ETPTA Inverse Photonic Crystals for the Detection of Alcohols
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the ETPTA IPhCs
2.3. Apparatus
3. Results and Discussion
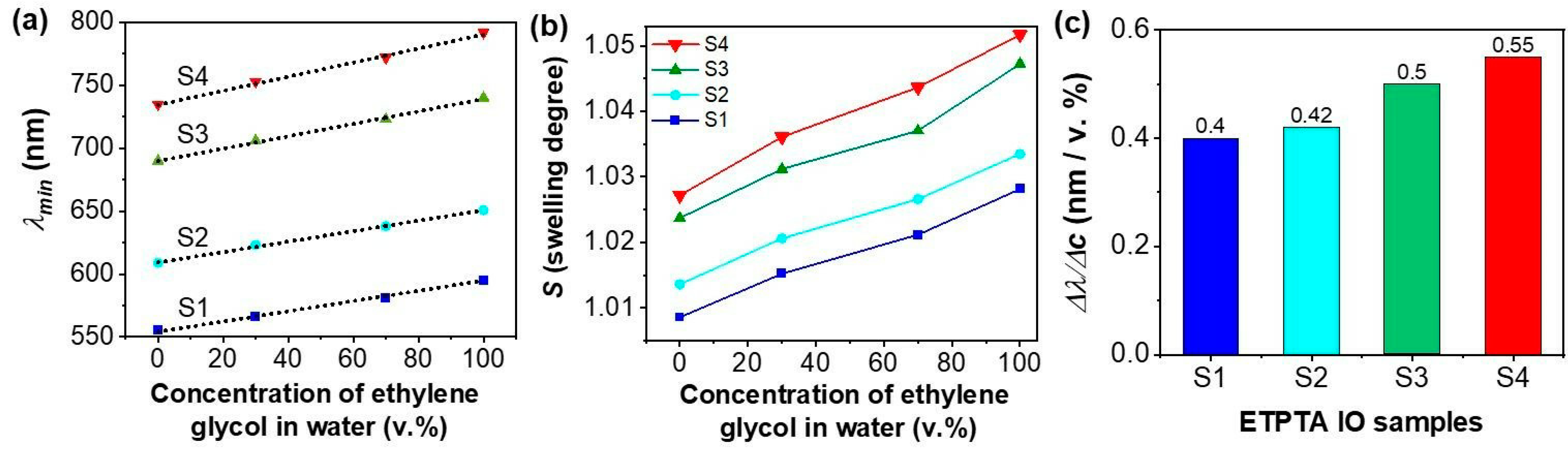
3.1. Characterization of ETPTA IPhCs
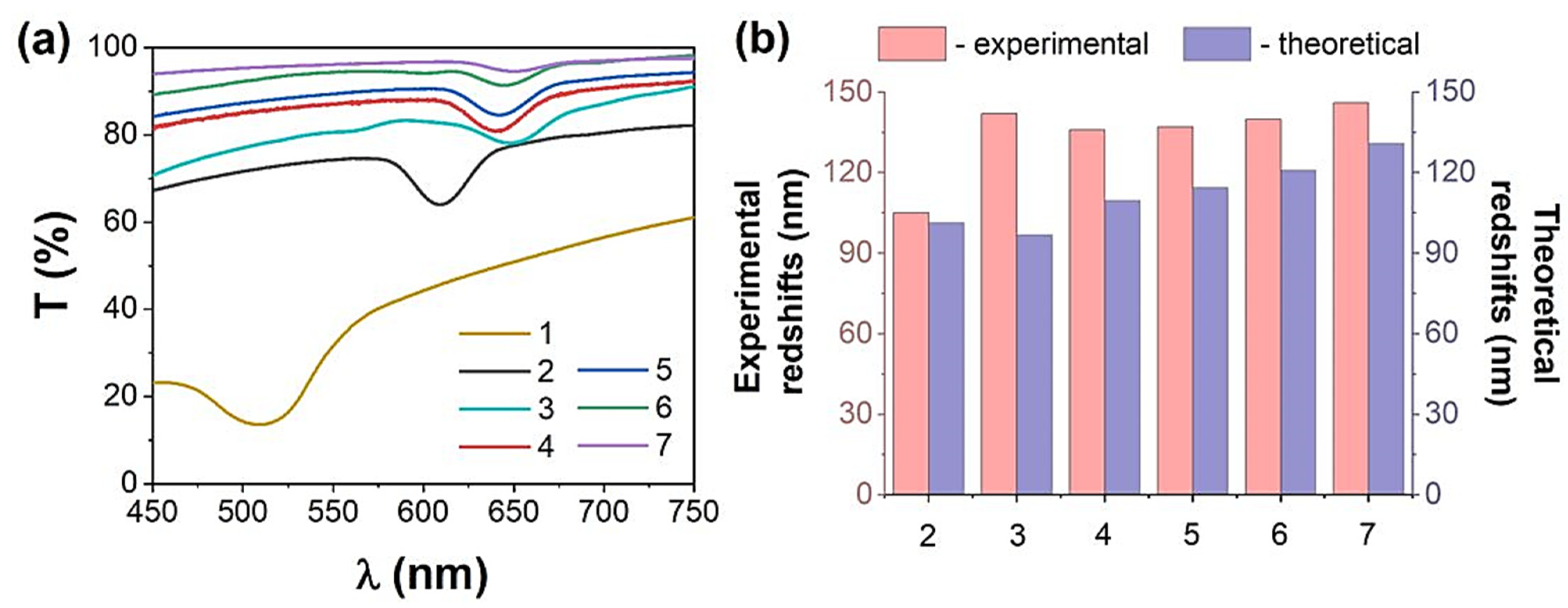
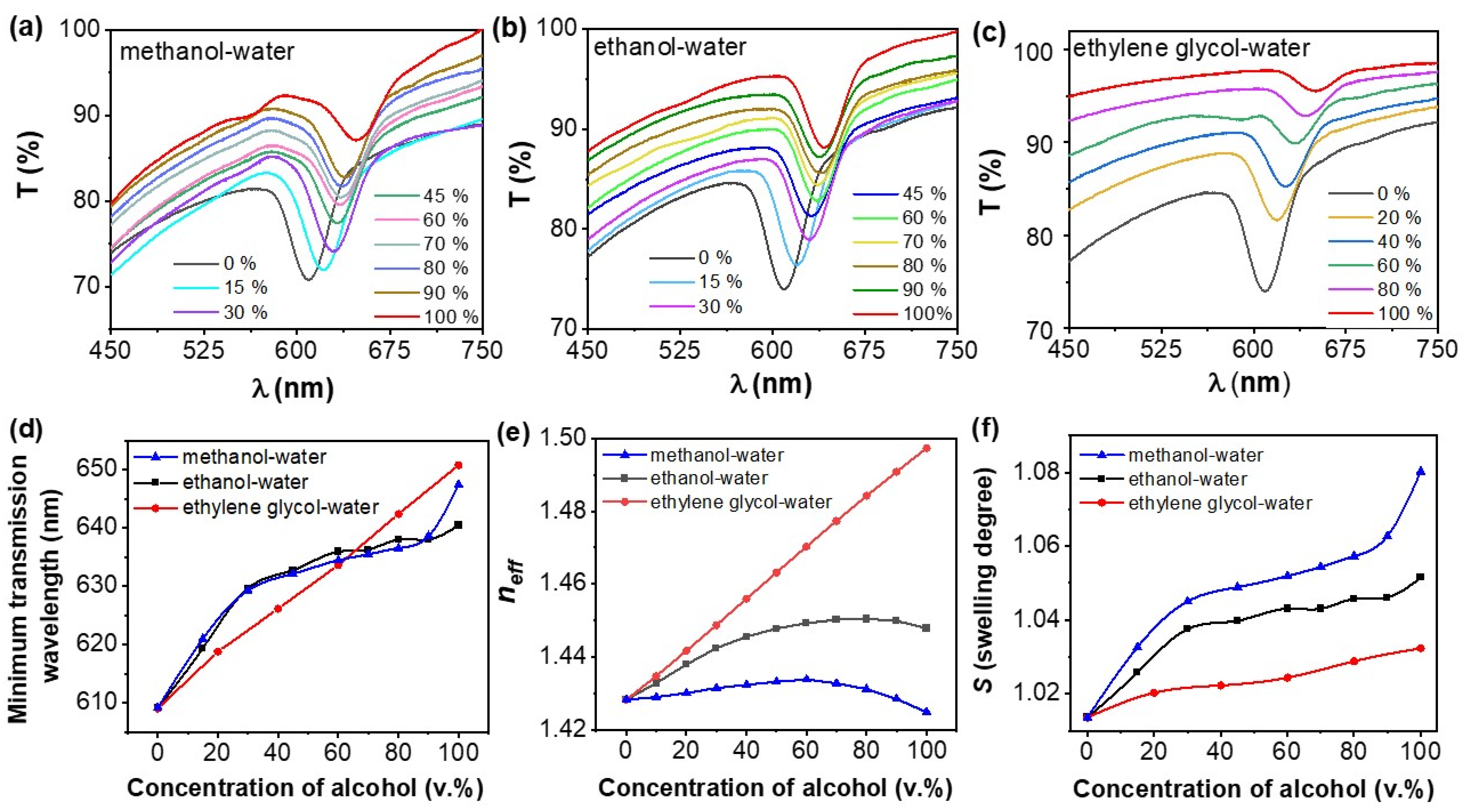
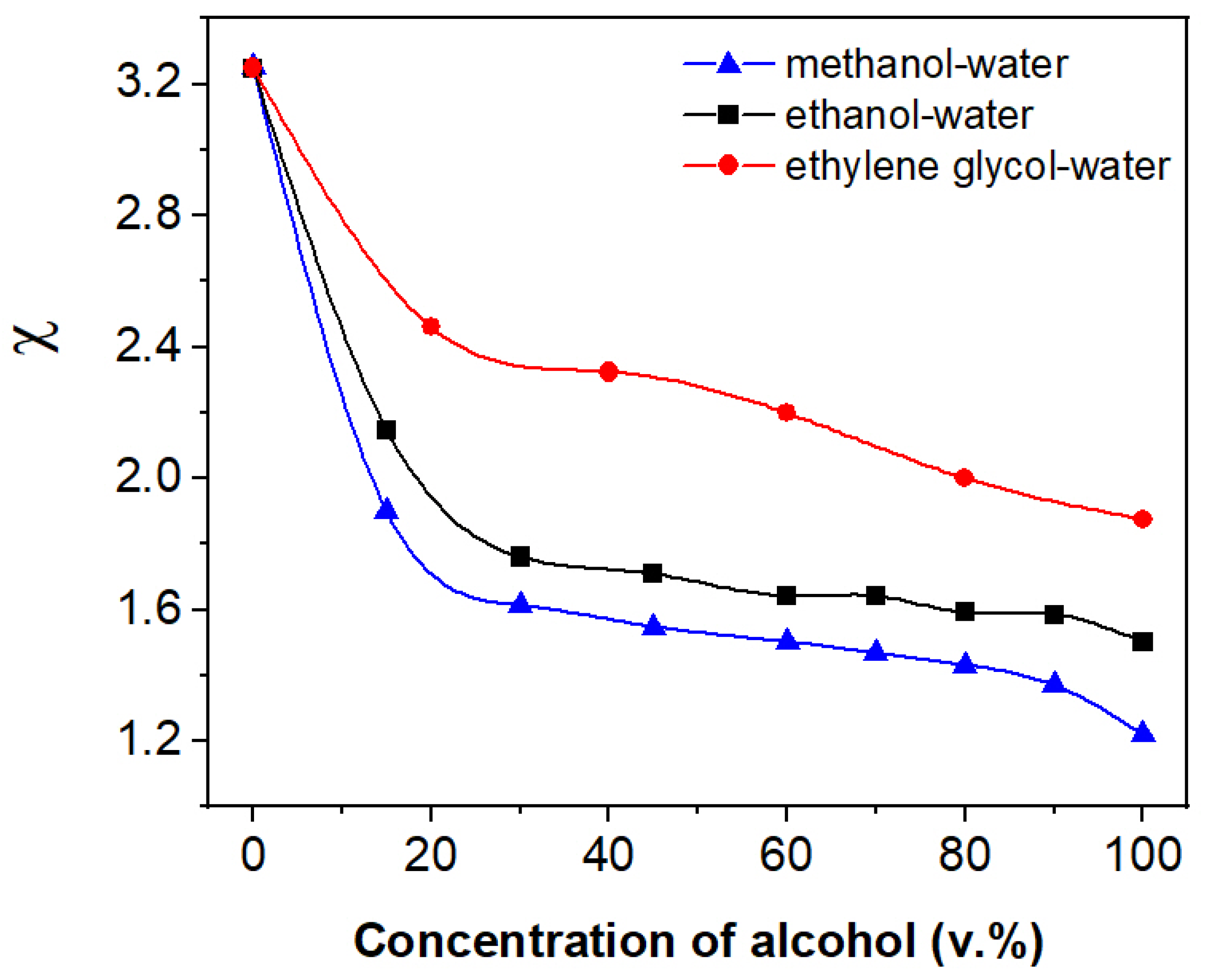
3.2. The Response of the ETPTA IPhCs to Alcohols
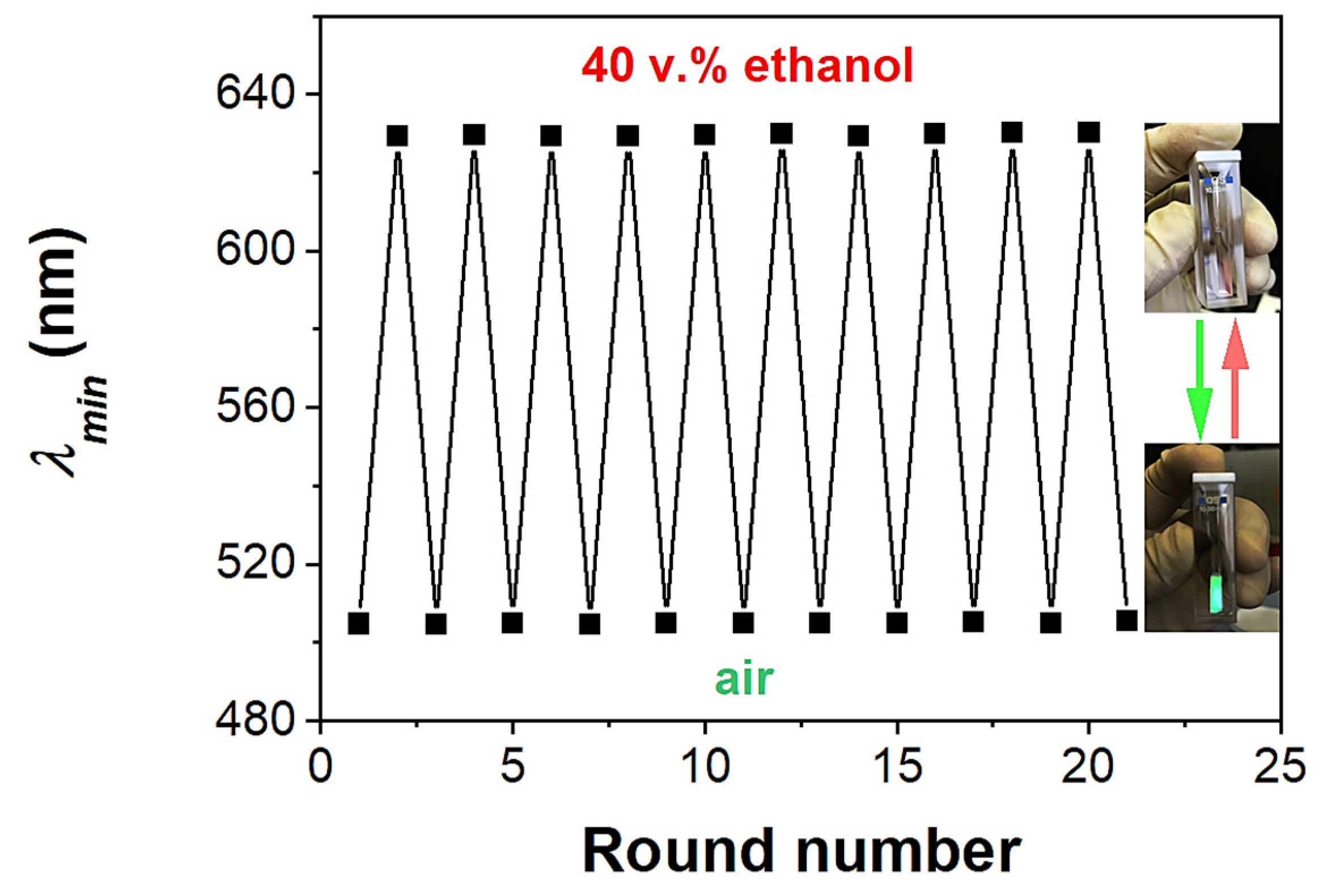
3.3. Recyclability of ETPTA IO Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuchuk, V.I.; Shirokova, I.Y.; Golikova, E.V. Physicochemical properties of water-alcohol mixtures of a homological series of lower aliphatic alcohols. Glass Phys. Chem. 2012, 38, 460–465. [Google Scholar] [CrossRef]
- Derlacki, Z.J.; Easteal, A.J.; Edge, A.V.J.; Woolf, L.A.; Roksandic, Z. Diffusion coefficients of methanol and water and the mutual diffusion coefficient in methanol-water solutions at 278 and 298 K. J. Phys. Chem. 1985, 89, 5318–5322. [Google Scholar] [CrossRef]
- Jimenez Rioboo, R.J.; Philipp, M.; Ramos, M.A.; Kruger, J.K. Concentration and temperature dependence of the refractive index of ethanol-water mixtures: Influence of intermolecular interactions. Eur. Phys. J. E 2009, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2500. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Chiappini, A.; Tran, L.T.N.; Trejo-García, P.M.; Zur, L.; Lukowiak, A.; Ferrari, M.; Righini, G.C. Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review. Micromachines 2020, 11, 290. [Google Scholar] [CrossRef]
- Guddala, S.; Kamanoor, S.A.; Chiappini, A.; Ferrari, M.; Desai, N.R. Experimental investigation of photonic band gap influence on enhancement of Raman-scattering in metal-dielectric colloidal crystals. J. Appl. Phys. 2012, 112, 084303. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Eremina, E.A.; Abdusatorov, B.A.; Klimonsky, S.O. Synthesis of SiO2@Ag core-shell photonic crystals for SERS application. J. Phys. Conf. Ser. 2019, 1410, 012165. [Google Scholar] [CrossRef]
- Almohamed, Y.; Barille, R.; Vodchits, A.I.; Voinov, Y.P.; Gorelik, V.S.; Kudryavtseva, A.D.; Orlovich, V.A.; Chernega, N.V. Reduction of the threshold of stimulated Raman scattering in Raman-active media introduced into pores of a globular photonic crystal. JETP Lett. 2015, 101, 365–370. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Abdusatorov, B.A.; Baranchikov, A.E.; Klimonsky, S.O. Surface-enhanced Raman scattering in ETPTA inverse photonic crystals with gold nanoparticles. Phys. Chem. Chem. Phys. 2021, 23, 20275–20281. [Google Scholar] [CrossRef]
- Wei, W.; Shi, A.; Wu, T.; Wei, J.; Guo, J. Thermo-responsive shape and optical memories of photonic composite films enabled by glassy liquid crystalline polymer networks. Soft Matter 2016, 12, 8534–8541. [Google Scholar] [CrossRef] [PubMed]
- Ashurov, M.; Gorelik, V.; Napolskii, K.; Klimonsky, S. Anodic Alumina Photonic Crystals as Refractive Index Sensors for Controlling the Composition of Liquid Mixtures. Photonic Sens. 2020, 10, 147–154. [Google Scholar] [CrossRef]
- Miller, B.H.; Liu, H.; Kolle, M. Scalable optical manufacture of dynamic structural colour in stretchable materials. Nat. Mater. 2022, 21, 1014–1018. [Google Scholar] [CrossRef]
- Ma, W.; Li, B.; Jiang, L.; Sun, Y.; Wu, Y.; Zhao, P.; Chen, G. A bioinspired, electroactive colorable and additive manufactured photonic artificial muscle. Soft Matter 2022, 18, 1617–1627. [Google Scholar] [CrossRef]
- Lee, K.; Asher, S.A. Photonic crystal chemical sensors: pH and ionic strength. Am. Chem. Soc. 2000, 122, 9534–9537. [Google Scholar] [CrossRef]
- Yang, Z.K.; Shi, D.J.; Zhang, X.D.; Liu, H.H.; Chen, M.Q.; Liu, S.R. Co-deposition motif for constructing inverse opal photonic crystals with pH sensing. RSC Adv. 2015, 5, 69263–69267. [Google Scholar] [CrossRef]
- Matsubara, K.; Watanabe, M.; Takeoka, Y. A thermally adjustable multicolor photochromic hydrogel. Angew. Chem. Int. Ed. 2007, 46, 1688–1692. [Google Scholar] [CrossRef]
- Bolshakov, E.S.; Schemelev, I.S.; Ivanov, A.V.; Kozlov, A.A. Photonic Crystals and Their Analogues as Tools for Chemical Analysis. J. Anal. Chem. 2022, 77, 875–898. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lim, J.-M.; Lee, S.-K.; Heo, C.-J.; Yang, S.-M. Biofunctional colloids and their assemblies. Soft Matter 2010, 6, 1092–1110. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Ezhov, A.A.; Kazakova, T.A.; Klimonsky, S.O. Self-assembly of lines of microscopic photonic crystals. J. Phys. Conf. Ser. 2018, 1124, 051008. [Google Scholar] [CrossRef]
- Froyen, A.A.F.; Schenning, A.P.H.J. A multifunctional structural coloured electronic skin monitoring body motion and temperature. Soft Matter 2023, 19, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yin, Y.; Pan, Z.; Chen, M.; Zhang, L.; Liu, Y.; Zhang, Y.; Gao, J. Fabrication of 3D Photonic Crystals from Chitosan That Are Responsive to Organic Solvents. Biomacromolecules 2014, 15, 4396–4402. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-L.; Tsai, H.-P.; Lin, K.-Y.; Chen, Y.-C.; Yang, H. Reusable macroporous photonic crystal-based ethanol vapor detectors by doctor blade coating. J. Colloid Interface Sci. 2017, 487, 360–369. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Z.; Xue, M.; Xue, F.; Wang, Q.; Meng, Z.; Lu, W.; Chen, W.; Qi, F.; Yan, Z. Cellulose photonic crystal film sensor for alcohols. Sens. Act. B 2015, 220, 222–226. [Google Scholar] [CrossRef]
- Kou, D.; Zhang, S.; Lutkenhaus, J.L.; Wang, L.; Tang, B.; Ma, W. Porous organic/inorganic hybrid one-dimensional photonic crystals for rapid visual detection of organic solvents. J. Mater. Chem. C 2018, 6, 2704–2711. [Google Scholar] [CrossRef]
- Alee, K.S.; Sriram, G.; Rao, D.N. Spectral and morphological changes of 3D polystyrene photonic crystals with the incorporation of alcohols. Opt. Mater. 2012, 34, 1077–1081. [Google Scholar] [CrossRef]
- Xu, M.; Goponenko, A.V.; Asher, S.A. Polymerized polyHEMA photonic crystals: pH and ethanol sensor materials. J. Am. Chem. Soc. 2008, 130, 3113–3119. [Google Scholar] [CrossRef]
- Pan, Z.; Ma, J.; Yan, J.; Zhou, M.; Gao, J. Response of inverse-opal hydrogels to alcohols. J. Mater. Chem. 2012, 22, 2018–2025. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, X.; Yang, Z.; Liu, S.; Chen, M. Fabrication of PAM/PMAA inverse opal photonic crystal hydrogels by a “sandwich” method and their pH and solvent responses. RSC Adv. 2016, 6, 85885–85890. [Google Scholar] [CrossRef]
- Guddala, S.; Alee, K.S.; Rao, D.N. Fabrication of multifunctional SnO2 and SiO2-SnO2 inverse opal structures with prominent photonic band gap properties. Opt. Mater. Express 2013, 3, 407–417. [Google Scholar] [CrossRef]
- Nishijima, Y.; Ueno, K.; Juodkazis, S. Inverse silica opal photonic crystals for optical sensing applications. Opt. Express 2007, 15, 12979–12988. [Google Scholar] [CrossRef] [PubMed]
- Klimonsky, S.O.; Abramova, V.V.; Sinitskii, A.S.; Tretyakov, Y.D. Photonic crystals based on opals and inverse opals: Synthesis and structural features. Russ. Chem. Rev. 2011, 80, 1191–1207. [Google Scholar] [CrossRef]
- Bertone, J.F.; Jiang, P.; Hwang, K.S.; Mittleman, D.M.; Colvin, V.L. Thickness Dependence of the Optical Properties of Ordered Silica-Air and Air-Polymer Photonic Crystals. Phys. Rev. Lett. 1999, 83, 300–303. [Google Scholar] [CrossRef]
- Ozaki, M.; Shimoda, Y.; Kasano, M.; Yoshino, K. Electric field tuning of the stop band in a liquid-crystal infiltrated polymer inverse opal. Adv. Mater. 2002, 14, 514–518. [Google Scholar] [CrossRef]
- Tuyen, L.D.; Wu, C.Y.; Anh, T.K.; Minh, L.Q.; Kan, H.-C.; Hsu, C.C. Fabrication and optical characterisation of SiO2 opal and SU-8 inverse opal photonic crystals. J. Exp. Nanosci. 2012, 7, 198–204. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Bakhia, T.; Saidzhonov, B.M.; Klimonsky, S.O. Preparation of inverse photonic crystals by ETPTA photopolymerization method and their optical properties. Jour. Phys. Conf. Ser. 2020, 1461, 012009. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Ikrami, S.A.; Klimonsky, S.O. Photonic crystal sensors for the composition of liquids based on films with the structure of inverse opal. Inorg. Mater. Appl. Res. 2020, 12, 915–921. [Google Scholar] [CrossRef]
- Abdurakhmonov, S.D.; Ashurov, M.S.; Klimonsky, S.O.; Tcherniega, N.V. Numerical Simulation of Optical Properties of Photonic Crystals with Inverse Opal Structure. Bull. Lebedev Phys. Inst. 2022, 49, 137–144. [Google Scholar] [CrossRef]
- Klimonsky, S.O.; Bakhia, T.; Knotko, A.V.; Lukashin, A.V. Synthesis of Narrow-Dispersed SiO2 Colloidal Particles and Colloidal Crystal Films Based on Them. Dokl. Chem. 2014, 457, 115–117. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V. Single-Crystal Colloidal Multilayers of Controlled Thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Cai, Z.; Teng, J.; Xiong, Z.; Li, Y.; Li, Q.; Lu, X.; Zhao, X.S. Fabrication of TiO2 Binary Inverse Opals without Overlayers via the Sandwich-Vacuum Infiltration of Precursor. Langmuir 2011, 27, 5157–5164. [Google Scholar] [CrossRef]
- Ashurov, M.; Baranchikov, A.; Klimonsky, S. Photonic crystal enhancement of Raman Scattering. Phys. Chem. Chem Phys 2020, 22, 9630–9636. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics: Section 8: Analytical Chemistry, 84th ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–128. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; p. 672. [Google Scholar]
- Li, J.L.; Zheng, T.S. A comparison of chemical sensors based on the different ordered inverse opal films. Sens. Act. B 2008, 131, 190–195. [Google Scholar] [CrossRef]
- Amrehn, S.; Wu, X.; Schumacher, C.; Wagner, T. Photonic crystal-based fluid sensors: Toward practical application. Phys. Status Solidi A 2015, 212, 1266–1272. [Google Scholar] [CrossRef]
- Yan, P.; Fei, G.T.; Shang, G.L.; Wu, B.; Zhang, L.D. Fabrication of one-dimensional alumina photonic crystals with a narrow band gap and their application to high-sensitivity sensors. J. Mater. Chem. C 2013, 1, 1659–1664. [Google Scholar] [CrossRef]
- Abdurakhmonov, S.D.; Ashurov, M.S.; Klimonsky, S.O.; Tcherniega, N.V.; Gorelik, V.S. Numerical Simulation of Optical Properties of One-Dimensional Photonic Crystals Based on Anodic Aluminum Oxide. Bull. Lebedev Phys. Inst. 2022, 49, 294–301. [Google Scholar] [CrossRef]
- Guo, D.-L.; Fan, L.-X.; Wang, F.-H.; Huang, S.-Y.; Zou, X.-W. Porous Anodic Aluminum Oxide Bragg Stacks as Chemical Sensors. J. Phys. Chem. C 2008, 112, 17952–17956. [Google Scholar] [CrossRef]
- Lee, J.; Bae, K.; Kang, G.; Choi, M.; Baek, S.; Yoo, D.-S.; Lee, C.-W.; Kim, K. Graded-lattice AAO photonic crystal heterostructure for high Q refractive index sensing. RSC Adv. 2015, 5, 71770–71777. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Santos, A. On the precise tuning of optical filtering features in nanoporous anodic alumina distributed Bragg reflectors. Sci. Rep. 2018, 8, 4642. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystal based sensor for organic solvents and for solvent-water mixtures. Sensors 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed]
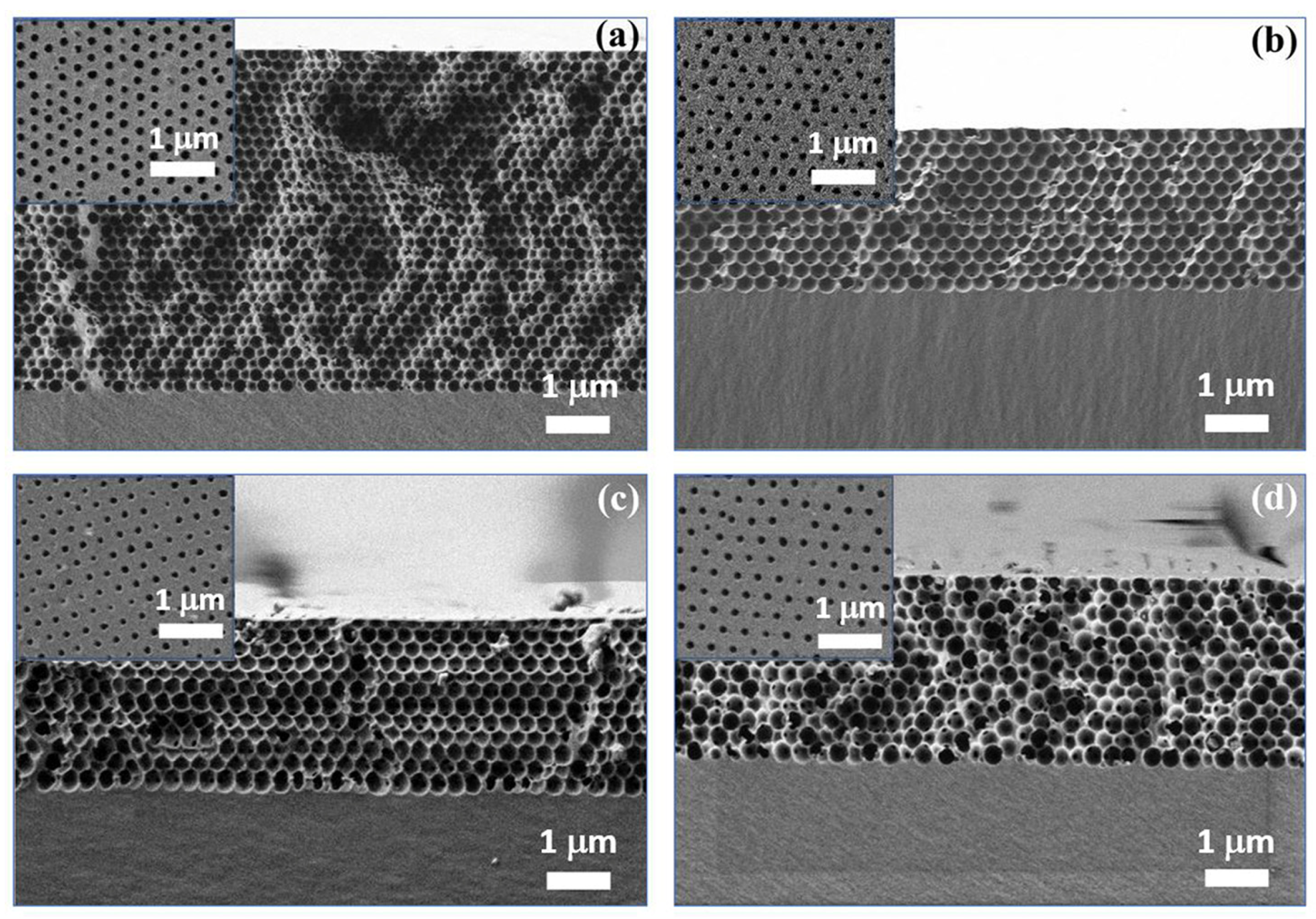
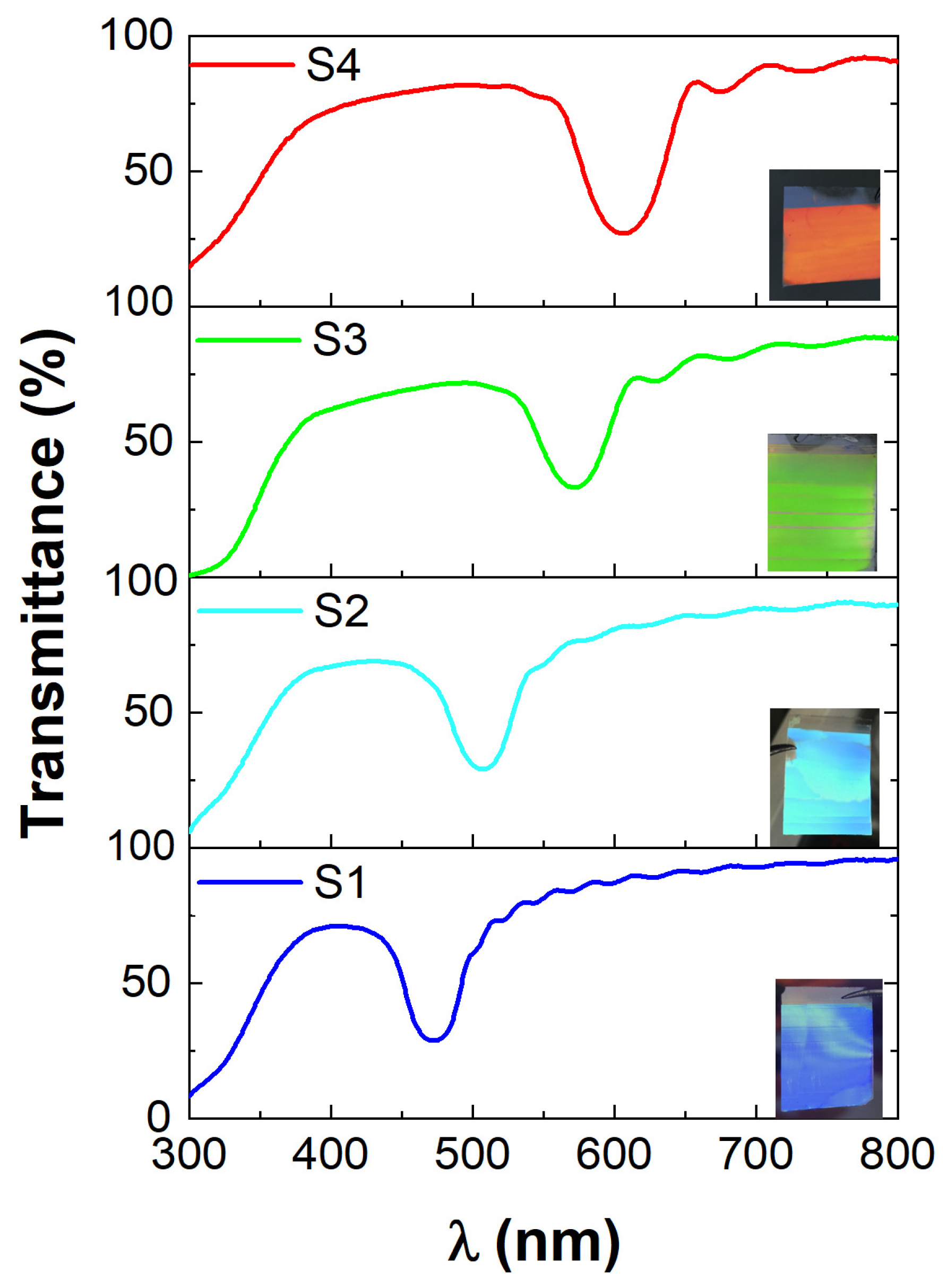
| Sample Number | Diameter of the Spherical Voids, D, nm 1 | Wavelength of Transmittance Minimum of the IPhCs, λmin, nm 2 | The Thickness of the IPhC Films, d, μm 1,2 |
|---|---|---|---|
| S1 | 240 ± 8 | 470 | 5.60 ± 0.10 |
| S2 | 260 ± 7 | 508 | 3.72 ± 0.15 |
| S3 | 290 ± 8 | 572 | 1.95 ± 0.15 |
| S4 | 310 ± 9 | 607 | 3.03 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashurov, M.; Kutrovskaya, S.; Baranchikov, A.; Klimonsky, S.; Kavokin, A. ETPTA Inverse Photonic Crystals for the Detection of Alcohols. Condens. Matter 2023, 8, 68. https://doi.org/10.3390/condmat8030068
Ashurov M, Kutrovskaya S, Baranchikov A, Klimonsky S, Kavokin A. ETPTA Inverse Photonic Crystals for the Detection of Alcohols. Condensed Matter. 2023; 8(3):68. https://doi.org/10.3390/condmat8030068
Chicago/Turabian StyleAshurov, Matin, Stella Kutrovskaya, Alexander Baranchikov, Sergey Klimonsky, and Alexey Kavokin. 2023. "ETPTA Inverse Photonic Crystals for the Detection of Alcohols" Condensed Matter 8, no. 3: 68. https://doi.org/10.3390/condmat8030068
APA StyleAshurov, M., Kutrovskaya, S., Baranchikov, A., Klimonsky, S., & Kavokin, A. (2023). ETPTA Inverse Photonic Crystals for the Detection of Alcohols. Condensed Matter, 8(3), 68. https://doi.org/10.3390/condmat8030068








