. Horm. Behav. 2014, 66, 667–673. [Google Scholar] [CrossRef]
- Primus, R.J.; Kellogg, C.K. Gonadal Hormones during Puberty Organize Environment-Related Social Interaction in the Male Rat. Horm. Behav. 1990, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cooke, B.M.; Woolley, C.S. Effects of Prepubertal Gonadectomy on a Male-Typical Behavior and Excitatory Synaptic Transmission in the Amygdala. Dev. Neurobiol. 2009, 69, 141–152. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Zehr, J.L.; Schulz, K.M.; Lorenz, B.H.; DonCarlos, L.L.; Sisk, C.L. Pubertal Hormones Modulate the Addition of New Cells to Sexually Dimorphic Brain Regions. Nat. Neurosci. 2008, 11, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Morris, J.R.; Juraska, J.M. Neuron Number Decreases in the Rat Ventral, but Not Dorsal, Medial Prefrontal Cortex between Adolescence and Adulthood. Neuroscience 2007, 144, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Schneider, A.; Doncarlos, L.L.; Breedlove, S.M.; Jordan, C.L. Astrocytes in the Rat Medial Amygdala Are Responsive to Adult Androgens. J. Comp. Neurol. 2012, 520, 2531–2544. [Google Scholar] [CrossRef]
- Reid, S.N.M.; Juraska, J.M. Sex Differences in the Gross Size of the Rat Neocortex. J. Comp. Neurol. 1992, 321, 442–447. [Google Scholar] [CrossRef]
- Reid, S.N.M.; Juraska, J.M. Sex Differences in the Number of Synaptic Junctions in the Binocular Area of the Rat Visual Cortex. J. Comp. Neurol. 1995, 352, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.L.; Sodhi, J.; Juraska, J.M. Ovarian Hormones after Postnatal Day 20 Reduce Neuron Number in the Rat Primary Visual Cortex. J. Neurobiol. 2002, 52, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Antonio Muñoz-Cueto, J.; Miguel García-Segura, L.; Ruiz-Marcos, A. Developmental Sex Differences and Effect of Ovariectomy on the Number of Cortical Pyramidal Cell Dendritic Spines. Brain Res. 1990, 515, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Willing, J.; Juraska, J.M. The Timing of Neuronal Loss across Adolescence in the Medial Prefrontal Cortex of Male and Female Rats. Neuroscience 2015, 301, 268–275. [Google Scholar] [CrossRef]
- Koss, W.A.; Lloyd, M.M.; Sadowski, R.N.; Wise, L.M.; Juraska, J.M. Gonadectomy before Puberty Increases the Number of Neurons and Glia in the Medial Prefrontal Cortex of Female, but Not Male, Rats. Dev. Psychobiol. 2015, 57, 305–312. [Google Scholar] [CrossRef]
- Bell, H.C.; McCaffrey, D.R.; Forgie, M.L.; Kolb, B.; Pellis, S.M. The Role of the Medial Prefrontal Cortex in the Play Fighting of Rats. Behav. Neurosci. 2009, 123, 1158–1168. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Zhu, H.; Zhang, Q.; Lin, Z.; Hu, H. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science 2011, 334, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of Brain Development: Point of Vulnerability or Window of Opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021, 44, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Amateur, S.K.; McCarthy, M.M. Sexual Differentiation of Astrocyte Morphology in the Developing Rat Preoptic Area. J. Neuroendocrinol. 2002, 14, 904–910. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex Differences in Microglial Colonization of the Developing Rat Brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef]
- Mong, J.A.; Kurzweil, R.L.; Davis, A.M.; Rocca, M.S.; McCarthy, M.M. Evidence for Sexual Differentiation of Glia in Rat Brain. Horm. Behav. 1996, 30, 553–562. [Google Scholar] [CrossRef]
- Mong, J.A.; Glaser, E.; McCarthy, M.M. Gonadal Steroids Promote Glial Differentiation and Alter Neuronal Morphology in the Developing Hypothalamus in a Regionally Specific Manner. J. Neurosci. 1999, 19, 1464–1472. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Smith, S.H.; Schwarz, J.M. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J. Neuroimmune Pharmacol. 2012, 7, 24–41. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Zhang, A.; Cohen, O.S.; Page, D.T. Environmental Enrichment Rescues Social Behavioral Deficits and Synaptic Abnormalities in Pten Haploinsufficient Mice. Genes 2021, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Urruela, M.; Devine, D.P. Effects of Environmental Enrichment on Repetitive Behaviors in the Btbr T+tf/j Mouse Model of Autism. Autism Res. 2013, 6, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mouton, P.R.; Long, J.M.; Lei, D.L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and Gender Effects on Microglia and Astrocyte Numbers in Brains of Mice. Brain Res. 2002, 956, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M.A.M.; Earl, C.; Kaczmarczyk, E.; Ferrari, C.; Besedovsky, H.; Del Rey, A.; Pitossi, F.J.F.J.; Oertel, W.H.W.H.; Rey, A.; Pitossi, F.J.F.J.; et al. Microglial Activation with Atypical Proinflammatory Cytokine Expression in a Rat Model of Parkinson’s Disease. Eur. J. Neurosci. 2003, 18, 2731–2742. [Google Scholar] [CrossRef]
- Pott Godoy, M.C.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and Systemic IL-1 Exacerbates Neurodegeneration and Motor Symptoms in a Model of Parkinson’s Disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen Prevents the Lipopolysaccharide-Induced Inflammatory Response in Microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef]
- Williamson, L.L.; Chao, A.; Bilbo, S.D. Environmental Enrichment Alters Glial Antigen Expression and Neuroimmune Function in the Adult Rat Hippocampus. Brain. Behav. Immun. 2012, 26, 500–510. [Google Scholar] [CrossRef]
- van den Berg, H. Evaluating the Validity of Animal Models of Mental Disorder: From Modeling Syndromes to Modeling Endophenotypes. Hist. Philos. Life Sci. 2022, 44, 1–26. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in Translation: The Valley of Death across Preclinical and Clinical Divide–Identification of Problems and Overcoming Obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
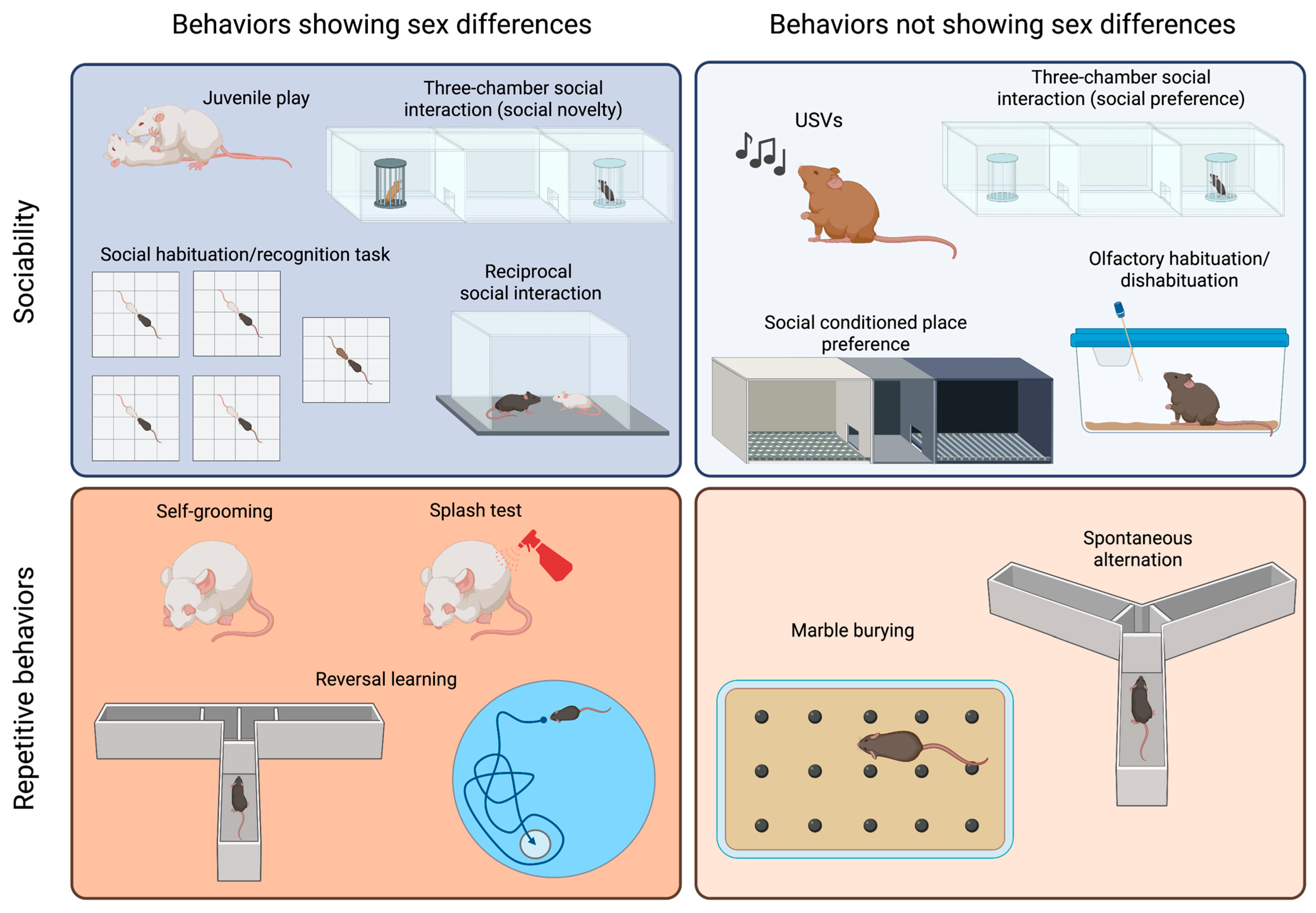
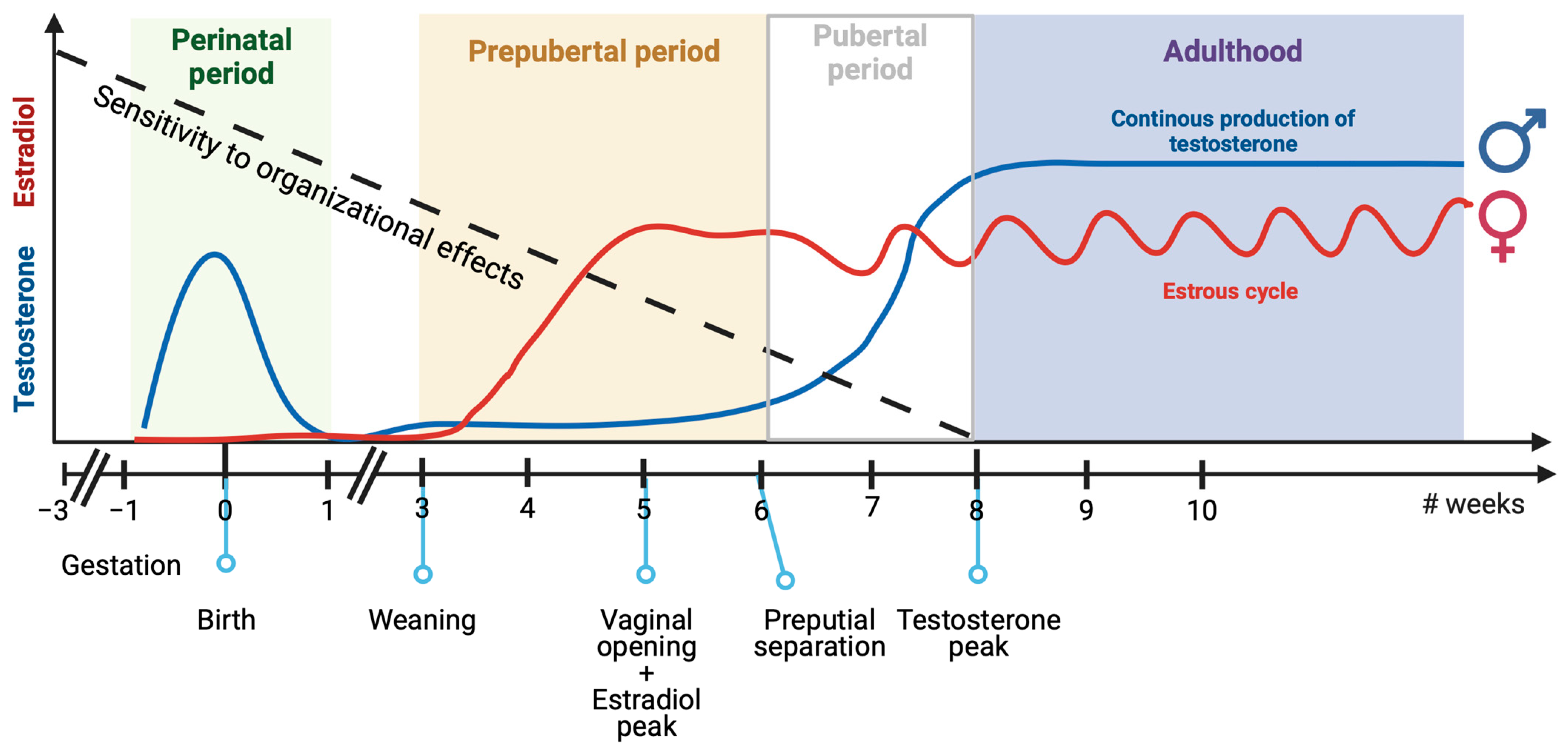
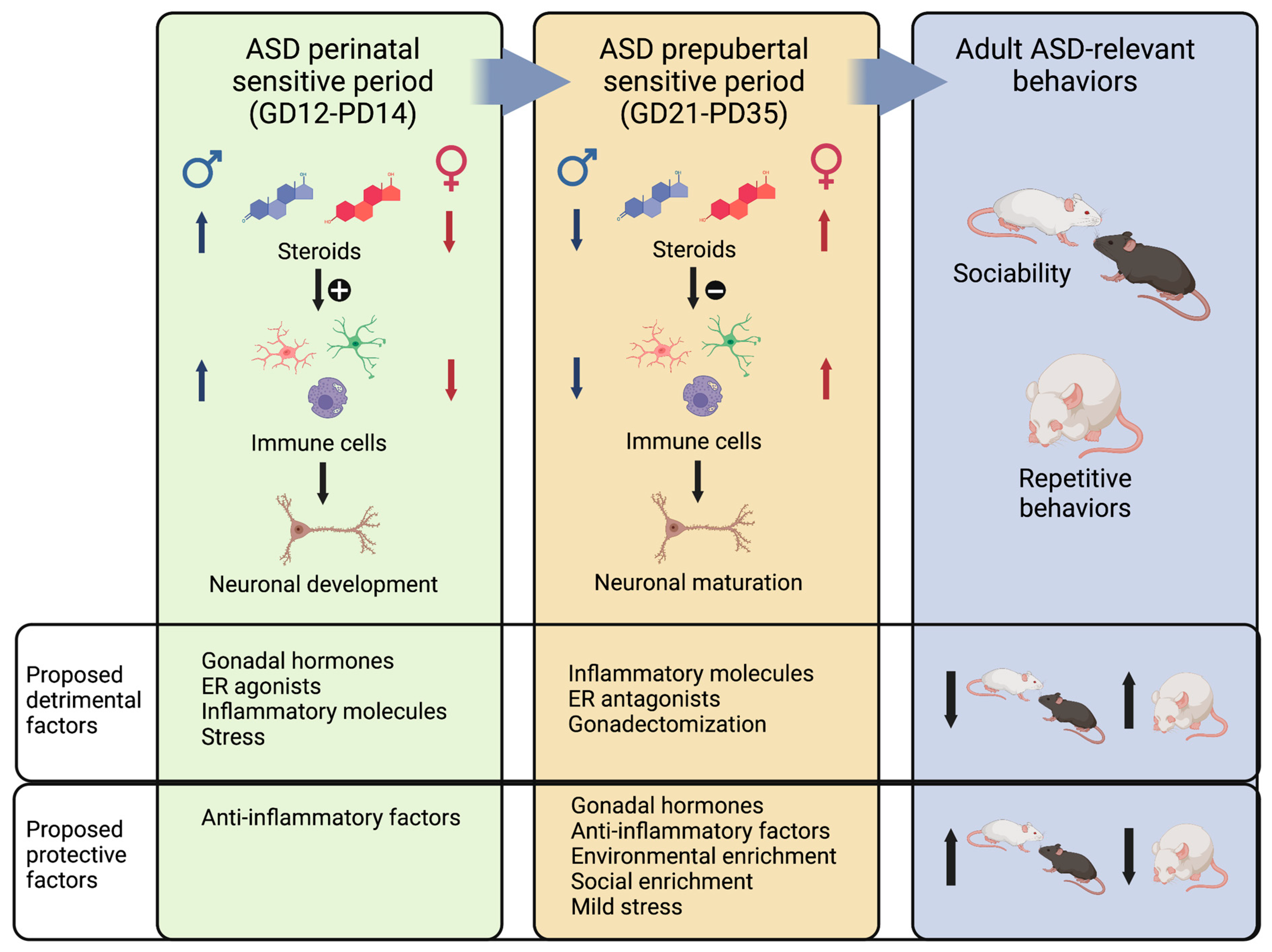
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murta, V.; Seiffe, A.; Depino, A.M. Sex Differences in Mouse Models of Autism Spectrum Disorders: Their Potential to Uncover the Impact of Brain Sexual Differentiation on Gender Bias. Sexes 2023, 4, 358-391. https://doi.org/10.3390/sexes4030024
Murta V, Seiffe A, Depino AM. Sex Differences in Mouse Models of Autism Spectrum Disorders: Their Potential to Uncover the Impact of Brain Sexual Differentiation on Gender Bias. Sexes. 2023; 4(3):358-391. https://doi.org/10.3390/sexes4030024
Chicago/Turabian StyleMurta, Verónica, Araceli Seiffe, and Amaicha Mara Depino. 2023. "Sex Differences in Mouse Models of Autism Spectrum Disorders: Their Potential to Uncover the Impact of Brain Sexual Differentiation on Gender Bias" Sexes 4, no. 3: 358-391. https://doi.org/10.3390/sexes4030024
APA StyleMurta, V., Seiffe, A., & Depino, A. M. (2023). Sex Differences in Mouse Models of Autism Spectrum Disorders: Their Potential to Uncover the Impact of Brain Sexual Differentiation on Gender Bias. Sexes, 4(3), 358-391. https://doi.org/10.3390/sexes4030024






