Abstract
Central precocious puberty (CPP) is due to the premature activation of the hypothalamic–pituitary–gonadal axis, which is responsible for the appearance of secondary sexual characteristics. It occurs before the age of 8 and 9 in girls and boys, respectively. CPP shows higher incidence in females than in males. Causes of CPP are similar in both sexes, but the idiopathic form is more frequent in girls, while organic forms are more frequent in males. Recent studies demonstrated a role of some genetic variants in the pathogenesis of CPP. The diagnostic evaluation based on accurate physical examination, assessment of the pituitary–gonadal axis, pelvic sonography in girls, and determination of bone age. Magnetic resonance of the central nervous system should be done in all boys and selected girls. Since the 1980s, pharmacologic treatment involves the use of gonadotropin-releasing hormone (GnRH) analogs. These drugs are characterized by few side effects and long-term safety. Many data are available on the outcome of GnRH analog treated female patients, while poor data are reported in boys. Adult height is improved in both sexes.
1. Introduction
Precocious puberty (PP) is a specific pediatric disease characterized by the appearance of secondary sexual characteristics at an abnormally early age in comparison with reference populations. It is usually defined as the onset of puberty before 8 years in girls and 9 years in boys. PP largely differentiates in its pathogenesis; the two main groups are related to activation or not of neuronal pathways driving hypothalamic–pituitary–gonadal axis activity [1,2,3].
Central precocious puberty (CPP) is the gonadotropin-dependent form of PP. It is due to premature awakening of the hypothalamic gonadotropin-releasing hormone (GnRH) generator with subsequent increase in amplitude and frequency of GnRH pulses, which determines the pubertal secretion pattern of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by gonadotropin cells in the pituitary gland [1,2]. LH and FSH are responsible of the premature increase in gonadal sex steroids secretion, determining the development of secondary sexual characteristics. CPP may have important physical and psycho-social consequences [1,2,3].
Girls’ preponderance of CPP is clearly documented and criteria for clinical decisions in females are frequently extrapolated to males. In this paper, some findings of CPP are reviewed, showing similarities and differences in the two sexes.
2. Epidemiology
The true epidemiology of CPP is unknown. A US study estimated that PP in the general population was between 1:5000 to 1:10,000 children [4]. In Europe, a Danish national study reported the prevalence of CPP as 0.2% for girls and less than 0.05% for boys [5]. Spanish and French studies showed different annual incidence of CPP in both sexes [6,7]. A low incidence of CPP was found in a first study from Korea (Table 1) [8]. A second paper from Korea, including girls aged ≤9 years and boys aged ≤10 years, showed high values mainly in girls in comparison with the previous Korean study (Table 1) [8,9]. A higher incidence of CPP in girls in comparison with boys was reported in all the studies (Table 1), suggesting that males are less prone than females to develop CPP.

Table 1.
Incidence of central precocious puberty (CPP) in boys and girls.
Geographical and genetic differences as well as environmental factors may be involved in the different epidemiological results from various studies (Table 1). In addition, data reported in Table 1 were obtained with different methods of assessment and may not be representative of the total susceptible population. Excessive weight gain during childhood as well as adoption or immigration from underdeveloped countries are risk factors for the development of precocious pubertal onset [1,2,6]. They may be involved in the differences among the various reports. Recent data of an Italian cohort have reported an increased incidence of precocious and accelerated puberty in girls during the COVID-19 pandemic [10], suggesting that even acute environmental factors may trigger precocious pubertal onset. Similar data are not yet reported in boys.
3. Etiology
The etiology of CPP is summarized in Table 2. CPP may be due to organic disorders of the central nervous system (CNS) or to specific genetic variants [1,2,3]. Several brain abnormalities are involved in CPP (Table 2). Some syndromes may also be associated with early or precocious pubertal onset [11]. CPP is defined as “idiopathic” (or “isolated”) when no identifiable cause is found. This latter form is more frequent in females than in males (Table 3) [1,2].

Table 2.
Main causes and conditions associated with CPP.
Some authors suggested an increase of idiopathic CPP in boys. A Turkish study revised a series of 100 boys with CPP during a period of 10 years [12]. They showed that the diagnoses of CPP in boys gradually increased by each year of the study. However, cases of organic CPP remained the same throughout the observational period, whereas the idiopathic cases increased until 2009, after which they reached a plateau [12]. In total, there was no underlying cause in 74% of cases, while an organic cause was observed in a minority of boys (26%) [12].
A high percentage of idiopathic CPP was also found in two series of 71 and 138 boys from Korea [13,14] (Table 3). On the contrary, another Korean series of 23 boys reported a 74% of organic CPP (Table 3) [15]. A recent US paper confirmed that the majority (64%) of a series of 50 boys with CPP had organic forms [16]. No change in the incidence of male CPP after accounting for the increase in the clinic volume during the period 2001–2010 was documented [16]. A recent large French study from single center confirmed a low proportion of boys presenting with idiopathic CPP, while this diagnosis occurred in the majority of girls (Table 3) [17].

Table 3.
Organic vs. idiopathic CPP in boys and girls.
Table 3.
Organic vs. idiopathic CPP in boys and girls.
| Author | Year | Country | N * | Organic/Idiopathic (%) | F:M Ratio | |
|---|---|---|---|---|---|---|
| Boys | Girls | |||||
| Thamdrup [18] | 1961 | Netherland | 56 | 64/36 | 24/76 | 4.1:1 |
| UCSF ° [19] | 1981 | USA | 205 | 67/33 | 27/73 | 4.2:1 |
| Bridges et al. [20] | 1994 | UK | 95 | 100/- | 6/94 | 23.0:1 |
| ISGPP ^ [21,22] | 2000 | Italy | 473 | 40/60 | 18/82 | 9.5:1 |
| Chemaitilly et al. [23] | 2001 | France | 256 | 73/27 | 19/81 | 8.8:1 |
| Klein et al. [24] | 2001 | USA | 98 | 83/17 | 32/68 | 4.4:1 |
| Lee et al. [25] | 2011 | USA | 54 | — | — | 9.8:1 |
| Jaruratanasirikul et al. [26] | 2011 | Thailand | 73 | 100/- | 15/85 | 13.6:1 |
| Soriano-Guillén et al. [6] | 2010 | Spain | 250 | 33/67 | 11/89 | 9.4:1 |
| Alikasifoglu et al. [12] | 2015 | Turkey | 100 | 26/74 | — | — |
| Lee et al. [13] | 2018 | Korea | 71 | 38/62 | — | — |
| Yoon et al. [14] | 2018 | Korea | 138 | 6/132 | — | — |
| Choi et al. [15] | 2013 | Korea | 23 | 6/17 | — | — |
| Topor et al. [16] | 2018 | USA | 50 | 64/36 | — | — |
| Harbulot et al. [17] | 2021 | France | 395 | 60/40 | 12/88 | 10.6:1 |
* Total boys + girls; ° UCSF, University of California; San Francisco, USA; ^ ISGPP, Italian Study Group for Physiopathology of Puberty.
The onset of puberty seems to be earlier in organic than in idiopathic CPP in both sexes [1,2,3,17]. Albeit the odds of detecting an underlying pathology are very high in boys with a very early presentation of pubertal findings, there is consensus that all boys with CPP should undergo brain imaging to exclude a central cause [27]. This recommendation should be operative until more data are available. Brain imaging in girls with CPP is a much-discussed issue. Recent meta-analyses cast doubt on the benefit of routine brain magnetic resonance in girls with CPP older than 6 years of age without any neurological concerns [28,29].
A main organic cause of CPP is hypothalamic hamartoma (HH), which is a congenital and benign brain mass (Figure 1, left image). This lesion develops on the inferior surface of hypothalamus with a thin stalk that arises from tuber cinereum. Parahypothalamic tumors, which attach to the anterior part of this gland, more frequently cause CPP. HH may be associated with gelastic seizures and/or psychiatric disorders [30]. Each child with HH should be fully evaluated for neuro-developmental disorders. Males with HH present a CPP with an average age of presentation around 3.5 years, about one year later than girls (2.5 years) [31]. Another relatively frequent cause of CPP is optic glioma due to type 1 neurofibromatosis [Figure 1, right image] [11,19,32].
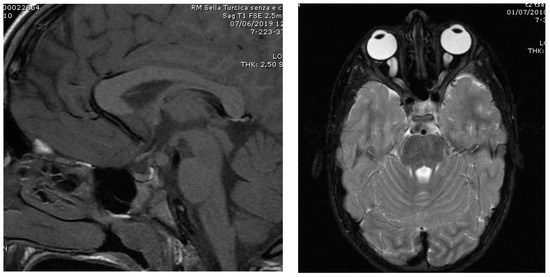
Figure 1.
Left image: Hypothalamic hamartoma in a boy with CPP (onset of pubertal development at the age of 7.0 years; mean testis volume, ml: 8; LH, IU/L: 1.4; FSH IU/L: 1.7; Testosterone, nmol/L: 3.5). Right image: Optic glioma in a boy with neurofibromatosis type 1 and CPP (onset of pubertal development at the age 5.6 years; mean testis volume, ml: 10; LH, IU/L: 3.7; FSH IU/L: 1.9; Testosterone, nmol/L: 5.8).
In the few last years, some genetic causes of CPP have been identified [33]. Genetic variants in the kisspeptin pathway, MKRN3 and Delta-like homolog 1 (DLK1) have been reported in both familial and sporadic cases of CPP [33,34,35,36,37]. Kisspeptin is a peptide hormone expressed by hypothalamus and it serves as a ligand of the kisspeptin receptor (KISS1R), which is a G-protein receptor expressed on the surface of GnRH secreting neurons. If kisspeptin levels increase, the amplitude and frequency of GnRH pulsatility increase. In CPP, gain-of-function mutations of the kisspeptin gene and KISS1R are found [2,33]. MKRN3 loss-of-function mutations may also cause early activation of reproductive axis in children. MKRN3 mutations have been reported in 33% of children with family members with CPP; they represent the most frequent genetic cause of familial CPP, even if an imprinting pattern of inheritance of MKRN3 from an asymptomatic father was described [36,38]. A high frequency of genetic mutations (deleterious MKNR3 variants: 8/20; KISS1 activating mutation: 1/20) in boys with CPP previously classified as idiopathic has been described [34]. The patients with CPP due to MKRN3 variants had classical features of CPP with puberty onset at a borderline age. This suggests that gene mutations may be an unrecognized cause of apparent idiopathic CPP. DLK1 is responsible for differentiation of pituitary cells and it is also implicated in pathogenesis of CPP in girls, but at present, no male cases related to this gene mutations have been reported [33]. Currently, the occurrence of sound discrepancies among sexes are not clear.
It is unknown if identification of the genetic basis of CPP may have implications for medical treatment, but genetic analysis should be included in the CPP diagnostic workup, at least when familial inheritance is present [2]. Positive genetic analyses have clear implications for future reproductive counselling.
4. Clinical Presentation
When CPP is suspected, a complete patient history should be taken, including information about growth patterns since birth, past medical, family, social and psychological history and age of onset of physical pubertal changes. Information about pubertal development of the family members is important to understand if underlying genetic causes may be involved. Possible exposure to exogenous hormones or endocrine disruptors, previous or current central nervous system symptoms and the presence of syndromes associated with PP should be investigate [1,2,3].
The evaluation of a child with PP requires an accurate physical examination with measurements of height, weight and height velocity. In girls, estrogen determines the development of breasts (thelarche), enlargement of labia majora and minora and an increase and redistribution of body fat, predominantly in the hips. In boys, the first physical change is an increase of mean testicular volume more than 3 mL. Testosterone is responsible for the testicular, penis and cricoid cartilage growth, facial hair development, changes in body fat distribution and muscle mass. Transient pubertal gynecomastia can occur in 40% of boys. Increase in growth velocity (pubertal spurt) and advancement of bone age are additional features of CPP. They are largely related to estrogen action on bone. In boys, estrogen arises from aromatization of testosterone; thus, these findings may be delayed in comparison with girls [1,2,3,19].
Skin examination may show neurofibromas, café-au-lait spots (namely, coast of California spots) and axillary/pubic freckling in children with CPP due to type 1 neurofibromatosis. In these cases, optic gliomas may be present. If irregular café-au-lait spots (namely, coast of Maine spots) are observed, peripheral precocious puberty due to McCune-Albright syndrome should be suspected [1,2,39].
5. Laboratory Assessment
Final diagnosis of CPP depends on the demonstration of pubertal levels of gonadotropins and sex steroids [1,2]. The sensitivity of early morning serum LH (before 10.00 am) is between 60–100% and depends on cut-off values and laboratory methodology [1,40]. A GnRH stimulation test (100 μg e.v.) may be necessary to clearly document pubertal LH and FSH levels, because as many as 35.7% of girls with CPP initially presented low basal LH values [40]. Various basal and peak cut-off limits have been reported (Table 4). Thus, each center should be aware of its laboratory methodology and its appropriate reference range. Distinct cut-off values for boys and girls are rarely reported (Table 4).

Table 4.
Suggested basal and GnRH-stimulated (100 µg/i.v.) LH cut-off values for diagnosis of CPP in some studies by using different assay methods.
Table 4.
Suggested basal and GnRH-stimulated (100 µg/i.v.) LH cut-off values for diagnosis of CPP in some studies by using different assay methods.
| Author | Method | LH (IU/L) | |||
|---|---|---|---|---|---|
| Girls | Boys | ||||
| B | P | B | P | ||
| Brito et al. [41] | IFMA | 0.6 | 6.9 | 0.6 | 9.6 |
| Lee et al. [42] | IRMA | 1.1 | 5 | — | — |
| Neely et al. [43] | ICMA | 0.15 | 5 | 0.15 | 5 |
| Pasternak et al. [44] | ICMA | 0.1 | 4.9 | — | — |
| Resende et al. [45] | IFMA | 0.6 | 4.2 | 0.6 | 3.3 |
| ICMA | 0.1 | 3.3 | 0.3 | 4.1 | |
| Wankanit et al. [46] | CMIA | 0.2 | 5.0 * | — | — |
IFMA = immunoflorimetric; IRMA = immunoradiometric; ICMA = immunochemiluminescence; CMIA = chemiluminescent maicriparticle immunoassay; * after triptorelin 100 µg/s.c.
Neely et al. [43] found the same cut-off values in both the sexes, while Brito et al. [41] indicated a higher cut-off in boys in comparison with girls. Resende et al. [45] reported a higher cut-off in girls than in boys by using an IFMA method as in Brito et al. [41], while they found the contrary with an ICMA assay [45] (Table 4). Thus, it remains unclear if a true difference of LH peak after GnRH stimulation is operative between boys and girls. In some countries, GnRH drug is not available and GnRH analogs (0.1 mg) were used to evaluate activation of pituitary-gonadal axis [40,46]. Schemes of sampling and cut-offs values remain not well standardized with this approach.
Standard estradiol assays have low sensitivity and large overlap between prepubertal and early pubertal levels. Serum total testosterone assay is sensitive to diagnose PP, but its isolated measurement does not permit the differential diagnosis between central or peripheral forms of PP [1,40]. Adrenal hormonal profile may be done if an adrenal disorder driving a secondary CPP is suspected. Interpretation of endocrine tests should be made in strict conjunction with clinical data [1,2,3,19,40].
6. Clinical Consequences of Untreated CPP
Impaired adult height is the main long-term somatic consequence of untreated CPP (Table 5). Albeit smaller series are available in boys than in girls, a poorer auxological outcome is likely in males, suggesting a more aggressive disease (Table 5), which may be related to the lower occurrence of idiopathic form in male sex (Table 3). The adult height in untreated men is about −2.5/−3.0 standard deviation scores (SDS) below the normal mean (Table 5) [18,47,48,49,50,51]. Adult height in men results in at least –1 SDS below the mean adult height of females with untreated CPP (Table 5). Other concerns related to CPP are altered body proportions in adulthood, with an upper: lower ratio >1 [1,19] and psycho-social distress (decreased peer interaction, social withdrawal, impairment in school performance, altered behavioral development, increased aggression, increased risk of sexual abuse) [1,2,19].

Table 5.
Adult height (AH) in untreated boys and girls with CPP.
Table 5.
Adult height (AH) in untreated boys and girls with CPP.
| Author | Year | Adult Height, Males | Adult Height, Females | ||||
|---|---|---|---|---|---|---|---|
| n | cm | SDS a | n | cm | SDS a | ||
| Thamdrup [18] | 1961 | 8 | 155.4 ± 8.3 | −2.9 | 26 | 151.3 ± 8.8 | −1.8 |
| Sigurjonsdottir et al. [47] | 1968 | 11 | 156.0 ± 7.3 | −2.8 | 40 | 152.7 ± 8.0 | −1.6 |
| Bovier-Lapierre et al. [48] | 1972 | 5 | 155.8 ± 2.8 | −2.8 | 4 | 150.5 ± 1.6 | −1.9 |
| Paul et al. [49] | 1995 | 4 | 159.6 ± 8.7 | −3.7 b | 8 | 153.8 ± 6.8 | −2.4 |
| Pisa [50] | 2008 | 4 | 156.0 ± 4.7 | −2.8 | 7 | 151.4 ± 4.7 | −1.8 |
| Swaiss et al. [51] | 2017 | 2 | 149.0 ± 12.7 | −3.9 | 11 | 151.2 ± 8.4 | −1.8 |
a SDS, standard deviation score vs. reference values of Tanner et al. [52]; b According to the NCSH reference values [49].
Early engagement in risk-taking behavior (such as smoking, alcohol or drug abuse and early unprotected sex) are additional concerns related to CPP [2,19,53]. These items remain poorly investigated, mainly in boys.
7. Therapy
Management of CPP should be primarily directed to the treatment of the organic cause when this is possible. Children with no treatable organic cause or with idiopathic forms may undergo medical treatment [1,2,3,19,27,39]. The goal of therapy is the attainment of effective and selective suppression of pubertal gonadotropin secretion to induce prepubertal sex steroid levels and to stop premature sexual maturation [1,2,3,27,39]. In addition, treatment should permit the attainment of an adult height adequate for each child in relation to their genetic target by suppressing the accelerated skeletal maturation to a larger extent than growth velocity [1,2,3]. Prompt reversal of the suppression after the discontinuation of treatment, the absence of toxicity and/or side effects during administration and of interferences with reproductive function in adulthood must be considered in prescribing medical therapy for CPP [1,2,3,27].
GnRH analogs are the drug of choice because these drugs desensitize and down-regulate GnRH-receptors, suppress gonadotropin secretion and reduce gonadal steroids to prepubertal levels. Then, stabilization or recovery of the symptoms of CPP as well as improvement of adult height occur [1,2,3,27]. These drugs are synthetic decapeptides, which differ from the naïve GnRH for various hydrophobic D-amino acid substitutions of glycine 6, determining different potencies (Table 6). Glycine 10 can also be modified or substituted by an ehtylamide group, but this latter change is not needed for complete superagonist activity, while aminoacids 1–5 need to be conserved for the preservation of an agonist effect [54]. The result is a higher affinity of the agonist for the receptor and lower enzymatic degradation in comparison with naïve GnRH [54].

Table 6.
Main depot GnRH analogs used in children with CPP.
The depot formulations are able to provide a constant release of the peptide for weeks or months (Table 6) after an immediate peak release of the molecules [54,55,56,57,58]. Many long-term data are available on monthly formulations (1 injection/28 days), while few trials reported on the use of quarterly (11.25 mg/90 days), triptorelin 6-month (22.50 mg) or yearly histrelin implants (50 mg) [55,56,57]. A small volume of 45 mg subcutaneous leuprolide acetate formulation has been recently approved for both sexes; it effectively suppressed pubertal hormones and stopped or caused regression of pubertal progression [58]. The reduced injections frequency of very long-acting GnRH analogs has the potential advantage of improving compliance to treatment and increasing comfort for children [56].
Variable end-results are reported [57]. Table 7 and Table 8 summarized some data related to GnRH analog treatment of CPP in the two sexes. Relatively large series in girls are in Table 7. Few data are available for boys (Table 8).

Table 7.
Adult height in some series of girls treated with monthly GnRH analogues (only full papers with n ≥ 40 girls treated with a single drug are considered).
Table 7.
Adult height in some series of girls treated with monthly GnRH analogues (only full papers with n ≥ 40 girls treated with a single drug are considered).
| Triptorelin, 3.75 mg | Leuprorelin, 3.75 mg | ||||||
|---|---|---|---|---|---|---|---|
| Authors | AH | AH-PH | AH-TH | Authors | AH | AH-PH | AH-TH |
| cm | cm | ||||||
| Adan et al. [59] | 159.5 ± 5.3 | 3.5 | −1.7 | Brito et al. [60] | 155.3 ± 6.9 | −5.3 | −2.2 |
| Arrigo et al. [61] | 158.4 ± 5.8 | 2.9 | −2.9 | Cho et al. [62] | 161.5 ± 4.6 | 8.4 | 2.2 |
| Carel et al. [63] | 161.1 ± 5.9 | 4.7 | 1.0 | Lee et al. [25] | 160.1 ± 5.0 | 4.0 | 0.8 |
| Faienza et al. [64] | 160.6 ± 3.4 | 2.2 | −0.2 | Tanaka et al. [65] | 154.5 ± 5.7 | −0.4 | −0.4 |
| Heger et al. [66] | 160.6 ± 8.0 | 5.7 | −2.0 | Vuralli et al. [67] * | −0.6 ± 0.8 a | 2.0 | 0.6 |
| Kauli et al. [68] | 159.6 ± 6.3 | 2.7 | 1.9 | −0.7 ± 0.9 b | 1.0 | 0.2 | |
| Pasquino et al. [69] | 159.8 ± 5.3 | 9.5 | 2.4 | −1.0 ± 0.7 c | 0.6 | −0.5 | |
AH = adult height; AH-PH = adult height—predicted adult height at the start of GnRH therapy; AH-TH = adult height—mid-parental height; * data are expressed as SDS; age at start of GnRH analog (leuprorelin, 3.75 mg or 7.5 mg/28 days): a ≤6.4 years, b 6.4–8.3 years, c <8.3 years [67].

Table 8.
Final height in boys treated with GnRH analogues (only full papers with n ≥ 15 boys are considered).
Table 8.
Final height in boys treated with GnRH analogues (only full papers with n ≥ 15 boys are considered).
| Authors | GnRH Analog (Dose) | AH | AH-PH | AH-TH |
|---|---|---|---|---|
| cm | ||||
| Klein et al. [24] | Deslorelin (4 mg/kg/day) | 171.1 ± 8.7 | 15.0 | −7.2 |
| Mul et al. [70] | Triptorelin (3.75 mg/28 days) | 172.9 ± 6.6 | 6.2 | −2.2 |
| Shim et al. [71] | Leuprorelin or triptorelin (3.75 µg/28 days) | 173.4 ± 5.8 | 3.3 | 2.5 |
| Tanaka et al. [65] | Leuprorelin (10–90 µg/28 days) | 163.2 ± 13.0 | 1.1 | −4.4 |
AH = adult height; AH-PH = adult height—predicted adult height at the start of GnRH therapy; AH-TH = adult height—mid-parental height.
Adult height was improved in all the studies (Table 7 and Table 8) in comparison with the height of untreated patients (Table 5). Variable increase in final height over pre-treatment predicted height has been shown (Table 7 and Table 8) [56,57]. Overall, better results are shown in boys than in girls, but comparative trials between sexes are lacking.
8. Short- and Long-Term Safety
GnRH analogs in children with CPP generally demonstrated good tolerance and relatively few minor side effects [27]. There are some short-term side effects, such as pain, local skin reactions, gastrointestinal symptoms, and headache. Vaginal bleeding after the first administration can occur. Sterile abscess or anaphylaxis rarely occur [72]. Some boys developed a sonographic pattern suggestive of testicular microcalcification during GnRH analog administration [73]. An increase of adiposity during therapy with GnRH analogs may affect some children [72].
After the discontinuation of GnRH analogs, the hormonal suppression recovers and menses occur after a period of 12–18 months [3,55,56]. The development of polycystic ovary syndrome (PCOS) in treated girls is a subject of debate [72]. Magiakou et al. [74] found a prevalence of 17.2% and 30.8% in triptorelin-treated patients and untreated ones, respectively, without evidence of a predisposing effect to PCOS or menstrual irregularities played by GnRH analog therapy. Other authors reported that young women previously treated with analogs showed a hyperandrogenism, insulin resistance and increased prevalence of PCOS [62,75]. Likely, baseline characteristics of patients, modalities of treatment and different criteria to diagnose PCOS may be involved in the different results. Untreated and GnRH analog treated women with previous CPP experienced spontaneous and uncomplicated pregnancies with the delivery of healthy babies reviewed in [3,55]. Few data are available about adult males treated for CPP. Manasco et al. [73] showed that serum levels of testosterone increased progressively after discontinuation of treatment reaching values similar to the pre-treatment period in about three months. In the same patients, an adequate development of testis volume occurred [73]. Ramos et al. [76] reported successful paternity in three young men. In both sexes, the assessment of antimüllerian hormone before, during and after the discontinuation of GnRH analogs also suggests that treatment has no adverse effects on reproductive function [77,78].
9. Conclusions
CPP is an endocrine disease limited to pediatric age. Idiopathic CPP affects mainly girls, while an organic cause is likely in boys and in young children (age < 5–6 years). Its prevalence is increasing in both sexes [5,6,7,8,9]. Genetic background, environmental interferences, geographical differences and better diagnostic work-up may be involved. The “classic” endocrine diagnosis is based on the demonstration of a pubertal pattern of gonadotropin secretion after GnRH stimulation test, while some authors recently suggested that basal LH levels are adequate to diagnose precocious activation of reproductive axis [reviewed in 40]. The latter approach permits to reduce costs and overcome the lack of GnRH availability in some countries, but it may not allow an early diagnosis in some children. A standardization of two strategies will require additional studies, also considering the differences among the results obtained with different assays (Table 4). Whether the cut-offs are the same in boys and girls will be better clarified. Long-acting GnRH analogs are the ‘gold standard’ for the medical treatment of CPP [27]. Long-term results demonstrated improvements of adult height in both boys and girls. A recent systematic review and meta-analysis (98 studies, 5475 individuals) concluded that GnRH analog treatment increase adult height and decrease the body mass index in girls with idiopathic CPP in comparison with no treatment [79]. The same authors stated that GnRH analog treatment did not evidently increase the risk of PCOS [79]. Even better outcome in terms of adult height seems to present in males, but few data are available and poor evidence on the reproductive function in adulthood are available in male sex [50,73,76]. The very long-acting (quarterly, half yearly and yearly) GnRH analogs likely represent a key developmental step to optimize pharmacological treatment of CPP, but their use has been explored in relatively few trials, in particular regarding end results on adult height [55,56,57,58]. A comprehensive agreement on the criteria for discontinuation of therapy are not reached. Several parameters usually considered to evaluate the effects of treatment are interrelated; thus, they may not be sensitive enough [3,27]. It is reasonable that stopping therapy at a bone age close to the physiological age for puberty onset (11.5–12.5 years in girls; 13.0–14 years in boys) may permit to improve adult height, because residual growth capacity is likely better [3,55,56]. Strict auxological follow-up should be warranted to all children with CPP during GnRH analog administration, because some children show an excessive decrease of growth velocity during GnRH analog therapy [80,81]. Adjunctive treatment with growth hormone has been proposed to optimize growth velocity and adult height of these patients, and some papers reported a benefit of this associated therapy [81,82,83]. This associated therapy is not licensed in several countries if a clear growth hormone deficiency is not diagnosed. Large discrepancies have been reported on the improvement of adult height in children treated by GnRH analogs (Table 7 and Table 8). The lacking standardization of inclusion criteria, protocols of treatment monitoring, cut-off values to define optimal LH and FSH suppression during treatment as well as criteria to stop treatment and differences in mid-parental height and in adult height prediction methods may play roles in these discrepancies. Future longitudinal trials should explore these items. In addition, evidence regarding other key long-term outcomes (such as infertility and malignant or metabolic diseases) were judged to be very weak, suggesting high benefits and few side effects of GnRH analog treatment [79]. Finally, psychological difficulties related to precocious pubertal onset, presence of menarche in girls and aggressiveness in males represent the main issues for treatment [53], but sound data on these aspects are poorly evaluated, mainly in males. Long-term high-quality studies on these matters are needed.
Author Contributions
Conceptualization, C.M. and S.B.; methodology, C.M. and N.T.; software, C.M. and G.I.B.; validation, S.B. and D.P.; formal analysis, C.M., N.T. and S.B.; investigation, N.T. and S.B.; resources, D.P.; data curation, C.M., N.T. and G.I.B.; writing—original draft preparation, C.M.; writing—review and editing, N.T., S.B. and G.I.B.; supervision, S.B.; project administration, D.P. and S.B.; funding acquisition, not done. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable: This review did not report any original data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brito, V.N.; Spinola-Castro, A.M.; Kochi, C.; Kopacek, C.; Da Silva, P.C.A.; Guerra-Júnior, G. Central precocious puberty: Revisiting the diagnosis and therapeutic management. Arch. Endocrinol. Metab. 2016, 60, 163–172. [Google Scholar] [CrossRef]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Bertelloni, S.; I Baroncelli, G. Current pharmacotherapy of central precocious puberty by GnRH analogs: Certainties and uncertainties. Expert Opin. Pharmacother. 2013, 14, 1627–1639. [Google Scholar] [CrossRef]
- Gonzales, E.R. For puberty that comes too soon, new treatment highly effective. J. Am. Med. Assoc. 1982, 248, 1149–1152. [Google Scholar] [CrossRef]
- Teilmann, G. Prevalence and incidence of precocious pubertal development in Denmark: An epidemiologic study based on national registries. Pediatrics 2005, 116, 1323–1328. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Corripio, R.; Labarta, J.I.; Cañete, R.; Castro-Feijóo, L.; Espino, R.; Argente, J. Central precocious puberty in children living in Spain: Incidence, prevalence, and influence of adoption and immigration. J. Clin. Endocrinol. Metab. 2010, 95, 4305–4313. [Google Scholar] [CrossRef]
- Le Moal, J.; Rigou, A.; Le Tertre, A.; De Crouy-Channel, P.; Léger, J.; Carel, J.-C. Marked geographic patterns in the incidence of idiopathic central precocious puberty: A nationwide study in France. Eur. J. Endocrinol. 2018, 178, 33–41. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, K.; Won, S.; Lee, K.-W.; Park, M.-J. A significant increase in the incidence of central precocious puberty among Korean girls from 2004 to 2010. PLoS ONE 2015, 10, e0141844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.-S. Incidence and prevalence of central precocious puberty in Korea: An epidemiologic study based on a national database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; De Masi, S.; Bencini, E.; Losi, S.; Paci, S.; Parpagnoli, M.; Ricci, F.; Ciofi, D.; Azzari, C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital. J. Pediatr. 2020, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wannes, S.; Elmaleh-Bergès, M.; Simon, D.; Zénaty, D.; Martinerie, L.; Storey, C.; Gelwane, G.; Paulsen, A.; Ecosse, E.; De Roux, N.; et al. High prevalence of syndromic disorders in patients with non-isolated central precocious puberty. Eur. J. Endocrinol. 2018, 179, 373–380. [Google Scholar] [CrossRef]
- Alikasifoglu, A.; Vuralli, D.; Gonc, E.N.; Ozon, A.; Kandemir, N. Changing etiological trends in male precocious puberty: Evaluation of 100 cases with central precocious puberty over the last decade. Horm. Res. Paediatr. 2015, 83, 340–344. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Yang, A.; Cho, S.Y.; Jin, D.-K. Etiological trends in male central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 75–80. [Google Scholar] [CrossRef]
- Yoon, J.S.; So, C.H.; Lee, H.S.; Lim, J.S.; Hwang, J.S. The prevalence of brain abnormalities in boys with central precocious puberty may be overestimated. PLoS ONE 2018, 13, e0195209. [Google Scholar] [CrossRef]
- Choi, K.H.; Chung, S.J.; Kang, M.J.; Yoon, J.Y.; Lee, J.E.; Lee, Y.A.; Shin, C.H.; Yang, S.W. Boys with precocious or early puberty: Incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 183–190. [Google Scholar] [CrossRef]
- Topor, L.S.; Bowerman, K.; Machan, J.T.; Gilbert, C.L.; Kangarloo, T.; Shaw, N.D. Central precocious puberty in Boston boys: A 10-year single center experience. PLoS ONE 2018, 13, e0199019. [Google Scholar] [CrossRef]
- Harbulot, C.; Lessim, S.; Simon, D.; Martinerie, L.; Storey, C.; Ecosse, E.; De Roux, N.; Carel, J.-C.; Léger, J. Prevalence and clinical characteristics of isolated forms of central precocious puberty: A cohort study at a single academic center. Eur. J. Endocrinol. 2021, 184, 243–251. [Google Scholar] [CrossRef]
- Thamdrup, E. Precocious sexual development: A clinical study of one hundred children. Dan. Med. Bull. 1961, 8, 104–105. [Google Scholar]
- Grumbach, M.M.; Styne, D.M. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In Williams Textbook of Endocrinology, 9th ed.; Saunders W.B.: Philadelphia, PA, USA, 1998; pp. 1509–1625. [Google Scholar]
- Bridges, N.A.; Christopher, J.A.; Hindmarsh, P.C.; Brook, C.G. Sexual precocity: Sex incidence and aetiology. Arch. Dis. Child. 1994, 70, 116–118. [Google Scholar] [CrossRef]
- Cisternino, M.; Arrigo, T.; Pasquino, A.; Tinelli, C.; Antoniazzi, F.; Beduschi, L.; Bindi, G.; Borrelli, P.; De Sanctis, V.; Farello, G.; et al. Etiology and age incidence of precocious puberty in girls: A multicentric study. J. Pediatr. Endocrinol. Metab. 2000, 13, 695–701. [Google Scholar] [CrossRef]
- De Sanctis, V.; Corrias, A.; Rizzo, V.; Bertelloni, S.; Urso, L.; Galluzzi, F.; Pasquino, A.; Pozzan, G.; Guarneri, M.; Cisternino, M.; et al. Etiology of central precocious puberty in males: The results of the Italian study group for physiopathology of puberty. J. Pediatr. Endocrinol. Metab. 2000, 13, 687–694. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Trivin, C.; Adan, L.; Gall, V.; Sainte-Rose, C.; Brauner, R. Central precocious puberty: Clinical and laboratory features. Clin. Endocrinol. 2001, 54, 289–294. [Google Scholar] [CrossRef]
- Klein, K.O.; Barnes, K.M.; Jones, J.V.; Feuillan, P.P.; Cutler, G.B.C., Jr. Increased final height in precocious puberty after long-term treatment with LHRH agonists: The national institutes of health experience. J. Clin. Endocrinol. Metab. 2001, 86, 4711–4716. [Google Scholar] [CrossRef]
- Lee, P.A.; Neely, E.K.; Fuqua, J.; Yang, D.; Larsen, L.M.; Mattia-Goldberg, C.; Chwalisz, K. Efficacy of leuprolide acetate 1-month depot for central precocious puberty (CPP): Growth outcomes during a prospective, longitudinal study. Int. J. Pediatr. Endocrinol. 2011, 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Thaiwong, M. Outcome of gonadotropin-releasing analog treatment for children with central precocious puberty: 15-year experience in Southern Thailand. J. Pediatr. Endocrinol. Metab. 2011, 24, 519–523. [Google Scholar] [CrossRef]
- Carel, J.-C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef]
- Pedicelli, S.; Alessio, P.; Scirè, G.; Cappa, M.; Cianfarani, S. Routine screening by brain magnetic resonance imaging is not indicated in every girl with onset of puberty between the ages of 6 and 8 years. J. Clin. Endocrinol. Metab. 2014, 99, 4455–4461. [Google Scholar] [CrossRef]
- Cantas-Orsdemir, S.; Garb, J.L.; Allen, H.F. Prevalence of cranial MRI findings in girls with central precocious puberty: A systematic review and meta-analysis. J. Pediatr. Endocrinol. Metab. 2018, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Burcher, G.C.; Liang, H.; Lancaster, R.; Cross, J.H.; Tisdall, M.; Varadkar, S.; Spoudeas, H.A.; Caredda, E.; Bennett, S.; Heyman, I. Neuropsychiatric profile of pediatric hypothalamic hamartoma: Systematic review and case series. Dev. Med. Child Neurol. 2019, 61, 1377–1385. [Google Scholar] [CrossRef]
- Harrison, V.S.; Oatman, O.; Kerrigan, J.F. Hypothalamic hamartoma with epilepsy: Review of endocrine comorbidity. Epilepsia 2017, 58, 50–59. [Google Scholar] [CrossRef]
- Virdis, R.; Sigorini, M.; Laiolo, A.; Lorenzetti, E.; Street, M.; Villani, A.; Donadio, A.; Pisani, F.; Terzi, C.; Garavelli, L. Neurofibromatosis Type 1 and precocious puberty. J. Pediatr. Endocrinol. Metab. 2000, 13, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, R.S.; Eugster, E.A. Central precocious puberty: From genetics to treatment. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bessa, D.S.; Macedo, D.B.; Brito, V.N.; França, M.M.; Montenegro, L.R.; Cunha-Silva, M.; Silveira, L.G.; Hummel, T.; Bergadá, I.; Braslavsky, D.; et al. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology 2017, 105, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.B.; Brito, V.N.; Latronico, A.C. New causes of central precocious puberty: The role of genetic factors. Neuroendocrinology 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Abreu, A.P.; Dauber, A.; Macedo, D.B.; Noel, S.D.; Brito, V.N.; Gill, J.C.; Cukier, P.; Thompson, I.R.; Navarro, V.M.; Gagliardi, P.C.; et al. Central precocious puberty caused by mutations in the imprinted gene MKRN. N. Engl. J. Med. 2013, 368, 2467–2475. [Google Scholar] [CrossRef]
- Grandone, A.; Capristo, C.; Cirillo, G.; Sasso, M.; Umano, G.R.; Mariani, M.; Del Giudice, E.M.; Perrone, L. Molecular screening of MKRN3, DLK1, and KCNK9 genes in girls with idiopathic central precocious puberty. Horm. Res. Paediatr. 2017, 88, 194–200. [Google Scholar] [CrossRef]
- Simon, D.; Ba, I.; Mekhail, N.; Ecosse, E.; Paulsen, A.; Zenaty, D.; Houang, M.; Perelroizen, M.J.; De Filippo, G.-P.; Salerno, M.; et al. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur. J. Endocrinol. 2016, 174, 1–8. [Google Scholar] [CrossRef]
- Brito, V.N.; Latronico, A.C.; Arnhold, I.J.P.; Mendonça, B.B. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Braz. Soc. Endocrinol. Metab. 2008, 52, 18–31. [Google Scholar] [CrossRef]
- Ab Rahim, S.N.; Omar, J.; Ismail, T.S.T. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: A mini review. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.N.; Batista, M.C.; Borges, M.F.; Latronico, A.C.; Kohek, M.B.F.; Thirone, A.C.P.; Jorge, B.H.; Arnhold, I.J.P.; Mendonca, B.B. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J. Clin. Endocrinol. Metab. 1999, 84, 3539–3544. [Google Scholar] [CrossRef][Green Version]
- Resende, E.A.M.R.; Lara, B.H.J.; Reis, J.D.; Ferreira, B.P.; Pereira, G.A.; Borges, M.F. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J. Clin. Endocrinol. Metab. 2007, 92, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wankanit, S.; Mahachoklertwattana, P.; Pattanaprateep, O.; Poomthavorn, P. Basal serum luteinising hormone cut-off, and its utility and cost-effectiveness for aiding the diagnosis of the onset of puberty in girls with early stages of breast development. Clin. Endocrinol. 2020, 92, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, H.K.; Ko, J.H.; Kim, Y.J.; Hwang, J.S. Utility of basal luteinizing hormone levels for detecting central precocious puberty in girls. Horm. Metab. Res. 2012, 44, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Neely, E.; Hintz, R.L.; Wilson, D.M.; Lee, P.A.; Gautier, T.; Argente, J.; Stene, M. Normal ranges for immunochemiluminometric gonadotropin assays. J. Pediatr. 1995, 127, 40–46. [Google Scholar] [CrossRef]
- Pasternak, Y.; Friger, M.; Loewenthal, N.; Haim, A.; Hershkovitz, E. The utility of basal serum LH in prediction of central precocious puberty in girls. Eur. J. Endocrinol. 2012, 166, 295–299. [Google Scholar] [CrossRef]
- Sigurjonsdottir, T.J.; Hayles, A.B. Precocious puberty. A report of 96 cases. Am. J. Dis. Child. 1968, 115, 309. [Google Scholar] [CrossRef]
- Bovier-Lapierre, M.; Sempé, M.; David, M. Etiological, clinical and biological aspects of precocious puberty of central origin. Pediatrie 1972, 27, 587–609. [Google Scholar]
- Paul, D.; Conte, F.A.; Grumbach, M.M.; Kaplan, S.L. Long-term effect of gonadotropin-releasing hormone agonist therapy on final and near-final height in 26 children with true precocious puberty treated at a median age of less than 5 years. J. Clin. Endocrinol. Metab. 1995, 80, 546–551. [Google Scholar] [CrossRef][Green Version]
- Bertelloni, S.; Mul, D. Treatment of central precocious puberty by GnRH analogs: Long-term outcome in men. Asian J. Androl. 2008, 10, 525–534. [Google Scholar] [CrossRef]
- Swaiss, H.H.; Khawaja, N.M.; Farahid, O.H.; Batieha, A.M.; Ajlouni, K.M. Effect of gonadotropin-releasing hormone analogue on final adult height among Jordanian children with precocious puberty. Saudi Med. J. 2017, 38, 1101–1107. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- Wojniusz, S.; Callens, N.; Sütterlin, S.; Andersson, S.; De Schepper, J.; Gies, I.; Vanbesien, J.; De Waele, K.; Van Aken, S.; Craen, M.; et al. Cognitive, emotional, and psychosocial functioning of girls treated with pharmacological puberty blockage for idiopathic central precocious puberty. Front. Psychol. 2016, 7, 1053. [Google Scholar] [CrossRef]
- Lahlou, N.; Carel, J.-C.; Chaussain, J.-L.; Roger, M. Pharmacokinetics and pharmacodynamics of GnRH agonists: Clinical implications in pediatrics. J. Pediatr. Endocrinol. Metab. 2000, 13, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, S.; Mucaria, C.; Baroncelli, G.I.; Peroni, D. Triptorelin depot for the treatment of children 2 years and older with central precocious puberty. Expert Rev. Clin. Pharmacol. 2018, 11, 659–667. [Google Scholar] [CrossRef]
- Bereket, A. A critical appraisal of the effect of gonadotropin-releasing hormon analog treatment on adult height of girls with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2018, 9, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Tauber, M.; Patel, B.; Dutailly, P. Meta-Analysis of paediatric patients with central precocious puberty treated with intramuscular triptorelin 11.25 mg 3-month prolonged-release formulation. Horm. Res. Paediatr. 2017, 87, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Freire, A.; Gryngarten, M.G.; Kletter, G.B.; Benson, M.; Miller, B.S.; Dajani, T.S.; A Eugster, E.; Mauras, N. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J. Clin. Endocrinol. Metab. 2020, 105, e3660–e3671. [Google Scholar] [CrossRef]
- Adan, L.; Chemaitilly, W.; Trivin, C.; Brauner, R. Factors predicting adult height in girls with idiopathic central precocious puberty: Implications for treatment. Clin. Endocrinol. 2002, 56, 297–302. [Google Scholar] [CrossRef]
- Brito, V.N.; Latronico, A.C.; Cukier, P.; Teles, M.G.; Silveira, L.F.G.; Arnhold, I.J.P.; Mendonca, B.B. Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J. Clin. Endocrinol. Metab. 2008, 93, 2662–2669. [Google Scholar] [CrossRef]
- Arrigo, T.; Cisternino, M.; Galluzzi, F.; Bertelloni, S.; Pasquino, A.M.; Antoniazzi, F.; Borrelli, P.; Crisafulli, G.; Wasniewska, M.; De Luca, F. Analysis of the factors affecting auxological response to GnRH agonist treatment and final height outcome in girls with idiopathic central precocious puberty. Eur. J. Endocrinol. 1999, 141, 140–144. [Google Scholar] [CrossRef]
- Cho, A.Y.; Ko, S.Y.; Lee, J.H.; Kim, E.Y. Relationship between final adult height and birth weight after gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Carel, J.-C.; Roger, M.; Ispas, S.; Tondu, F.; Lahlou, N.; Blumberg, J.; Chaussain, J.-L. Final height after long-term treatment with triptorelin slow release for central precocious puberty: Importance of statural growth after interruption of treatment. J. Clin. Endocrinol. Metab. 1999, 84, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Acquafredda, A.; DelVecchio, M.; Lonero, A.; Gaeta, A.; Bulzis, P.S.; Corica, D.; Velletri, M.R.; De Luca, F.; et al. Metabolic outcomes, bone health, and risk of polycystic ovary syndrome in girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogues. Horm. Res. Paediatr. 2017, 87, 162–169. [Google Scholar] [CrossRef]
- Tanaka, T.; Niimi, H.; Matsuo, N.; Fujieda, K.; Tachibana, K.; Ohyama, K.; Satoh, M.; Kugu, K. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: Evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR japanese study group on central precocious puberty. J. Clin. Endocrinol. Metab. 2005, 90, 1371–1376. [Google Scholar] [CrossRef]
- Heger, S.; Partsch, C.-J.; Sippell, W.G. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: Final height, body proportions, body composition, bone mineral density, and reproductive function. J. Clin. Endocrinol. Metab. 1999, 84, 4583–4590. [Google Scholar] [CrossRef][Green Version]
- Vuralli, D.; Gonc, N.E.; Ozon, Z.A.; Kandemir, N.; Alikasifoglu, A. Which parameters predict the beneficial effect of GnRHa treatment on height in girls with central precocious puberty? Clin. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Kauli, R.; Galatzer, A.; Kornreich, L.; Lazar, L.; Pertzelan, A.; Laron, Z. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. Horm. Res. 1997, 47, 54–61. [Google Scholar] [CrossRef]
- Pasquino, A.M.; Pucarelli, I.; Accardo, F.; Demiraj, V.; Segni, M.; Di Nardo, R. Long-Term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogs: Impact on adult height, body mass index, bone mineral content, and reproductive function. J. Clin. Endocrinol. Metab. 2008, 93, 190–195. [Google Scholar] [CrossRef]
- Mul, D.; Bertelloni, S.; Carel, J.-C.; Saggese, G.; Chaussain, J.; Oostdijk, W. Effect of gonadotropin-releasing hormone agonist treatment in boys with central precocious puberty: Final height results. Horm. Res. 2002, 58, 1–7. [Google Scholar] [CrossRef]
- Shim, Y.S.; Lim, K.I.; Lee, H.S.; Hwang, J.S. Long-term outcomes after gonadotropin-releasing hormone agonist treatment in boys with central precocious puberty. PLoS ONE 2020, 15, e0243212. [Google Scholar] [CrossRef]
- Vincenzo, D.S.; Ashraf, T.S.; Salvatore, D.M.; Nada, S.; Heba, E.; De Sanctis, V.; Soliman, A.T.; Di Maio, S.; Soliman, N.; Elsedfy, H. Long-term effects and significant adverse drug reactions (ADRs) associated with the use of gonadotropin-releasing hormone analogs (GnRHa) for central precocious puberty: A brief review of literature. Acta Biomed. 2019, 90, 345–359. [Google Scholar]
- Manasco, P.K.; Pescovitz, O.H.; Feuillan, P.P.; Hench, K.D.; Barnes, K.M.; Jones, J.; Hill, S.C.; Loriaux, D.L.; Cutler, G.B. Resumption of puberty after long term luteinizing hormone-releasing hormone agonist treatment of central precocious puberty. J. Clin. Endocrinol. Metab. 1988, 67, 368–372. [Google Scholar] [CrossRef]
- Magiakou, M.A.; Manousaki, D.; Papadaki, M.; Hadjidakis, D.; Levidou, G.; Vakaki, M.; Papaefstathiou, A.; Lalioti, N.; Kanaka-Gantenbein, C.; Piaditis, G.; et al. The efficacy and safety of gonadotropin-releasing hormone analog treatment in childhood and adolescence: A single center, long-term follow-up study. J. Clin. Endocrinol. Metab. 2010, 95, 109–117. [Google Scholar] [CrossRef]
- Chiavaroli, V.; Liberati, M.; D’Antonio, F.; Masuccio, F.; Capanna, R.; Verrotti, A.; Chiarelli, F.; Mohn, A. GNRH analog therapy in girls with early puberty is associated with the achievement of predicted final height but also with increased risk of polycystic ovary syndrome. Eur. J. Endocrinol. 2010, 163, 55–62. [Google Scholar] [CrossRef]
- Ramos, C.O.; Latronico, A.C.; Cukier, P.; Macedo, D.B.; Bessa, D.S.; Cunha-Silva, M.; Arnhold, I.J.; Mendonca, B.B.; Brito, V.N. Long-Term outcomes of patients with central precocious puberty due to hypothalamic hamartoma after GnRHa treatment: Anthropometric, metabolic, and reproductive aspects. Neuroendocrinology 2018, 106, 203–210. [Google Scholar] [CrossRef]
- Grinspon, R.P.; Andreone, L.; Bedecarrás, P.; Ropelato, M.G.; Rey, R.A.; Campo, S.M.; Bergadá, I. Male central precocious puberty: Serum profile of anti-müllerian hormone and inhibin B before, during, and after treatment with GnRH analogue. Int. J. Endocrinol. 2013, 2013, 823064. [Google Scholar] [CrossRef]
- Nam, H.-K.; Kim, H.R.; Rhie, Y.-J.; Lee, K.-H. Serum anti-müllerian hormone levels in precocious puberty girls according to stage of GnRH agonist treatment. J. Korean Med. Sci. 2017, 32, 475–479. [Google Scholar] [CrossRef]
- Luo, X.; Liang, Y.; Hou, L.; Wu, W.; Ying, Y.; Ye, F. Long-term efficacy and safety of gonadotropin-releasing hormone analog treatment in children with idiopathic central precocious puberty: A systematic review and meta-analysis. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Şahin, N.M.; Dikmen, A.U.; Çetinkaya, S.; Aycan, Z. Subnormal growth velocity and related factors during GnRH analog therapy for idiopathic central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 239–246. [Google Scholar] [CrossRef]
- Pasquino, A.M.; Pucarelli, I.; Segni, M.; Matrunola, M.; Cerrone, F. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone analogues and growth hormone. J. Clin. Endocrinol. Metab. 1999, 84, 449–452. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Cheng, X.; Luo, Y.; Wen, Y. Effects and safety of combination therapy with gonadotropin-releasing hormone analogue and growth hormone in girls with idiopathic central precocious puberty: A meta-analysis. J. Endocrinol. Investig. 2016, 39, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Luo, Y.; Ye, J.; Shen, X. Comparative efficacy and safety of three current clinical treatments for girls with central precocious puberty: A network meta-analysis. Endocr. Pract. 2019, 25, 717–728. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).